Epitaxy-driven vertical growth of single-crystalline cobalt nanowire arrays by chemical vapor deposition†
Si-in
Kim
a,
Hana
Yoon
ab,
Hyoban
Lee
a,
Sunghun
Lee
a,
Younghun
Jo
c,
Sungyul
Lee
*d,
Jaebum
Choo
*e and
Bongsoo
Kim
*a
aDepartment of Chemistry, KAIST, Daejeon 305-701, Korea. E-mail: bongsoo@kaist.ac.kr; Fax: +82-42-350-2810
bEnergy Storage Department, KIER, Daejeon 305-343, Korea
cNano Materials Research Team, KBSI, Daejeon 305-333, Korea
dDepartment of Applied Chemistry, Kyung Hee University, Kyungki 446-701, Korea. E-mail: sylee@khu.ac.kr; Fax: +82-31-204-8122
eDepartment of Bionano Engineering, Hanyang University, Ansan, 426-791, Korea. E-mail: jbchoo@hanyang.ac.kr; Fax: +82-31-436-8188
First published on 23rd October 2014
Abstract
Highly oriented single-crystalline ferromagnetic Co nanowire (NW) arrays were synthesized on sapphire substrates via a single-step chemical vapor deposition (CVD) method. On an m-cut sapphire substrate, Co NWs were vertically grown in epitaxial relationship with the substrate without using any catalysts or templates. On an r-cut sapphire substrate, Co NWs were horizontally grown in two perpendicular directions. Furthermore, we report that the Co NWs were transformed into Co3O4 nanotubes by thermal annealing under dilute O2 conditions. Such formation of hollow structures is ascribed to favored outward diffusion of Co ions. The present vertically aligned arrays of single-crystalline Co NWs could be utilized for advanced magnetic memory applications owing to their uniform orientations.
Introduction
Metal nanowires (NWs) that are ferromagnetic at room temperature have played important roles in diverse applications of nanoelectronics and nanospintronics.1–5 In the fabrication of NW devices, while the bottom-up approach is simpler in synthesis procedures as well as capable of producing NWs with more diverse compositions than the top-down approach, it requires technologies to integrate as-grown materials into the desired platform.6 Well-ordered ferromagnetic NW arrays can be utilized for high-density magnetic information storage.1,4 In order to drive these ferromagnetic NWs into efficient device components, it is also critical to form single-crystalline NWs because the crystallinity of ferromagnetic NWs can significantly affect both the behavior of the magnetic domain wall inside the NW and subsequent implementation of the reliable device.7 Therefore, it is highly desirable to synthesize single-crystalline Co NWs in a specific orientation for future high-density magnetic recording devices and magnetic sensors.So far, the most common and well-studied technique for the synthesis of Co NWs has been the electrodeposition method based on a template having anisotropic channels such as anodic aluminum oxide (AAO), polycarbonate, and diblock copolymers.8–13 However, these methods require complex multi-step template preparation as well as post-synthesis NW purification processes. It is also rather difficult to synthesize single-crystalline NWs by these methods. Recently, Liakakos et al. reported direct epitaxial vertical growth of hexagonal close-packed (hcp) Co NWs on a metal film by reduction of the coordination compound in the solution phase,14 which was much more effective than previous methods. In these solution-based synthesis methods, vertically grown NWs aggregate into a bundle form as the solvent evaporates, making it rather hard to fabricate independent memory units of individual NWs.
Herein, we report epitaxial growth of Co NW arrays in vertical orientation on a sapphire substrate by a single-step chemical vapor deposition (CVD) method without using any templates. The CVD method only requires suitable substrates and precursors without template materials. It made possible synthesis of many useful single-crystalline metal NWs including Ni, Au, Pd, and AuPd cost-effectively with simple preparation steps.15,16 Furthermore, He et al. produced ultrathin Pb NWs in 6 nm pores of SBA-15 mesoporous silica substrates by the CVD method.17 This approach provides a simple synthesis process of aligned arrays of single-crystalline Co NWs. The single-crystallinity and ferromagnetism of Co NWs were confirmed by electron diffraction and superconducting quantum interference device (SQUID) measurement, respectively. Furthermore, as-synthesized Co NWs can be transformed into Co3O4 nanotubes through thermal annealing in the presence of dilute O2. It is anticipated that the aligned arrays of single-crystalline and ferromagnetic Co NWs could be quite valuable for the development of advanced magnetic memory applications by integration of nanomaterials. Subsequently fabricated Co3O4 nanotubes can be utilized as heterogeneous catalysts, gas sensors, and electrochromatic devices.18–20
Experimental
Synthesis of Co NWs
We synthesized Co NWs in a 1 inch diameter quartz tube using a horizontal hot-wall single/dual zone furnace (ESI, Fig. S1a†). The setup was equipped with pressure and mass flow controllers. The upstream (US) zone and downstream (DS) zone were used for vaporization of the precursor and NW growth, respectively. Anhydrous CoCl2 beads (0.05 g, 99.9% purity, Sigma-Aldrich) in an alumina boat used as Co precursors were placed at the center of the US heating zone. Prior to the start of the deposition, all Al2O3 substrates were cut by a diamond cutter to a 5 × 5 mm2 size, cleaned with acetone in an ultrasonic bath for 10 min, and then dried under nitrogen gas. The Co NWs grew on a rectangular Al2O3 substrate placed on carbon powder (99.9% purity, Alfa), which was located at ∼12 cm downstream from the precursors. The US zone and DS zone were then heated at 670 and 950 °C, respectively, for a reaction time of 10 min with Ar flow at 200 sccm, while the chamber pressure was maintained at 760 Torr during the reaction. No catalyst or template was used. The as-synthesized vertical Co NWs are not easily removed from the substrate due to their epitaxial relationship with the substrate. To prepare a TEM sample, we could disperse some vertical Co NWs in ethanol by ultrasonication for tens of minutes.Transformation from Co NWs into Co3O4 nanotubes
For fabrication of Co3O4 nanotubes, as-synthesized Co NWs grown on the Al2O3 substrate were placed at the center of the heating zone in the same furnace system (ESI, Fig. S1b†). NW crystal transformation was carried out at 250–600 °C at a flow rate of 250 sccm of 20% O2/Ar at atmospheric pressure for 10–30 min.Characterization
The XRD pattern of the specimen was recorded on a Rigaku D/max-rc (12 kW) diffractometer operating at 30 kV and 60 mA with filtered CuKα radiation. Field emission scanning electron microscopy (FESEM) images of Co NWs were collected on a Phillips XL30S and Nova 230. Transmission electron microscopy (TEM) images, high-resolution TEM (HRTEM) images, selected area electron diffraction (SAED) patterns, energy-dispersive X-ray spectrometry (EDS) and electron energy-loss spectroscopy (EELS) spectra were recorded on a JEOL JEM-2100F TEM operating at 200 kV. After the nanostructures were dispersed in ethanol, a drop of the solution was placed on a carbon film coated copper grid for TEM analysis. For the cross-section TEM study of Co NWs and the interface between the sapphire substrate and the Co NW, a cross-section was sliced and thinned using a focused ion beam (FIB). The temperature and field dependences of the magnetization were measured by using a commercial SQUID magnetometer (Quantum Design, MPMS7).Results and discussion
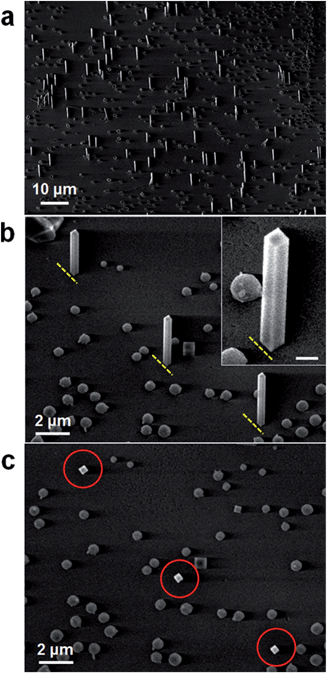
Fig. 1 shows representative scanning electron microscopy (SEM) images of as-synthesized NWs in vertical orientations on a large area of substrate. The Co NW arrays were successfully synthesized on an m-cut (10![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 0) sapphire substrate via the CVD method using a furnace without the catalyst and template, and have diameters of 100–250 nm and lengths of several μm. The magnified 45° tilted-view SEM images in Fig. 1b and the inset show that the NWs have clear facets and are well-aligned on a substrate as marked by the yellow dashed lines. The inset image illustrates a flat tip and rectangular cross-section of the NWs. The top-view SEM image of the NWs (Fig. 1c) for the same region in Fig. 1b confirms again that as-synthesized NWs have rectangular cross-sections (see the red circles). Small spherical Co particles are also synthesized on the substrate in addition to vertical Co NWs. It has been reported that the diameter of FeSi NWs could be controlled in a wide range by adjusting the amount of Si precursor.21 Similarly, we anticipate that the diameter of Co NWs can be controlled through the adjustment of reaction conditions such as the amount of CoCl2 precursor as well as their temperature, and that this diameter control could play a major role in sensing based applications.
0) sapphire substrate via the CVD method using a furnace without the catalyst and template, and have diameters of 100–250 nm and lengths of several μm. The magnified 45° tilted-view SEM images in Fig. 1b and the inset show that the NWs have clear facets and are well-aligned on a substrate as marked by the yellow dashed lines. The inset image illustrates a flat tip and rectangular cross-section of the NWs. The top-view SEM image of the NWs (Fig. 1c) for the same region in Fig. 1b confirms again that as-synthesized NWs have rectangular cross-sections (see the red circles). Small spherical Co particles are also synthesized on the substrate in addition to vertical Co NWs. It has been reported that the diameter of FeSi NWs could be controlled in a wide range by adjusting the amount of Si precursor.21 Similarly, we anticipate that the diameter of Co NWs can be controlled through the adjustment of reaction conditions such as the amount of CoCl2 precursor as well as their temperature, and that this diameter control could play a major role in sensing based applications.
The XRD pattern of vertically grown NW arrays indicates that face-centered cubic (fcc) Co with a lattice constant of 3.544 Å (space group Fm3m, JCPDS card no. 15-0806) is the only phase present except for sapphire substrate peaks (ESI, Fig. S2†). Indeed, it is well-known that Co has two kinds of phases.22
While the hcp Co structure is more stable than fcc Co at room temperature, Co with the fcc phase also exists at room temperature depending on the synthesis conditions since the energy difference of two structures is not quite large. Thus many Co nanostructures can have the fcc structure or mixed structures of hcp and fcc at room temperature.23
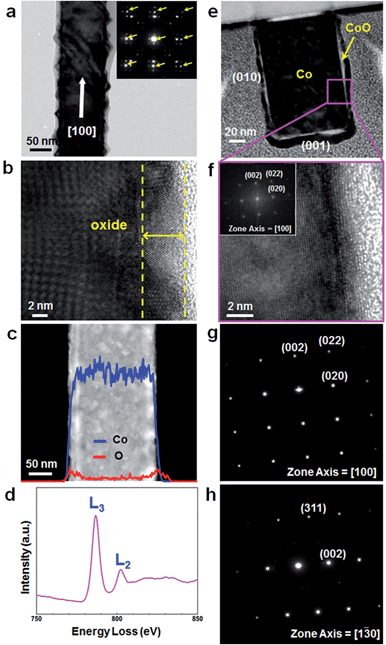
TEM investigation shows that rough oxide layers are formed on the surface of as-synthesized Co NWs presumably due to immediate surface oxidation.24,25 Diffraction spots from the Co single-crystal appear as multiple spots by the thin crystalline oxide layer on the NW surface (inset of Fig. 2a). Analysis of the spots marked by arrows indicates that the NW has the fcc Co phase with the [100] growth direction. The high-resolution TEM (HRTEM) image in Fig. 2b exhibits that the oxide layer is crystalline with a thickness of 5–10 nm indicated by yellow dashed lines. Nonuniform lattice fringes in the core region of the NW (Fig. 2b) are also due to the presence of a crystalline oxide layer. Energy-dispersive X-ray spectroscopy (EDS) line profile analysis shows that Co atoms are evenly distributed over the whole diameter of the NW, whereas O atoms are more highly concentrated in the outer region than the inner region of the NW (Fig. 2c). Such distribution of O atoms is consistent with the fact that as-synthesized Co NWs are covered with thin oxide layers. Cobalt oxide typically has three different compositions, CoO, Co2O3, and Co3O4, among which CoO and Co3O4 are more stable.26 The chemical compositions of oxides in the NW surface were measured by EDS at five points on the NW surface (ESI, Fig. S3†), showing that the Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O atomic ratio is close to 1
O atomic ratio is close to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 near the outermost surface and suggesting the structure of CoO. There might be a minor error in the oxygen EDS signal due to natural oxidation of the Cu grid.
1 near the outermost surface and suggesting the structure of CoO. There might be a minor error in the oxygen EDS signal due to natural oxidation of the Cu grid.
The electron energy-loss spectroscopy (EELS) measurement of as-synthesized Co NWs reveals the chemical bonding state of cobalt oxide over the NW surface (Fig. 2d). L3 and L2 peaks detected from the first transition-series metal elements are sensitively affected by the oxidation state of metals, and we can determine the oxidation state of Co in an oxide shell by comparing the area of these two peaks. L3 and L2 peaks have an area of 1.94 × 106 and 6.35 × 105, respectively, with an area ratio of 3.04. This value is closest to that of CoO among Co3O4 (2.43), CoO (2.90), and Co (3.77), and thus it is most likely that the chemical composition of the cobalt oxide layer is mainly CoO.27
Ferromagnetic Co NWs covered with the antiferromagnetic CoO layer can show the exchange bias effect at the interface by coupling of two materials. In this study, however, such a phenomenon was not observed in the hysteresis loop because the diameter of Co NWs (100–250 nm) is large compared to the thickness of the CoO layer (5–10 nm).
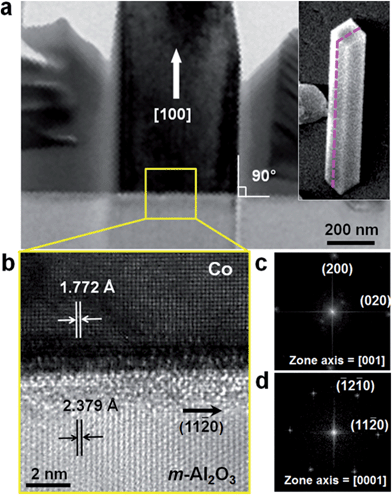
The crystallinity of Co NWs was investigated by cross-sectional TEM analysis after vertically grown NWs were transferred onto a silicon substrate by pressing and then sliced and thinned by the focused ion beam (FIB) technique. To protect the sample from the ion beam during milling, a Pt layer was deposited on the desired region by using FIB gas injection prior to ion milling. The low-resolution TEM image in Fig. 2e shows that the cross-section of the NW is a rectangle, consistent with the observation by SEM. An oxide layer is clearly observed in Fig. 2e and f. Fig. 2f is a magnified HRTEM image of the pink square region in Fig. 2e and displays uniform and clear lattice fringes of a Co NW. The fast Fourier transform (FFT) pattern (Fig. 2f, inset) and SAED patterns (Fig. 2g and h), observed at different zone axes, demonstrate that the Co NWs are defect-free single-crystalline and vertically grow along the [100] direction, and the side and top facets are all {100}.
Fig. 3a shows the cross-sectional TEM image of a vertical Co NW grown on an m-cut sapphire substrate, cut perpendicular to the substrate along the pink dashed line (in the inset). The upper part of the NW was damaged by ion milling during sample preparation. Fig. 3b is a HRTEM image of the interface between the NW and the substrate (see the yellow square in Fig. 3a), and Fig. 3c and d show the FFT patterns of the NW and substrate, respectively. Analysis of the HRTEM image and FFT patterns reveals that the epitaxial relationship between the vertical Co NW and the m-cut sapphire substrate is (200) Co//(1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 00) Al2O3. At the interface, the lattice mismatch between the 〈020〉 direction of Co and the 〈11
00) Al2O3. At the interface, the lattice mismatch between the 〈020〉 direction of Co and the 〈11![[2 with combining macron]](https://www.rsc.org/images/entities/char_0032_0304.gif) 0〉 direction of Al2O3 is 24.6% and that between the 〈001〉 direction of Co and the 〈0001〉 direction of Al2O3 is 17.8%. In a domain matching epitaxy, five layers of Co are matched with three layers of Al2O3 along the Co 〈020〉 direction with only a 0.56% mismatch, and five layers of Co are matched with four layers of Al2O3 along the Co 〈001〉 direction with 2.8% mismatch. While the growth of Co NWs through electrodeposition using a template with anisotropic channels has been intensively studied,8–13 direct synthesis of ordered Co NWs on the substrate has been quite rarely reported.14
0〉 direction of Al2O3 is 24.6% and that between the 〈001〉 direction of Co and the 〈0001〉 direction of Al2O3 is 17.8%. In a domain matching epitaxy, five layers of Co are matched with three layers of Al2O3 along the Co 〈020〉 direction with only a 0.56% mismatch, and five layers of Co are matched with four layers of Al2O3 along the Co 〈001〉 direction with 2.8% mismatch. While the growth of Co NWs through electrodeposition using a template with anisotropic channels has been intensively studied,8–13 direct synthesis of ordered Co NWs on the substrate has been quite rarely reported.14
To make these Co NWs more applicable to 3-dimensional magnetic memory devices, both uniform homogeneity and higher density are required. Since the vertical NW growth propensity is mostly provided by direct impingement to the substrate from the vapor, to increase the density of vertical NWs, it is needed to increase the impingement rate to the substrate from the vapor, in other words, the density of the Co atoms in the vapor. Further optimization can be achieved through control of experimental parameters, such as substrate temperature, vapor flux of Co, chamber pressure, and furnace heating rate. In addition, since it is known that the Co epitaxial thin film can be grown in either the fcc phase or the hcp phase on a sapphire substrate depending on the substrate temperature, we expect that hcp-structured Co NWs having a higher magnetic crystalline anisotropy could also be obtained at higher substrate temperature than currently used for the synthesis of fcc-structured Co NWs.28
Interestingly, horizontal Co NWs aligned in two directions were synthesized when an r-cut (1![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) 02) sapphire was employed as a substrate instead of an m-cut sapphire while other experimental conditions were kept the same (ESI, Fig. S4†). We found that the as-synthesized horizontal Co NW has an hcp crystal structure unlike a vertical Co NW. The horizontal Co NWs have a hexagonal cross-section and an oxide layer at the surface, and grow along the [0001] direction. Furthermore, Co NWs possessing several twin planes as well as twin-free NWs were synthesized at the same time with stacking faults observed in both NWs. The two orientations of the horizontal NWs perpendicularly crossing on an r-cut sapphire substrate could be explained as follows. Fine lattice match is retained when the Co lattice is rotated by 90° with respect to the favorable substrate lattice. It is well-known that the interfacial energy between the NW and the substrate varies depending on the crystal orientation of the substrate and the atomic distribution matching, and consequently may affect the growth orientation and crystal structure of the Co NWs.29 A detailed analysis of the mechanism is currently in progress.
02) sapphire was employed as a substrate instead of an m-cut sapphire while other experimental conditions were kept the same (ESI, Fig. S4†). We found that the as-synthesized horizontal Co NW has an hcp crystal structure unlike a vertical Co NW. The horizontal Co NWs have a hexagonal cross-section and an oxide layer at the surface, and grow along the [0001] direction. Furthermore, Co NWs possessing several twin planes as well as twin-free NWs were synthesized at the same time with stacking faults observed in both NWs. The two orientations of the horizontal NWs perpendicularly crossing on an r-cut sapphire substrate could be explained as follows. Fine lattice match is retained when the Co lattice is rotated by 90° with respect to the favorable substrate lattice. It is well-known that the interfacial energy between the NW and the substrate varies depending on the crystal orientation of the substrate and the atomic distribution matching, and consequently may affect the growth orientation and crystal structure of the Co NWs.29 A detailed analysis of the mechanism is currently in progress.
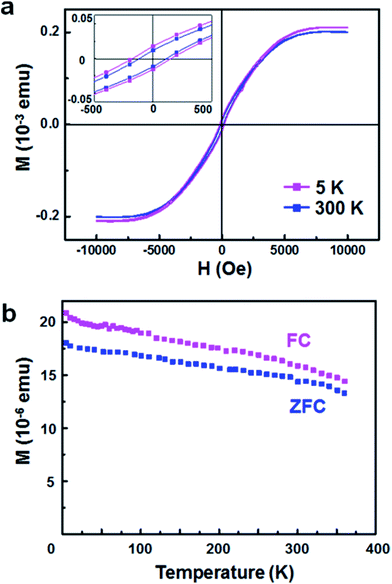
Magnetic properties of vertical Co NW arrays on a sapphire substrate were examined by using a SQUID magnetometer. Fig. 4a shows magnetic field-dependent magnetization (M–H) curves measured at 5 and 300 K. The two M–H curves exhibit the hysteresis loop with a coercive field (HC) of approximately 180 and 120 Oe at 5 and 300 K, respectively. The temperature-dependent magnetization (M–T) curves measured after field cooling (FC) and zero-field cooling (ZFC) under a 500 Oe magnetic field are indicated in Fig. 4b. The M–T curves obtained in a temperature range from 5 to 370 K show nonzero magnetization up to room temperature in both FC and ZFC measurements. The hysteresis loop and M–T curves reveal that as-synthesized Co NWs are ferromagnetic at room temperature, which is consistent with Curie temperature (TC) reported in bulk Co (∼1388 K).30
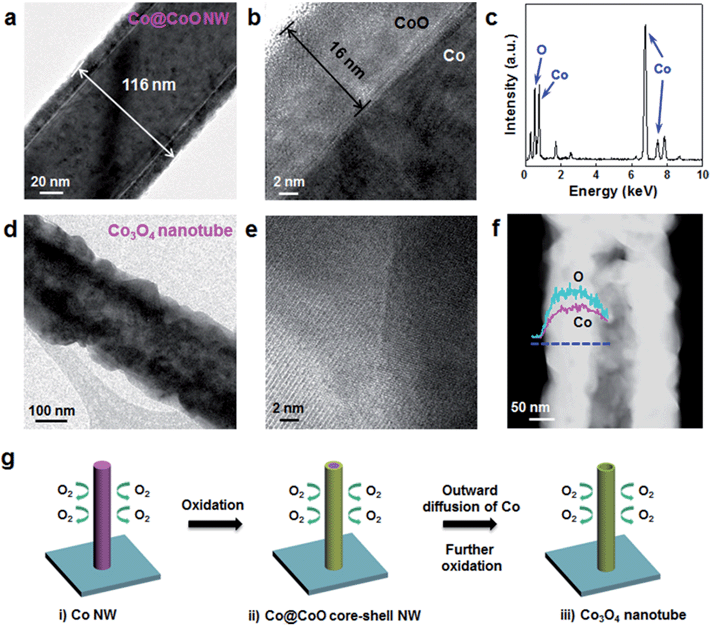
Co NWs were transformed into Co3O4 nanotubes by thermal annealing at 250–600 °C under 20% O2 conditions for 10–30 min. TEM results for the nanostructures obtained from the reaction at 250 °C for 30 min are shown in Fig. 5a–c. While the oxide layer thickness of as-synthesized Co NWs in Fig. 1 was about 5 nm, that of thermally oxidized NWs increased to ∼16 nm (see the low- and high-resolution TEM images in Fig. 5a and b). The EDS spectrum (Fig. 5c) of a shell region of NW in Fig. 5b reveals that the Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O atomic ratio is 47.3
O atomic ratio is 47.3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 52.8%, close to 1
52.8%, close to 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Other peaks were attributed to Si, Cu and C from the EDS detector and TEM grid. When we increase the annealing temperature to 400 °C, the oxide shell thickness further increased to ∼50 nm after 10 min reaction time (ESI, Fig. S5†). When the Co NWs were annealed at 600 °C for 10 min, they were converted to nanotubes (Fig. 5d–f). The HRTEM image in Fig. 5e indicates that the nanotube is polycrystalline, consisting of multiple crystalline layers. The clear contrast of the scanning TEM (STEM) image (Fig. 5f) demonstrates again the nanotube morphology, in which the inside is empty along the longitudinal direction. TEM-EDS line profile analysis shows that the atomic composition of this nanotube is Co
1. Other peaks were attributed to Si, Cu and C from the EDS detector and TEM grid. When we increase the annealing temperature to 400 °C, the oxide shell thickness further increased to ∼50 nm after 10 min reaction time (ESI, Fig. S5†). When the Co NWs were annealed at 600 °C for 10 min, they were converted to nanotubes (Fig. 5d–f). The HRTEM image in Fig. 5e indicates that the nanotube is polycrystalline, consisting of multiple crystalline layers. The clear contrast of the scanning TEM (STEM) image (Fig. 5f) demonstrates again the nanotube morphology, in which the inside is empty along the longitudinal direction. TEM-EDS line profile analysis shows that the atomic composition of this nanotube is Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O = 36.7
O = 36.7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 63.2. Because stable compounds of cobalt oxide are CoO and Co3O4, it is likely that the nanotubes have crystal structures of Co3O4. The EDS result (Fig. 5f) indicates that Co and O elements as marked by pink and cyan lines, respectively, have a constant Co
63.2. Because stable compounds of cobalt oxide are CoO and Co3O4, it is likely that the nanotubes have crystal structures of Co3O4. The EDS result (Fig. 5f) indicates that Co and O elements as marked by pink and cyan lines, respectively, have a constant Co![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) O ratio and the nanostructure has a hollow structure.
O ratio and the nanostructure has a hollow structure.
Transformation of Co NWs into Co3O4 nanotubes, inferred from the TEM results, occurred by the thermal annealing process following the scheme in Fig. 5g. First, Co is gradually oxidized into CoO from the surface of the NW by heating under dilute O2 conditions at an early stage of annealing, forming a Co@CoO core–shell NW with an oxide layer of tens of nanometers (Fig. 5a and S5†).31,32 The formation of cobalt oxide nanotubes can be explained by the nanoscale Kirkendall effect, which is attributed to the difference in diffusion rates between the cation and anion.33 Since the outward diffusion of Co is much faster than inward diffusion of O in the oxide layer, a tendency to form interior nanocavities is incurred at the interface of Co/oxide.34 CoO is finally further oxidized to Co3O4. Such an oxidation process of 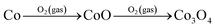 has been well observed in thin Co foil and Co nanoparticles.31,32
has been well observed in thin Co foil and Co nanoparticles.31,32
Conclusions
We have reported for the first time the epitaxial growth of single-crystalline Co NWs individually aligned in the vertical direction on m-cut sapphire substrates via a rapid and versatile CVD method without employing any catalysts or templates. Horizontal Co NWs are grown on r-cut sapphire substrates. Furthermore, we demonstrated that Co3O4 nanotubes could be readily fabricated by thermal annealing of Co NWs via CoO as an intermediate material under dilute O2 conditions. It is anticipated that well-aligned single-crystalline Co NW arrays can be utilized as valuable materials including development of advanced magnetic memory applications. Additionally, Co3O4 nanotubes are potentially applicable to catalyst and sensing devices.Acknowledgements
We acknowledge support from National Research Foundation (NRF) N01130942 (B.K.), NRF K20904000004-13A0500-00410 (J.C.), NRF 2012R1A2A2A02013289 and KISTI supercomputing Center (2014) (S.L.).Notes and references
- X. Kou, X. Fan, R. K. Dumas, Q. Lu, Y. Zhang, H. Zhu, X. Zhang, K. Liu and J. Q. Xiao, Memory Effect in Magnetic Nanowire Arrays, Adv. Mater., 2011, 23, 1393 CrossRef CAS PubMed.
- H.-T. Huang, T.-R. Ger, Y.-H. Lin and Z.-H. Wei, Single Cell Detection using a Magnetic Zigzag Nanowire Biosensor, Lab Chip, 2013, 13, 3098 RSC.
- W. Gao, S. Sattayasamitsathit, K. M. Manesh, D. Weihs and J. Wang, Magnetically Powered Flexible Metal Nanowire Motors, J. Am. Chem. Soc., 2010, 132, 14403 CrossRef CAS PubMed.
- S. S. P. Parkin, M. Hayashi and L. Thomas, Magnetic Domain-Wall Racetrack Memory, Science, 2008, 320, 190 CrossRef CAS PubMed.
- M. M. Maqableh, X. Huang, S.-Y. Sung, K. S. M. Reddy, G. Norby, R. H. Victora and B. J. H. Stadler, Low-Resistivity 10 nm Diameter Magnetic Sensors, Nano Lett., 2012, 12, 3102 CrossRef PubMed.
- M. C. P. Wang and B. D. Gates, Directed Assembly of Nanowires, Mater. Today, 2009, 12, 34 CrossRef CAS.
- F. Garcia-Sanchez, H. Szambolics, A. P. Mihai, L. Vila, A. Marty, A. M. Attane, J.-C. Toussaint and L. D. Buda-Prejbeanu, Effect of Crystalline Defects on Domain Wall Motion under Field and Current in Nanowires with Perpendicular Magnetization, Phys. Rev. B: Condens. Matter Mater. Phys., 2010, 81, 134408 CrossRef.
- X. Huang, L. Li, X. Luo, X. Zhu and G. Li, Orientation-Controlled Synthesis and Ferromagnetism of Single Crystalline Co Nanowire Arrays, J. Phys. Chem. C, 2008, 112, 1468 CAS.
- P. Yang, M. An, C. Su and F. Wang, Fabrication of Cobalt Nanowires from Mixture of 1-Ethyl-3-Methylimidazolium Chloride Ionic Liquid and Ethylene Glycol using Porous Anodic Alumina Template, Electrochim. Acta, 2008, 54, 763 CrossRef CAS PubMed.
- L. Cattaneo, S. Franz, F. Albertini, P. Ranzieri, A. Vicenzo, M. Bestetti and P. L. Cavallotti, Electrodeposition of Hexagonal Co Nanowires with Large Magnetocrystalline Anisotropy, Electrochim. Acta, 2012, 85, 57 CrossRef CAS PubMed.
- J. Qin, J. Nogues, M. Mikhaylova, A. Roig, J. S. Munoz and M. Muhammed, Differences in the Magnetic Properties of Co, Fe, and Ni 250–300 nm Wide Nanowires Electrodeposited in Amorphous Anodized Alumina Templates, Chem. Mater., 2005, 17, 1829 CrossRef CAS.
- L. Vila, P. Vincent, L. D. Pra, G. Pirio, E. Minoux, L. Gangloff, S. Demoustier-Champagne, N. Sarazin, E. Ferain, R. Legras, L. Piraux and P. Legagneux, Growth and Field-Emission Properties of Vertically Aligned Cobalt Nanowire Arrays, Nano Lett., 2004, 4, 521 CrossRef CAS.
- T. Thurn-Albrecht, J. Schotter, G. A. Kastle, N. Emley, T. Shibauchi, L. Krusin-Elbaun, K. Guarini, C. T. Black, M. T. Tuominen and T. P. Russell, Ultrahigh-Density Nanowire Arrays Grown in Self-Assembled Diblock Copolymer Templates, Science, 2000, 290, 2126 CrossRef CAS.
- N. Liakakos, T. Blon, C. Achkar, V. Vilar, B. Cormary, R. P. Tan, O. Benamara, G. Chaboussant, F. Ott, B. Warot-Fonrose, E. Snoeck, B. Chaudret, K. Soulantica and M. Respaud, Solution Epitaxial Growth of Cobalt Nanowires on Crystalline Substrates for Data Storage Densities beyond 1Tbit/in2, Nano Lett., 2014, 14, 3481 CrossRef CAS PubMed.
- K. T. Chan, J. J. Kan, C. Doran, L. Ouyang, D. J. Smith and E. E. Fullerton, Oriented Growth of Single-Crystal Ni Nanowires onto Amorphous SiO2, Nano Lett., 2010, 10, 5070 CrossRef CAS PubMed.
- Y. Yoo, K. Seo, S. Han, K. S. K. Varadwaj, H. Kim, J. Ryu, H. Lee, J. Ahn, H. Ihee and B. Kim, Steering Epitaxial Alignment of Au, Pd, and AuPd Nanowire Arrays by Atom Flux Change, Nano Lett., 2010, 10, 432 CrossRef CAS PubMed.
- M. He, C. H. Wong, P. L. Tse, Y. Zheng, H. Zhang, F. L. Y. Lam, P. Sheng, X. Hu and R. Lortz, Giant Enhancement of the Upper Critical Field and Fluctuations above the Bulk Tc in Superconducting Ultrathin Lead Nanowire Arrays, ACS Nano, 2013, 7, 4187 CrossRef CAS PubMed.
- Y. Liang, Y. Li, H. Wang, J. Zhou, J. Wang, T. Regier and H. Dai, Co3O4 Nanocrystals on Graphene as a Synergistic Catalyst for Oxygen Reduction Reaction, Nat. Mater., 2011, 10, 780 CrossRef CAS PubMed.
- H. Nguyen and S. A. El-Safty, Meso- and Macroporous Co3O4 Nanorods for Effective VOC Gas Sensors, J. Phys. Chem. C, 2011, 115, 8466 CAS.
- X. H. Xia, J. P. Tu, J. Zhang, X. H. Huang, X. L. Wang and X. B. Zhao, Improved Electrochromic Performance of Hierarchically Porous Co3O4 Array Film through Self-Assembled Colloidal Crystal Template, Electrochim. Acta, 2010, 55, 989 CrossRef CAS PubMed.
- S. Kim, H. Yoon, K. Seo, Y. Yoo, S. Lee and B. Kim, Truncated Tetrahedron Seed Crystals Initiating Stereoaligned Growth of FeSi Nanowires, ACS Nano, 2012, 6, 8652 CrossRef CAS PubMed.
- X. W. Wang, G. T. Fei, B. Wu, L. Chen and Z. Q. Chu, Structural Stability of Co Nanowire Arrays Embedded in the PAAM, Phys. Lett. A, 2006, 359, 220 CrossRef CAS PubMed.
- B. W. Lee, R. Alsenz and A. Ignatiev, Surface Structures of the Two Allotropic Phases of Cobalt, Phys. Rev. B: Condens. Matter Mater. Phys., 1978, 17, 1510 CrossRef CAS.
- S. Jia, C.-H. Hsia and D. H. Son, In situ Study of Room-Temperature Oxidation Kinetics of Colloidal Co Nanocrystals Investigated by Faraday Rotation Measurement, J. Phys. Chem. C, 2011, 115, 92 CAS.
- D.-H. Ha, L. M. Moreau, C. R. Bealing, H. Zhan, R. G. Hennig and R. D. Robinson, The Structural Evolution and Diffusion during the Chemical Transformation from Cobalt to Cobalt Phosphide Nanoparticles, J. Mater. Chem., 2011, 21, 11498 RSC.
- K. Deori and S. Deka, Morphology Oriented Surfactant Dependent CoO and Reaction Time Dependent Co3O4 Nanocrystals from Single Synthesis Method and Their Optical and Magnetic properties, CrystEngComm, 2013, 15, 8465 RSC.
- B. D. Yuhas, D. O. Zitoun, P. J. Pauzauskie, R. He and P. Yang, Transition-Metal Doped Zinc Oxide Nanowires, Angew. Chem. Int. Ed., 2006, 45, 420 CrossRef CAS PubMed.
- M. Ohtake, O. Yabuhara, Y. Nukaga and M. Futamoto, Preparation of Co(0001)hcp and (111)fcc Films on Single-Crystal Oxide Substrates, J. Phys.: Conf. Ser., 2011, 303, 012016 CrossRef.
- Y. Yoo, I. Yoon, H. Lee, J. Ahn, J. P. Ahn and B. Kim, Pattern-Selective Epitaxial Growth of Twin-Free Pd Nanowires from Supported Nanocrystal Seeds, ACS Nano, 2010, 4, 2919 CrossRef CAS PubMed.
- R. Cao, R. Deng, J. Tang, S. Song, Y. Lei and H. Zhang, Cobalt and Nickel with Various Morphologies: Mineralizer-Assisted Synthesis, Formation Mechanism, and Magnetic Properties, CrystEngComm, 2011, 13, 223 RSC.
- D.-H. Ha, L. M. Moreau, S. Honrao, R. G. Hennig and R. D. Robinson, The Oxidation of Cobalt Nanoparticles into Kirkendall-Hollowed CoO and Co3O4: The Diffusion Mechanisms and Atomic Structural Transformations, J. Phys. Chem. C, 2013, 117, 14303 CAS.
- M. Martin, U. Koops and N. Lakshmi, Reactivity of Solid Studied in situ XAS and XRD, Solid State Ionics, 2004, 172, 357 CrossRef CAS PubMed.
- Z. Yang, I. Lisiecki, M. Walls and M. -P. Pileni, Nanocrystallinity and the Ordering of Nanoparticles in Two-Dimensional Superlattices: Controlled Formation of Either Core–Shell (Co/CoO) or Hollow CoO Nanocrystals, ACS Nano, 2013, 7, 1342 CrossRef CAS PubMed.
- T. Li, S. Yang, L. Huang, B. Gu and Y. Du, A Novel Process from Cobalt Nanowire to Co3O4 Nanotube, Nanotechnology, 2004, 15, 1479 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental setup (S1). XRD pattern of Co NW arrays (S2). Chemical composition of cobalt oxide on the NW surface (S3). Horizontal Co NWs grown on an r-cut sapphire substrate (S4). Co@CoO NWs obtained by thermal annealing at 400 °C (S5). See DOI: 10.1039/c4tc01765j |
| This journal is © The Royal Society of Chemistry 2015 |

![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) 0] (h).
0] (h).