Open Access Article
Open Access ArticleChiral resolution with frozen aqueous amino acids†
Satsuki
Takahashi
,
Makoto
Harada
and
Tetsuo
Okada
*
Department of Chemistry, Tokyo Institute of Technology, Meguro-ku, Tokyo 152-8551, Japan. E-mail: tokada@chem.titech.ac.jp; Fax: +81-5734-2612; Tel: +81-5734-2612
First published on 21st October 2015
Abstract
Frozen aqueous amino acids are screened to determine their chiral resolution using ice chromatography. Freezing allows convenient preparation of functional solid materials without organic syntheses. Frozen proline and leucine resolve the enantiomers of 1,1′-bi-2-naphthol (BINOL), while other amino acids tested do not show chiral selectivity. The effects seen when changing the pH of an amino acid solution before freezing suggest that the amino group of the amino acid plays an important role as a hydrogen bond acceptor. The molecular mechanism of chiral resolution is examined using Gaussian 9.0. A single proline molecule does not show chiral resolution capability. In contrast, two proline molecules fixed on the ice surface through the carboxyl groups show preferable interaction with the R enantiomer of BINOL. In the optimized structure of the Pro molecules on the ice surface, the distance between carboxyl oxygen and water oxygen on the ice surface ranges from 0.2533 to 0.2594 nm. The amino nitrogen atoms interact with the OH groups in BINOL with the distances of 0.2668 and 2.770 nm for the R-enantiomer and 0.2711 and 0.2717 nm for S-enantiomer. The preferable interaction with the R-enantiomer of BINOL is in accordance with the ice chromatography results, which reveal a stronger retentivity for the R enantiomer. These results strongly suggest that amino acids are expelled from the ice phase upon freezing, and form aggregates that provide multiple hydrogen bonding sites to enhance chiral resolution.
Chiral separation is an essential technique used for the development of medical drugs, synthetic chemistry, and studies of biological activities. Chiral chromatography is a reliable method and is widely employed for analytical and preparative purposes. Scientific and industrial needs have facilitated the development of useful stationary phases; some of them are commercially available.1,2 Polysaccharides and cyclodextrin derivatives are known as efficient chiral selectors.2,3 In most of the chiral stationary phases, selectors are chemically anchored on a solid support such as silica gel or polymer beads. The chemically bonded chiral stationary phases are usually prepared through multi-step organic syntheses. It would be convenient and useful if these expensive and time-consuming processes could be replaced by a simpler preparation method of chiral stationary phases.
We have developed ice chromatography, in which ice particles are used for liquid chromatographic stationary phases.4,5 Any water-soluble compound can be incorporated into the ice stationary phase by freezing its aqueous solution. This simple method allows the preparation of functional stationary phases without synthetic processes. Ice stationary phases containing β-cyclodextrin exhibit chiral separation capability.6 One of the most interesting characteristics of the β-cyclodextrin-doped ice stationary phase is that chiral recognition occurs in the liquid phase coexistent with ice, rather than on the ice surface. Chiral separation cannot be obtained when the ice stationary phase is doped only with β-cyclodextrin but does occur when a salt is incorporated in the stationary phase. In salt-doped ice, the liquid phase is developed at temperatures higher than the eutectic point of the system.5,7 β-Cyclodextrin is dissolved in the liquid phase and resolves the chirality of solutes. Another advantage of ice chromatography is that the liquid phase volume in the ice stationary phase can be controlled on the basis of the phase diagram of the system.5
Ice chromatography provides information on molecular behavior at ice interfaces. We have previously found anomalous enhancement in crown ether complexation in frozen electrolyte solutions8 and have also revealed the characteristics of hydrogen bond formation of solute molecules on the ice surface through ice chromatographic measurements.4 Ice chromatography provides limited resolution, mainly because of the sintering of ice particles during chromatographic runs, but can act as an efficient probe of the ice interface, which is difficult to approach by other methods. In addition, as noted above, freezing allows convenient preparation of functional solid phases without requiring complex organic syntheses.6 Thus, ice chromatography is effective in finding novel functionality of a frozen solid phase and in evaluating molecular interactions occurring on its surface.
In the present paper, frozen amino acids are examined for chiral resolution, and the interactions occurring on the frozen phase are discussed. The ability of simple amino acids in chiral resolution is known to be limited.9 However, as shown in the following part, frozen aqueous solutions of some amino acids resolve the chirality of 1,1′-bi-2-naphthol (BINOL) employed as a test solute. The chiral resolution capability of frozen amino acid depends on the pH of the original amino acid solution before freezing. This allows us to predict the interactions involved in the chiral resolution. The interactions predicted from ice chromatographic experiments are discussed on the basis of the calculations using Gaussian 9.0.
Experimental section
Ice chromatographic screening of effective amino acids
Chiral ice chromatographic experiments were performed by a similar procedure as employed in our previous work.4–6,8 The chromatographic system was composed of a Shimadzu HPLC pump (model LC-20AT), a Rheodyne injection valve equipped with a 100 μL sample loop, a JASCO circular dichroism (CD) detector (model CD-2095 Plus), and a Thermo Scientific low-temperature bath (model HAAKE Phoenix II P1-C41P). The frozen amino acid stationary phase was prepared by introducing droplets of an aqueous amino acid (5.0 mM) into liquid nitrogen. Frozen amino acid particles with sizes smaller than 75 μm (a photo is given in Fig. S1†) were collected by sieving and were hand-packed into a PEEK column (7.5 mm i.d × 150 mm in length) at a low temperature. The packed column was immersed in the low temperature bath maintained at an appropriate temperature. The mobile phase (5% diethyl ether in hexane) was precooled by passing through a coiled tube connected between the pump and the sample injector. The flow rate was 0.80 mL min−1.Molecular orbital (MO) calculations
All of the MO calculations were performed with Gaussian 09A, Rev.D.01, for Windows. The molecular structures of proline (Pro) and BINOL were constructed with GaussView5 for Windows and were optimized with the 3-21G basis set.The chiral recognition of frozen aqueous amino acids was assumed to occur on the basal (0001) plane of the ice surface. The structure of the basal plane had the following characteristics; 0.96 Å (the bond length of O–H in water), 2.76 Å (the distance between two adjacent oxygen atoms), 109.47° (angle of O–O–O), and 60° (dihedral angle of O–O–O–O).10 It was assumed that hydrogen atoms were located between two oxygen atoms. The ice surface structure was fixed and was not optimized when the interaction between Pro and BINOL was evaluated.
Results and discussion
Ice chromatographic screening of frozen amino acids for chiral resolution of BINOL
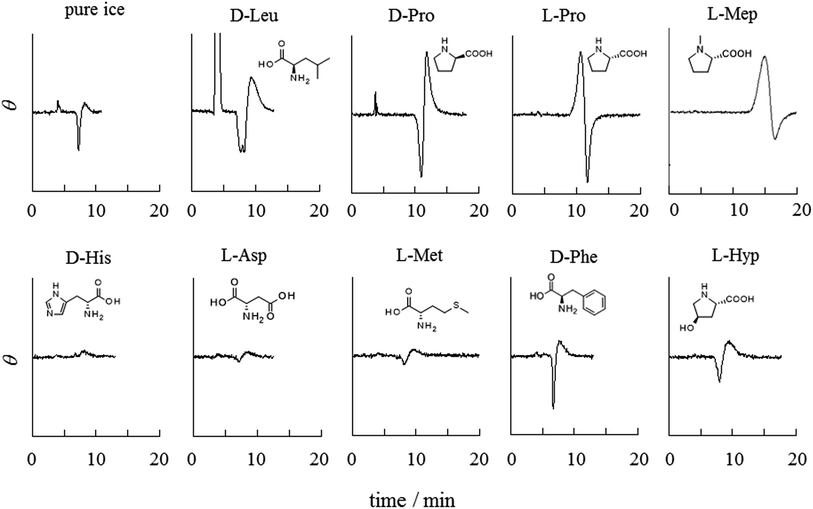
Various frozen amino acids were examined as ice chromatographic stationary phases to select effective amino acids for the chiral resolution of BINOL. Fig. 1 summarizes chromatograms of racemic BINOL with frozen 5.0 mM aqueous amino acids. Baseline perturbations due to the elution of BINOL are seen in all of the chromatograms, even for the achiral pure ice stationary phase. However, when chirality is resolved, a clear differential peak appears; positive and negative peak intensities become larger as the resolution is enhanced. Chromatography of a single enantiomer of BINOL has confirmed that the S-enantiomer is eluted earlier than the R-enantiomer when an L-amino acid is used as a chiral selector and that S-BINOL gives a positive peak in CD detection. Frozen proline (Pro), N-methylproline (Mep), and leucine (Leu) resolve the chirality of BINOL, while no chiral resolution is observed with the other amino acids tested. The elution order of the BINOL enantiomers is reversed for frozen L- and D-Pro stationary phases, indicating that the chiral resolution originates from the amino acids (the same assessment for Leu will be shown below). No clear dependence of chiral resolution on the amino acid concentration was not confirmed in the range of 1–10 mM. However, chiral separation becomes poorer as the concentration of amino acids increases past 10 mM. What causes the low resolution is discussed later.The structural characteristic common to Pro, Mep, and Leu is that the essential amino (–NH2) and carboxyl (–COOH) groups are located on one side of each molecule and the other side is occupied by hydrocarbon moieties. In contrast, other amino acids tested in this work have polar groups other than the –NH2 and –COOH groups in the molecules. The most obvious contrast is seen for Pro and hydroxyproline (Hyp). The latter has a hydroxyl group on the five-membered ring in a molecule, but otherwise the molecular structure is the same. Interestingly, despite such a subtle difference in a molecular structure, the chiral resolution ability of Pro is entirely lost by the introduction of a hydroxyl group to the molecule.
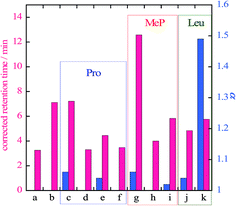
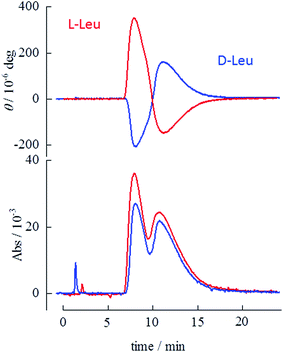
Retention times and separation ratios of the BINOL enantiomers obtained with various frozen amino acid stationary phases are summarized in Fig. 2. The effects of the pH of the original solutions before freezing are also indicated in this figure. For Pro and Mep, low pH results in poor resolution and lowers the retention time. The retention times obtained with pH ∼ 2 (Fig. 2d and h) are almost equal to that with the pure ice stationary phase, suggesting no interaction between the solute and amino acid in the stationary phase at acidic conditions. High pH (∼10) slightly reduces the retention time but the separation ratio is comparable to that for intermediate pH (i.e. no pH adjustment). For Leu, high pH (Fig. 2k) leads to a higher separation ratio than that for intermediate pH. Chiral separation with alkaline Leu stationary phases is shown in Fig. 3. The BINOL enantiomers are clearly resolved with this stationary phase. Reversed polarity in CD detection is also confirmed for frozen L- and D-Leu.
The liquid phase that coexists with ice has received attention to understand the interfacial and thermodynamic nature of ice11–13 and to facilitate functionality of ice.5,6,14 As noted above, β-cyclodextrin recognizes chirality in the liquid phase developed in the ice stationary phase.6 The addition of KCl was examined to confirm the contribution of the liquid phase to chiral separation with frozen amino acids. As shown in Fig. 2f, the retention time decreases, and chiral resolution is entirely lost by the addition of KCl to the frozen Pro stationary phase. The retention time obtained with KCl-doped frozen Pro is the same as that obtained with pure ice, suggesting that no interaction occurs between BINOL and Pro dissolved in the liquid phase. Temperature effects on the chiral resolution with frozen Pro/KCl are shown in Fig. S2.† No resolution is seen at −8.0 and −10.0 °C because of the development of the liquid phase. A further decrease in the temperature enhances the retention and allows, to some extent, the resumption of the chiral resolution. The baseline dent, which is seen after the positive signal at −12.5 or −15.0 °C, is obviously due to the elution of an enantiomer giving a negative CD signal. The liquid phase is not developed at the temperatures lower than the eutectic point of KCl/water, −10.6 °C.15 This strongly implies that chiral recognition of frozen Pro does not occur in the liquid phase, but on the surface of the frozen phase. As noted above, chiral resolution became poorer as the concentration of an amino acid increased past 10 mM. High concentration of an amino acid facilitates the development of the liquid in the frozen phase, and its chiral recognition ability is hereby lowered.
Ice chromatographic study has indicated that molecules adsorbed on the ice surface can provide additional hydrogen bonding sites and enhance solute retention.4 Hydrogen bond formation thus takes place at the interface of the ice and the mobile phase. The chiral resolution by frozen amino acids should also be due to the hydrogen bonding between the amino acid and solute molecules at this interface. Therefore, once amino acids are dissolved in the liquid phase, the chirality of the solute is no longer recognized. While amino acids adopt zwitterionic forms in aqueous solution, the ionization should be suppressed at the ice interface with apolar organic phases. Thus, the neutral forms of –NH2 and –COOH groups in an amino acid molecule may act as hydrogen bond donor or acceptor for chiral recognition. Low pH in an original aqueous solution before freezing facilitates the protonation of the –NH2 group, and its hydrogen bond acceptor abilities should be drastically lowered. This suggests that the –NH2 group plays an important role in the chiral recognition in frozen amino acid solutions. Fig. 2 indicates that low pH eliminates chiral resolution but high pH does not. In addition, the introduction of a methyl group on the nitrogen atom in the Pro molecule does not eliminate the chiral resolution of Pro at low or intermediate pH. These results imply that the –NH2 group may act as a hydrogen bond acceptor but its hydrogen bond donation is not critical in the determination of chiral resolution of frozen amino acids.
Amino acids, including Pro and Leu and its derivatives, have been used as selectors in chiral chromatography.9,16–19 However, simple amino acids rarely act as effective chiral selectors. Successful chiral separation has been carried out by introducing additional π–π interaction.16,17,19 Leu derivatives with a dinitrophenyl or naphthyl group, for example, provide good chiral resolution for enantiomers of various aromatic compounds.19 Coordination bond formation of an anchored amino acid is also often employed for chiral separation.20,21 Effects of coordination bonds on the chiral resolution of frozen amino acids were studied with frozen Pro-Cu2+ stationary phase as shown in Fig. S3.† Chiral resolution provided by this stationary phase is much poorer than that with frozen Pro. For comparison, chromatograms obtained with a commercial chiral column with Pro-Cu2+ (Daicel CHIRALPAK WH, 4.6 mm i.d. × 250 mm) as the chiral selector are also depicted in Fig. S4.† Although BINOL enantiomers are resolved, chiral resolution is poor in a wide temperature range, and not better than the separation demonstrated in Fig. 3. Thus, the coordination bond (liquid-exchange) does not enhance the chiral recognition occurring in the frozen stationary phase. The mechanism of chiral resolution in frozen amino acids is different from the ones provided by unmodified amino acids.
Molecular computations for interaction on ice surface
As shown above, some amino acids resolve chirality of BINOL in the frozen state. Molecular calculations with Gaussian 9.0 were performed to give a further insight into the molecular processes occurring at the interface between the frozen aqueous amino acid and the mobile phase.22–24 Because hexane is a dominant component of the mobile phase, the calculation was performed in vacuum and no solvent effects were taken into consideration. L-Pro was chosen for calculation as an amino acid selector incorporated in the frozen phase.The interaction between a single L-Pro molecule and each enantiomer of BINOL was calculated. Fig. S5† shows the optimized structures of the complexes of R- and S-enantiomers of BINOL with L-Pro. Naturally, the two hydroxyl groups in a BINOL molecule form hydrogen bonds with the carboxyl and amino group in the L-Pro molecule. In this configuration, the total energy is only 0.3 kJ mol−1 smaller for the R-enantiomer than for the S-enantiomer of BINOL. This difference is so small that the BINOL enantiomers are not resolved. This result agrees with the chromatographic work previously reported for simple amino acid stationary phases.9
Considering that the chiral resolution occurs at the interface between the frozen phase and the organic mobile phase, we constructed a model in which L-Pro molecules interact with the ice surface through the hydrogen bonds; these hydrogen bonds are formed between the carboxyl groups in Pro molecules, and the dangling bonds on the ice surface, as illustrated in Fig. 4. As noted in the Experimental section, the basal plane was assumed for the ice surface interacting with L-Pro. The ice surface structure was fixed and was not optimized during calculations. The L-Pro molecule interacts with the dangling bonds on the ice basal plane through the hydrogen bond at the carboxyl group. If an L-Pro molecule were anchored on the ice surface through the hydrogen bonds of the carboxyl group (the distance between carboxyl oxygen and water oxygen on the ice surface ranged from 0.2533 to 0.2594 nm), a BINOL molecule could interact with the Pro through the amino groups. As discussed above, a single Pro molecule did not resolve BINOL chirality. Therefore, two L-Pro molecules were fixed on the ice surface and their interaction with BINOL was evaluated.
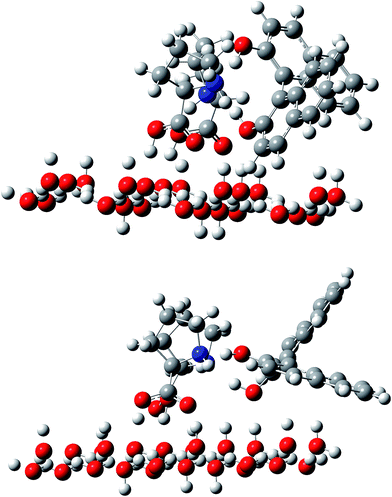 | ||
| Fig. 4 Optimized geometries of two Pro molecules fixed on the ice surface, which interact with the R (top) and S (bottom) enantiomers of BINOL. | ||
Ice chromatographic measurements have suggested the important involvement of the amino group in an L-Pro molecule as a hydrogen bond acceptor rather than a donor. A BINOL molecule was therefore arranged to form hydrogen bonds between the amino nitrogen in Pro and an OH group in BINOL. The optimized structures for the R- and S-enantiomer of BINOL are illustrated in Fig. 4. The N–O distance between Pro and BINOL was 0.2668 and 2.770 nm for the R-enantiomer and 0.2711 and 0.2717 nm for S-enantiomer. The total energy difference indicates that the interaction of the R-enantiomer with the Pro dimer fixed on the ice surface is 41.6 kJ mol−1 more preferable than that of the S-enantiomer. This result satisfactorily explains ice chromatographic results that have shown greater retention of the R-enantiomer on the L-Pro doped ice stationary phase.
Amino acid-based chiral separation has been performed with polymeric or oligomeric amino acid surfactants.25–29 Micellar electrokinetic chromatography using buffers containing this type of surfactants as selectors allows high chiral resolution. Polymeric micelles with oligomeric amino acid head groups provide better separation than analogous monomeric ones,26 indicating that multiple hydrogen bonds are formed between a solute and stacked amino acid molecules on the micelle surface. When the aqueous solutions are frozen, amino acid molecules are expelled from the ice phase and concentrated in the surface grain boundary between ice crystals. In the absence of the liquid phase, condensed amino acids can form aggregates. Aggregates of amino acids enhance the chiral recognition.30 Thus, the chiral recognition of frozen amino acids may possibly arise from their aggregation induced by the freeze concentration.
Conclusions
Freezing is an efficient way to anchor functional molecules on the solid support without help of organic syntheses. Although the chiral resolution ability of amino acids is not very high, aggregation, which is induced by methods such as freezing, improves chiral resolution ability. Thus, the present method is not only useful for screening the molecular-based chiral resolution of a selector but also creates novel functionality as molecular assemblies. In addition, ice chromatography has shown its effectiveness in probing the molecular interactions occurring at the ice interfaces that are difficult to access by other methods. In the present study, we have focused on frozen amino acids and their chiral resolution. This approach can be extended to any frozen system and allows exploration of unknown molecular systems. Hence, the present method is efficient not only for screening of the useful functionality of solid materials prepared by freezing, but also searching and evaluating interfacial molecular interactions. We hope that ice chromatography becomes a general choice in studies of ice interfaces.Acknowledgements
This work was supported by SENTAN from Japan Science and Technology Agency and by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science.References
- A. Cavazzini, L. Pasti, A. Massi, N. Marchetti and F. Dondi, Anal. Chim. Acta, 2011, 706, 205–222 CrossRef CAS PubMed.
- Y. Okamoto and Y. Kaida, J. Chromatogr. A, 1994, 666, 403–419 CrossRef CAS.
- Z. Juvancz and J. Szejtli, Trends Anal. Chem., 2002, 21, 379–388 CrossRef CAS.
- Y. Tasaki and T. Okada, Anal. Chem., 2006, 78, 4155–4160 CrossRef CAS PubMed.
- Y. Tasaki and T. Okada, Anal. Chem., 2011, 83, 9593–9599 CrossRef CAS PubMed.
- T. Shamoto, Y. Tasaki and T. Okada, J. Am. Chem. Soc., 2010, 132, 13135–13137 CrossRef CAS PubMed.
- T. Hashimoto, Y. Tasaki, M. Harada and T. Okada, Anal. Chem., 2011, 83, 3950–3956 CrossRef CAS PubMed.
- Y. Tasaki and T. Okada, J. Am. Chem. Soc., 2012, 134, 6128–6131 CrossRef CAS PubMed.
- J. Huang, P. Zhang, H. Chen and T. Li, Anal. Chem., 2005, 77, 3301–3308 CrossRef CAS PubMed.
- F. Petrenko and R. W. Whitworth, Physics of Ice, Oxford University Press, New York, 1999 Search PubMed.
- H. Cho, P. B. Shepson, L. A. Barrie, J. P. Cowin and R. Zaveri, J. Phys. Chem. B, 2002, 106, 11226–11232 CrossRef CAS.
- V. Sadtchenko and G. E. Ewing, Can. J. Chem., 2003, 81, 333–341 CAS.
- Y. Tasaki and T. Okada, J. Phys. Chem. C, 2008, 112, 2618–2623 CAS.
- T. Y. Tasaki and T. Okada, Anal. Sci., 2009, 25, 177–181 CrossRef PubMed.
- R. R. Cohen-Adad, P. Vallee and J. W. Lorimer, Solubility Data Ser., 1991, 47, 225–243 Search PubMed.
- M. H. Hyun, M. H. Kang and S. C. Han, J. Chromatogr. A, 2000, 868, 31–39 CrossRef CAS PubMed.
- M. H. Hyun, J. B. Lee and Y. D. Kim, J. High Resolut. Chromatogr., 1998, 21, 69–71 CrossRef CAS.
- G. Landek, M. Vinković, D. Kontrec and V. Vinković, Chromatographia, 2006, 64, 469–473 CAS.
- W. H. Pirkle and W. E. Bowen, Tetrahedron: Asymmetry, 1994, 5, 773–776 CrossRef CAS.
- B. Natalini, R. Sardella, A. Macchiarulo and R. Pellicciari, J. Chromatogr. B, 2008, 875, 108–117 CrossRef CAS PubMed.
- Q.-H. Wan, P. N. Shaw, M. C. Davies and D. A. Barrett, J. Chromatogr. A, 1997, 786, 249–257 CrossRef CAS.
- G. Bian, H. Fan, H. Huang, S. Yang, H. Zong, L. Song and G. Yang, Org. Lett., 2015, 17, 1369–1372 CrossRef CAS PubMed.
- L. S. Moon, M. Pal, Y. Kasetti, P. V. Bharatam and R. S. Jolly, J. Org. Chem., 2010, 75, 5487–5498 CrossRef CAS PubMed.
- J. R. Mora, A. J. Kirby and F. Nome, J. Org. Chem., 2012, 77, 7061–7070 CrossRef CAS PubMed.
- E. Billiot, J. Macossay, S. Thibodeaux, S. A. Shamsi and I. M. Warner, Anal. Chem., 1998, 70, 1375–1381 CrossRef CAS PubMed.
- F. H. Billiot, E. J. Billiot and I. M. Warner, J. Chromatogr. A, 2001, 922, 329–338 CrossRef CAS PubMed.
- J. L. I. Haynes, E. J. Billiot, H. H. Yarabe, I. M. Warner and S. A. Shamsi, Electrophoresis, 2000, 21, 1597–1605 CrossRef CAS PubMed.
- Y. Hedeland, M. Hedeland, U. Bondesson and C. Pettersson, J. Chromatogr. A, 2003, 984, 261–271 CrossRef CAS PubMed.
- S. J. Thibodeaux, E. Billiot and I. M. Warner, J. Chromatogr. A, 2002, 966, 179–186 CrossRef CAS PubMed.
- H. Li, J. Cheng, H. Deng, E. Zhao, B. Shen, J. W. Y. Lam, K. S. Wong, H. Wu, B. S. Li and B. Z. Tang, J. Mater. Chem. C, 2015, 3, 2399–2404 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ay02522b |
| This journal is © The Royal Society of Chemistry 2016 |