Visible light-induced ion-selective optodes based on a metastable photoacid for cation detection†
Parth K.
Patel
and
Karin Y.
Chumbimuni-Torres
*
Department of Chemistry, University of Central Florida, Orlando, FL 32816-2366, USA. E-mail: Karin.ChumbimuniTorres@ucf.edu
First published on 16th November 2015
Abstract
A new platform of ion-selective optodes is presented here to detect cations under thermodynamic equilibrium via ratiometric analysis. This novel platform utilizes a ‘one of a kind’ visible light-induced metastable photoacid as a reference ion indicator to achieve activatable and controllable sensors. These ion-selective optodes were studied in terms of their stability, sensitivity, selectivity, and theoretical aspects.
Ion selective optodes (ISOs) have been extensively studied to monitor different cations for biomedical and environmental applications.1–11 In recent years, there has been considerable interest to study ISOs that are activatable, reversible and controllable for their use in ion sensing applications that require localized detection of free ions without perturbing their surroundings.12–15 Attempts have been made to convert the traditional passive mode ISOs into active mode by the use of photoactive compounds such as spiropyrans and photoacid generators.16–19 However, the use of spiropyrans in an ISO requires UV light to activate the sensor, which can result in cellular damage when applied in biomedical applications.20 In addition, UV light photodegrades the active compound in the ISO, shortening the sensor's lifetime.13–17 Moreover, photoacid generators in an ISO undergo photolysis, making the sensor irreversible.18,19 To overcome photodegradation and irreversibility for such ISOs, we propose to use a ‘one of a kind’ visible light activatable and controllable ISO based on a metastable photoacid (mPAH) to detect cations under equilibrium conditions by ratiometric analysis.
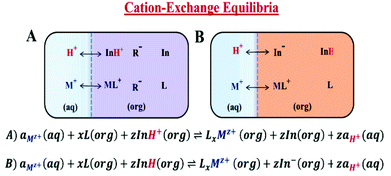
A typical ISO consists of a neutral basic indicator (In) that is selective to a reference ion (H+), an ionophore (L) that is selective to the cation of interest, and anionic additives (R−) that maintain electroneutrality in the plasticized polymer matrix (Scheme 1A).21–23 The response mechanism of these ISOs is dictated by mass transfer equilibria (cation-exchange process) between an organic (ISO) and an aqueous phase.24,25 Consequently, the detection of the cation of interest, after achieving thermodynamic equilibrium, is made by measuring the changes in the indicators’ optical properties, either by absorption or fluorescence spectroscopy.26 Nevertheless, prior to cation detection, these ISOs need an external source of protons for the cation-exchange process to proceed, resulting in a response mechanism with multiple steps. In contrast, the proposed tricyanofuran based mPAH (CF3PhTCF-PAH) acts as a neutral acidic indicator (InH), providing protons for the cation-exchange process. Considering that mPAH is a photoactive compound, it photodissociates its proton upon irradiation and thermally undergoes proton reassociation with a dissociated state that is sufficiently long-lived,27,28 thus eliminating the need for an external source of protons, while the dissociated state of the mPAH acts as an anionic additive to maintain electroneutrality within the ISO as shown in Scheme 1B.22 Hence, an ISO based on the proposed mPAH exhibits a one-step response mechanism.
We have recently shown that merocyanine based mPAH linked to an acrylate polymer backbone can be utilized in an ISO to detect calcium ions.29 This merocyanine based mPAH is a neutrally charged acidic indicator which exhibits a longer equilibrium response time (in the order of hours). Consequently, the detection of calcium ions was performed under non-equilibrium conditions.29 Moreover, we have shown that by modifying the merocyanine based mPAH with an appropriate functional group (electron donating group), the equilibrium response time can be shortened to the order of minutes.30 However, the charged nature of the merocyanine based mPAH was optimal only with a polar plasticizer, such as 2-nitrophenyl octyl ether (o-NPOE), to reduce ion-pair formation that affects selectivity.29o-NPOE absorbs under 400 nm, interfering with the absorbance peak of the deprotonated state merocyanine based mPAH, and inhibited ratiometric analysis. In contrast, the non-charged CF3PhTCF-PAH has shown ideal compatibility with a non-polar plasticizer, such as bis(2-ethylhexyl)sebacate (DOS) which does not interfere optically, allowing ratiometric analysis to further increase the sensor sensitivity and signal reproducibility.31
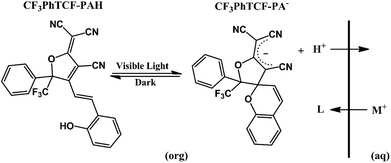
As shown in Scheme 2, it is expected that CF3PhTCF-PAH within the ISO would undergo oxidative photoreaction under visible light irradiation, resulting in a stable carbanion state (CF3PhTCF-PA−) of the mPAH along with its photodissociated proton.32 Likewise, the photodissociated protons would be exchanged when exposed to cations of interest, as the CF3PhTCF-PA− state is sufficiently long-lived to allow for the diffusion mediated cation-exchange process.
CF3PhTCF-PAH was synthesized according to a literature procedure.32–34 The calcium and sodium ISOs proposed here contain CF3PhTCF-PAH (7.5 mmol kg−1), and calcium ionophore IV (22.5 mmol kg−1) or sodium ionophore X (7.5 mmol kg−1) within poly(vinyl chloride) (33 wt%) and DOS (66 wt%).
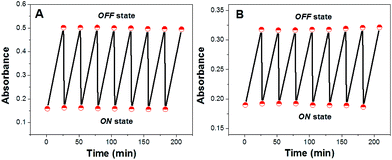
At first, these ISOs were exposed to their respective buffer solutions without any cation of interest. As shown in Fig. 1, the ISOs based on CF3PhTCF-PAH were stable over repeated activation cycles between the ON (deprotonated form) and OFF states (protonated form) without any loss of the absorbance signal, indicating no observable photodegradation.
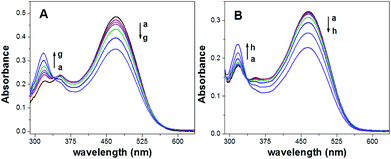
Fig. 2 shows the absorption spectra of the ISOs based on CF3PhTCF-PAH towards different concentrations of calcium (Fig. 2A) and sodium (Fig. 2B) ions at thermodynamic equilibrium (25 minutes in the dark) once the ISOs were activated by visible light (470 nm) for 1 minute. From both absorption spectra, it is observed that as the concentration of the cation of interest increases, there is a gradual decrease in the CF3PhTCF-PAH peak (470 nm) and a gradual increase in the CF3PhTCF-PA− peak (318 nm). These absorbance changes allow for ratiometric analysis (protonated and deprotonated absorbance peaks of the CF3PhTCF-PAH) to indirectly correlate the activity of the cation of interest.
The experimental data obtained were then compared to the theoretical response function. The theoretical response function (eqn (1)), in terms of the activity of protons and the cation of interest, was generated from the ion-exchange equilibria (Scheme 1B). This was derived by utilizing mass balance (eqn (2)), charge balance (eqn (3)), degree of deprotonation using the ratio of absorbance at 470 nm and 318 nm (eqn (4)), and the ion-exchange equilibrium constant (eqn (5)) equations. This is analogous to that of traditional ISO theory which was established in the early 1990s.21–23
Theoretical response function for cation:
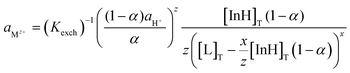 | (1) |
Mass balance equations:
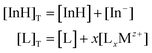 | (2) |
Charge balance equation:
| [In−] = z[LxMz+] | (3) |
Degree of deprotonation (α):
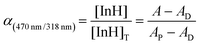 | (4) |
Cation-exchange equilibrium constant (Kexch) for the neutral acidic indicator:
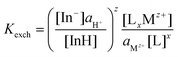 | (5) |
The subscript “T” indicates the total concentration of CF3PhTCF-PAH ([InH]T) and the ionophore ([L]T). The activity of the cation of interest and the proton is denoted as aMz+ and aH+. Also, “x” and “z” denote the value of the ionophore chelating with the cation of interest and charge of the cation of interest, respectively. The ratio of absorbance for CF3PhTCF-PAH is denoted as A, the protonated state of CF3PhTCF-PAH in 1 M hydrochloric acid as AP, and the deprotonated state of CF3PhTCF-PAH in 1 M sodium hydroxide as AD; for 25 minutes in the dark after activation.
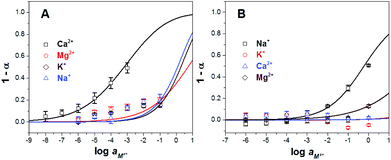
As shown in Fig. 3, the experimental data present a strong correlation with the theoretical response curve. From the resulting cation response curves, the experimental limit of detection (LOD) for calcium and sodium ions was 2.6 × 10−6 M and 2.3 × 10−3 M, respectively. These values were obtained by intersecting two extrapolated segments of the response curve as indicated in the literature.35 Furthermore, the cation-exchange constant (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kexch) for calcium and sodium ISOs was −9.3 ± 0.3 and −5.3 ± 0.1, respectively. The ionophore–cation complex ratio for calcium ISO was 3 to 1 and for sodium ISOs was 1 to 1.36,37
Kexch) for calcium and sodium ISOs was −9.3 ± 0.3 and −5.3 ± 0.1, respectively. The ionophore–cation complex ratio for calcium ISO was 3 to 1 and for sodium ISOs was 1 to 1.36,37
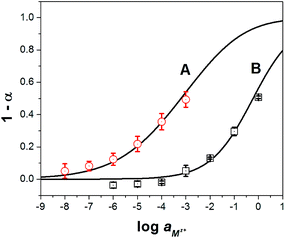 | ||
Fig. 3 Experimental ( and □) and theoretical (lines) responses of ISOs based on CF3PhTCF-PAH for the detection of (A) calcium, and (B) sodium ions for 25 minutes in the dark after activation (n = 3). and □) and theoretical (lines) responses of ISOs based on CF3PhTCF-PAH for the detection of (A) calcium, and (B) sodium ions for 25 minutes in the dark after activation (n = 3). | ||
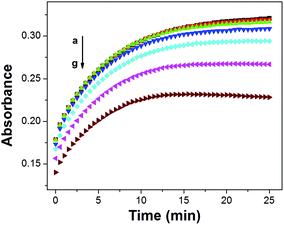
It was hypothesized that ISOs which contain no anionic additive, cannot maintain constant ionic strength within the ISO.23 As a result, the cation-exchange constant cannot be retained, due to each change in the activity of protons and the activity of the cation of interest.23 However, the changes in the ionic strength within the ISO based on CF3PhTCF-PAH, containing no additional anionic additive, were negligible as observed in the kinetic data for different concentrations of sodium ions (Fig. 4), where stable responses over time were obtained at thermodynamic equilibrium. It is noteworthy that the interactions between the negatively and positively charged components for such an ISO do occur, after a certain threshold (1 − α > 0.5), as the ionic strength within the ISO changes drastically. Accordingly, higher concentrations of cations are not shown in Fig. 3 because the thermodynamic equilibrium was not maintained, and thus the upper detection limit was 6.3 × 10−4 M and 0.6 M for calcium and sodium ions, respectively (at α = 0.5).
Furthermore, these ISOs based on CF3PhTCF-PAH were studied in terms of selectivity towards interfering cations (J) by a separate solution method.25,38 As shown in Fig. 5, all interfering ions were highly discriminated for both ISOs, calcium (Fig. 5A) and sodium (Fig. 5B). Thus, the selectivity coefficient values for the calcium ISO towards magnesium, sodium and potassium ions were −3.8, −3.1 and −3.3, respectively. When compared to other ISOs, containing the same ionophore and spiropyran as the reference indicators, the selectivity coefficients were −2.9 (magnesium ion), −6.6 (sodium ion) and −8.9 (potassium ion).16 The log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KM,J values for the sodium ISO towards potassium, calcium and magnesium ions were −4.5, −6.6 and −2.7, respectively. These values are comparable to the selectivity coefficient of sodium against the potassium ion (−2.4), calcium ion (−4.0) and magnesium ion (−4.1) containing spiropyran as a reference indicator.16 The selectivity coefficient (log
KM,J values for the sodium ISO towards potassium, calcium and magnesium ions were −4.5, −6.6 and −2.7, respectively. These values are comparable to the selectivity coefficient of sodium against the potassium ion (−2.4), calcium ion (−4.0) and magnesium ion (−4.1) containing spiropyran as a reference indicator.16 The selectivity coefficient (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) KM,J) values were calculated using eqn (6), and is analogous to that of ISO theory at α = 0.5.23
KM,J) values were calculated using eqn (6), and is analogous to that of ISO theory at α = 0.5.23
Selectivity coefficient for interfering cations:
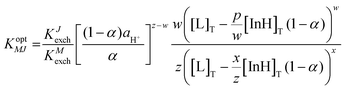 | (6) |
The high discrimination observed may be due to the proton affinity towards CF3PhTCF-PA− (deprotonated form) to be greater than the binding energy of interfering cations by the respective ionophores. Consequently, these ISOs inhibit the competitive behaviour between the interfering cations and protons during the cation-exchange process, even at high concentrations. As a result, an ISO based on CF3PhTCF-PAH may also be utilized for practical purposes which demands negligible interactions towards interfering cations.
In conclusion, this novel visible light activatable and controllable ISO based on CF3PhTCF-PAH that does not exhibit photodegradation, showed good stability, selectivity and reproducibility. Furthermore, the responses of these ISOs towards calcium and sodium ions were standardized following the cation-exchange equilibria. Similarly, other cations can be detected using this platform by interchanging the ionophore within the polymer matrix. Also, we aim to optimize this type of ISO in terms of size reduction and sensitivity. Likewise, we expect that this novel mPAH (CF3PhTCF-PAH) may act as a substitute to neutral basic indicators for their use in cation sensing applications that provide control using visible light as needed.
The authors acknowledge the Colleges of Sciences and the Department of Chemistry at the University of Central Florida (USA) for financial support for this research. We also acknowledge Dr Yi Liao (Florida Institute of Technology, USA) to provide tricyanofuran based metastable photoacids for preliminary studies, Dr Eric Bakker (University of Geneva, Switzerland) and Dr Philippe Buhlmann (University of Minnesota, USA) for advice on ion-selective optode theory.
Notes and references
- W. E. Morf, K. Seiler, B. Rusterholz and W. Simon, Anal. Chem., 1990, 62, 738 CrossRef CAS.
- K. Wang, K. Seiler, W. E. Morf, U. E. Spichiger, W. Simon, E. Lindner and E. Pungor, Anal. Sci., 1990, 6, 715 CrossRef CAS.
- K. Seiler, K. Wang, E. Bakker, W. E. Morf, B. Rusterholz, U. E. Spichiger and W. Simon, Clin. Chem., 1991, 37, 1350 CAS.
- M. Lerchi, E. Bakker, B. Rusterholz and W. Simon, Anal. Chem., 1992, 64, 1534 CrossRef CAS.
- H. Hisamoto, N. Miyashita, K. Watanabe, E. Nakagawa, N. Yamamoto and K. Suzuki, Sens. Actuators, B, 1995, 29, 378 CrossRef CAS.
- M. Lerchi, F. Orsini, Z. Cimerman, E. Pretsch, D. A. Chowdhury and S. Kamata, Anal. Chem., 1996, 68, 3210 CrossRef CAS PubMed.
- M. R. Shortreed, S. Dourado and R. Kopelman, Sens. Actuators, B, 1997, 38, 8 CrossRef CAS.
- J. M. Dubach, D. I. Harjes and H. A. Clark, J. Am. Chem. Soc., 2007, 129, 8418 CrossRef CAS PubMed.
- B. Kuswandi, Nuriman, H. H. Dam, D. N. Reinhoudt and W. Verboom, Anal. Chim. Acta, 2007, 591, 208 CrossRef CAS PubMed.
- A. A. Ensafi and M. Fouladgar, Sens. Actuators, B, 2009, 136, 326 CrossRef CAS.
- M. T. Bamsey, A. Berinstain and M. A. Dixon, Sens. Actuators, B, 2014, 190, 61 CrossRef CAS.
- G. Mistlberger, G. A. Crespo, X. J. Xie and E. Bakker, Chem. Commun., 2012, 48, 5662 RSC.
- X. J. Xie, G. Mistlberger and E. Bakker, J. Am. Chem. Soc., 2012, 134, 16929 CrossRef CAS PubMed.
- X. J. Xie, G. Mistlberger and E. Bakker, Anal. Chem., 2013, 85, 9932 CrossRef CAS PubMed.
- X. J. Xie and E. Bakker, ACS Appl. Mater. Interfaces, 2014, 6, 2666 CAS.
- G. Mistlberger, X. J. Xie, M. Pawlak, G. A. Crespo and E. Bakker, Anal. Chem., 2013, 85, 2983 CrossRef CAS PubMed.
- G. Mistlberger, M. Pawlak, E. Bakker and I. Klimant, Chem. Commun., 2015, 51, 4172 RSC.
- A. Shvarev, J. Am. Chem. Soc., 2006, 128, 7138 CrossRef CAS PubMed.
- X. J. Xie, G. Mistlberger and E. Bakker, Sens. Actuators, B, 2014, 204, 807 CrossRef CAS.
- V. Adler, A. Schaffer, J. Kim, L. Dolan and Z. Ronai, J. Biol. Chem., 1995, 270, 26071–26077 CrossRef CAS PubMed.
- K. Seiler and W. Simon, Sens. Actuators, B, 1992, 6, 295 CrossRef CAS.
- K. Seiler and W. Simon, Anal. Chim. Acta, 1992, 266, 73 CrossRef CAS.
- E. Bakker and W. Simon, Anal. Chem., 1992, 64, 1805 CrossRef CAS.
- X. J. Xie and E. Bakker, Anal. Bioanal. Chem., 2015, 407, 3899 CrossRef CAS PubMed.
- E. Bakker, P. Buhlmann and E. Pretsch, Chem. Rev., 1997, 97, 3083 CrossRef CAS PubMed.
- P. Buhlmann, E. Pretsch and E. Bakker, Chem. Rev., 1998, 98, 1593 CrossRef PubMed.
- Z. Shi, P. Peng, D. Strohecker and Y. Liao, J. Am. Chem. Soc., 2011, 133, 14699 CrossRef CAS PubMed.
- V. K. Johns, Z. Z. Wang, X. X. Li and Y. Liao, J. Phys. Chem. A, 2013, 117, 13101 CrossRef CAS PubMed.
- V. K. Johns, P. K. Patel, S. Hassett, P. Calvo-Marzal, Y. Qin and K. Y. Chumbimuni-Torres, Anal. Chem., 2014, 86, 6184 CrossRef CAS PubMed.
- P. K. Patel, V. K. Johns, D. M. Mills, J. E. Boone, P. Calvo-Marzal and K. Y. Chumbimuni-Torres, Electroanalysis, 2015, 27, 677 CrossRef CAS.
- S. Peper, I. Tsagkatakis and E. Bakker, Anal. Chim. Acta, 2001, 442, 25 CrossRef CAS.
- V. K. Johns, P. Peng, J. DeJesus, Z. Z. Wang and Y. Liao, Chem. – Eur. J., 2014, 20, 689 CrossRef CAS PubMed.
- M. Q. He, T. M. Leslie and J. A. Sinicropi, Chem. Mater., 2002, 14, 2393 CrossRef CAS.
- S. Liu, M. A. Haller, H. Ma, L. R. Dalton, S. H. Jang and A. K. Y. Jen, Adv. Mater., 2003, 15, 603 CrossRef CAS.
- E. Bakker, M. Willer and E. Pretsch, Anal. Chim. Acta, 1993, 282, 265–271 CrossRef CAS.
- P. Gehrig, B. Rusterholz and W. Simon, Chimia, 1989, 43, 377 CAS.
- D. Diamond, G. Svehla, E. M. Seward and M. A. Mckervey, Anal. Chim. Acta, 1988, 204, 223 CrossRef CAS.
- Y. Umezawa, P. Buhlmann, K. Umezawa, K. Tohda and S. Amemiya, Pure Appl. Chem., 2000, 72, 1851 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental methods. See DOI: 10.1039/c5an02206a |
| This journal is © The Royal Society of Chemistry 2016 |