 Open Access Article
Open Access ArticleSugared biomaterial binding lectins: achievements and perspectives†
P.
Bojarová
* and
V.
Křen
Laboratory of Biotransformation, Institute of Microbiology, Academy of Sciences of the Czech Republic, Vídeňská 1083, CZ 14220 Prague 4, Czech Republic. E-mail: bojarova@biomed.cas.cz
First published on 14th April 2016
Abstract
Lectins, a distinct group of glycan-binding proteins, play a prominent role in the immune system ranging from pathogen recognition and tuning of inflammation to cell adhesion or cellular signalling. The possibilities of their detailed study expanded along with the rapid development of biomaterials in the last decade. The immense knowledge of all aspects of glycan–lectin interactions both in vitro and in vivo may be efficiently used in bioimaging, targeted drug delivery, diagnostic and analytic biological methods. Practically applicable examples comprise photoluminescence and optical biosensors, ingenious three-dimensional carbohydrate microarrays for high-throughput screening, matrices for magnetic resonance imaging, targeted hyperthermal treatment of cancer tissues, selective inhibitors of bacterial toxins and pathogen-recognising lectin receptors, and many others. This review aims to present an up-to-date systematic overview of glycan-decorated biomaterials promising for interactions with lectins, especially those applicable in biology, biotechnology or medicine. The lectins of interest include galectin-1, -3 and -7 participating in tumour progression, bacterial lectins from Pseudomonas aeruginosa (PA-IL), E. coli (Fim-H) and Clostridium botulinum (HA33) or DC-SIGN, receptors of macrophages and dendritic cells. The spectrum of lectin-binding biomaterials covered herein ranges from glycosylated organic structures, calixarene and fullerene cores over glycopeptides and glycoproteins, functionalised carbohydrate scaffolds of cyclodextrin or chitin to self-assembling glycopolymer clusters, gels, micelles and liposomes. Glyconanoparticles, glycan arrays, and other biomaterials with a solid core are described in detail, including inorganic matrices like hydroxyapatite or stainless steel for bioimplants.
1. Introduction
The complexity of the carbohydrate structure conceals a bulk of biological information and its decoding belongs to the major challenges in current interdisciplinary science. The sugar message imprinted on most cellular surfaces in living organisms is translated by a range of specific molecules, among which lectins occupy a privileged position. Understanding of the principle of how the sugar code is cracked by lectins gives us the power to encompass the fundamentals of life. Therefore, a thorough study of lectin–carbohydrate interactions represents a yet unexplored route for applications like drug design, in vivo imaging, targeted drug delivery, diagnostic, and analytic methods.The subject of glycan-decorated biomaterials has been reviewed in current years.1–3 However, most authors presented just isolated aspects of this topic and, to the best of our knowledge, no recent work has given a comprehensive overview of biomaterial binding lectins and applications resulting thereof. This review aims to cover all types of glycan-decorated biomaterials including polymers, saccharide and non-saccharide scaffolds as well as solid carriers like glycoarrays, which have shown promising potential in lectin-mediated interactions. The hallmark of glycan-coated materials is multivalency, provided by simultaneous presentation of multiple sugar epitopes in a particular pick and arrangement. Since the monovalent lectin–glycan interaction is often relatively weak, i.e., with the association constant (Ka) in a micro- to millimolar range,4 the biological response in vivo is amplified by the cluster glycoside effect.5 Through the multivalent display of sugar ligands, the sugar–lectin interaction is enhanced by several orders of magnitude, resulting in Ka values of up to 109 M−1.6 Thus, glycomaterials successfully mimic the natural design, which makes them utmost effective and precise tools in biology and medicine; for example, in analysis using glyco-biosensors7 and microarrays,8 in magnetic resonance imaging9 as well as in targeted treatment of tumour tissue.10
2. Lectin ligands of sugar-coated biomaterials – biological ABC
The roots of the term “lectin” can be traced back to the 1950s, when William C. Boyd, an American immunochemist, recognised the need to distinguish a special group of proteins. They were not produced in response to antigens like antibodies but still selectively interacted with specific sugar structures without changing their biological nature.11 The word “lectin” comes from Latin lēctus, (legere, lat. read, pick; perfect passive). Lectins encompass carbohydrate-binding proteins besides antibodies, transport proteins, and enzymes,12 which are either secreted or localised on the cell surface and recognise specific glycan motifs presented typically on protein or lipid backbones. In the literature, they are often considered within a larger group of GBPs (glycan-binding proteins). The diversity of the lectin group reflects on their ubiquity in all parts of the living universe, from bacteria and viruses to plants, animals and humans. Merely the animal/human group includes fourteen 3D folds. The lectin family as such has been extensively reviewed elsewhere.13,14 Here we focus on three lectin groups that are most intensively studied from the viewpoint of interacting with glycomaterials: galectins, C-type lectins, and siglecs. They represent the majority of ca. 70 known mammalian GBPs and they all have irreplaceable roles in the immune system.8 As such, they are well documented in terms of their glycan specificity and cell-specific presentation.152.1. Galectins
Galectins are widely spread animal lectins typical of high evolutionary conservation of a carbohydrate-recognition domain (CRD) and sequence similarity.16,17 They specifically recognise β-galactose-containing glycans and participate in basic cellular processes such as cell growth, development and apoptosis, inter-cell adhesion, trafficking and signalling18,19 (Fig. 1). The involvement of galectins in defence and pathophysiological processes like infection, atherosclerosis and cancerogenesis has been thoroughly documented in recent years.20,21 So far, fifteen members of the galectin family have been described (Gal-1–15).22 They all feature a globular fold comprising two antiparallel β-sheets (5–6 strands each) and one or two concave-shaped CRD domains; sometimes also one non-lectin domain. Since the CRD domain is highly conserved, the differences in binding affinity are usually given by variations in β-sheets and the surrounding loop regions. The galectin function is based on supramolecular assembly into oligomeric structures, leading to cross-linking into “lattices” on the cell surface, which activates various signalling pathways.23 For example, the bivalent galectin-1 can induce specific pro-apoptotic glycoprotein receptors through crosslinking and thus affect the homeostasis and survival of T-cells.24 It is the major regulator of immune responses involving T-cell disorders, inflammation, allergies, and host–pathogen interactions; it participates in tumour metastasis, immunoregulation as well as in neurodegenerative pathologies.25 The roles of galectin-1 and -3 in tumour progression and proliferation, angiogenesis, resistance to drugs and masking against recognition by the immune system have recently been reviewed.26,27 Besides the role as a cancer marker, galectin-3 is implicated in a range of metabolic disorders, such as complications of diabetes;28 its expression accompanies processes connected to heart disease and stroke, fibrosis, and tissue repair.29 Upon binding to multivalent ligands, galectin-3 assembles into pentamers.30 More specialised than the previously mentioned counterparts, galectin-9 is involved in the regulation of the glucose level in blood and in related metabolic disorders like diabetes.31 Human galectin-7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 32 is involved in the metabolism of keratocytes and related epithelia formation, development and regeneration at the level of cornea and epidermis; it also contributes to the regulation of apoptosis.33
32 is involved in the metabolism of keratocytes and related epithelia formation, development and regeneration at the level of cornea and epidermis; it also contributes to the regulation of apoptosis.33
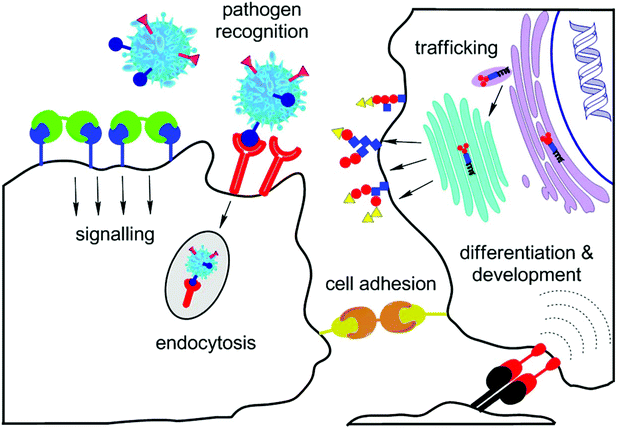 | ||
| Fig. 1 Important roles of lectins in vivo: inter-cellular signalling and trafficking, recognition of pathogens, cellular adhesion, and cell differentiation and development. | ||
2.2. C-type lectins and Siglecs
The C-type (calcium-dependent) lectin receptors (CLRs) are the biggest and most varied family of animal lectins. Their carbohydrate recognition domain is typical of binding sugar ligands by ligating them to Ca2+ ions.34 The diverse group of C-type lectins comprises endocytic receptors, selectins, collectins, and proteoglycans, both of secreted and transmembrane types. The degree of conservation varies throughout the family – receptors for adhesion and endocytosis of endogenous mammalian glycans are often conserved whereas pathogen-binding receptors on immune cells show more variability.35 Besides their function in cell adhesion, and glycoprotein metabolism, C-type lectins also strongly participate in immune response36 and pathogen recognition37 (Fig. 1). It was shown that pathogens and tumour antigens abuse CLRs in order to escape recognition by the host system leading to degradation.38 The communication of CLRs and Toll-like receptors of dendritic cells results either in the onset of inflammatory response or in maintaining tolerance by the defense system.39 Thus, CLRs are able to modify signalling pathways activated by Toll-like receptors. This behaviour is typically observed in DC-SIGN (dendritic cell specific intercellular adhesion molecule-3-grabbing nonintegrin)40 and the macrophage galactose receptor (MGL)41 and it shows a new pathway to antiviral and anticancer therapeutics as also described in section 7.2.Siglecs (sialic acid-binding immunoglobulin-like lectins) are a group of membrane proteins of type 1 that selectively bind glycans containing sialic acid.42 They are ranked under I-type lectins since they contain a homologous immunoglobulin-like domain. They form two distinct groups: (1) an evolutionary conserved group consisting of sialoadhesin/Siglec-1, CD22/Siglec-2, and myelin-associated glycoprotein/MAG/Siglec-4, and (2) CD33-related siglecs (CD33/Siglec-3 and Siglec-5 to -13). To date, thirteen siglec family representatives have been found in humans, particularly on immune cells like B-cells, monocytes, and dendritic cells.15 Siglecs are involved in cell signalling and adhesion, and they are supposed to participate in pathogen recognition and endocytosis43 (Fig. 1).
3. Glycomaterials – mode d'emploi
The expansive development of glycomaterials would not have been possible, were it not for novel synthetic methods like automated solid-phase synthesis,44 programmable one-pot synthesis,45 and ingenious multi-enzyme synthetic methods,46 which amplified the pool of glycans required by the high-throughput approach (Fig. 2). The use of synthetic glycans is particularly valuable when, for example, the binding nuances around a known cancer glyco-motif are examined. Anyway, the major challenge still remains to assemble a sufficient bulk of diverse carbohydrate motifs suitable for display. Especially needed are structures containing naturally occurring glycans, which are recognised as ligands by biologically or medically interesting GBPs. Such glycans may be isolated directly from natural sources but there arises the problem of sufficient purification and of reliable and easy structural verification. Despite these bottlenecks, natural glycans represent the most significant stock of carbohydrate structures for biomedical research to date. Commonly used natural glycans comprise milk oligosaccharides, proteoglycans, glycans, and their fragments released from glycolipids and glycoproteins by means of chemical or enzymatic degradation as well as bacterial and plant polysaccharides.47 The diversity of glycan libraries may further be increased by means of genetic engineering (Fig. 2 and Table 1), such as by mass production of glycophages containing sugar epitopes of interest, using recombinant bacteria.48 Advantageously, these glycan-displaying phages are readily isolated from bacterial supernatants, and are highly suitable for high-throughput screening methodologies.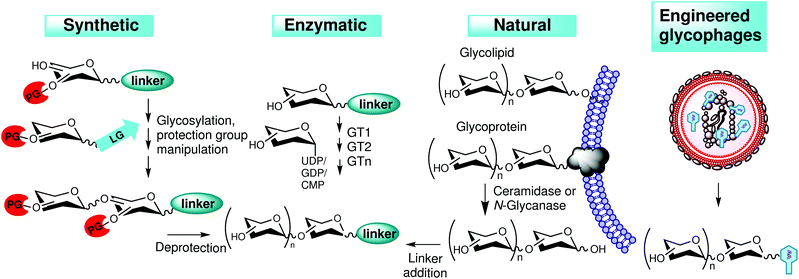 | ||
| Fig. 2 Generation of glycans for carbohydrate libraries. Glycans prepared by synthetic or enzymatic methods, by genetic engineering or from natural sources. LG, leaving group; PG, protecting group. | ||
| Research group | Glycans | Characteristics | Reference |
|---|---|---|---|
| Feizi | ∼600 | Amino-linked neoglycolipids | Liu et al.50 |
| Cummings | ∼200 | Natural fluorescently tagged glycans | Song et al.51 |
| Derda | 86 | Glycopeptides with Man-WYD motif | Ng et al.52 |
| Lepenies | 52 | Glycans binding CLRs | Maglinao et al.53 |
| Percec | 51 | Dendrimers with Man/Gal/Lac | Percec et al.54 |
| Winssinger | 50 | Glycans tagged to peptide-nucleic acids | Huang et al.55 |
| 33 | Novoa et al.56 | ||
| Paulson | 44 | 9-Acyl substituted sialosides | Blixt et al.57 |
| Smith | 26 | High-Man phosphorylated N-glycans | Song et al.58 |
| Paulson | 26 | Glycans with Neu5Acα-2,6-Gal | Nycholat et al.59 |
| Wong | 24 | Sialosides binding influenza HA | C. C. Wang et al.60 |
| Boons | 23 | Asymmetrically branched N-glycans | Z. Wang et al.61 |
| DeLisa | 8 | Fluorescent engineered glycophages | Çelik et al.48 |
Successful determination of the specificity of a particular GBP consists of presenting an exhaustive choice of glycans and comparing the strength of binding of individual structural features. Ideally, the complete glycome of the target tissue or cell should be displayed. The cellular glycome is estimated to comprise ca. 100 to 500 thousand glycans49 but the crucial structural information is contained within a limited number of structural variations in strategic positions of the glycan chain. Therefore, the glycan libraries of ca. 500–600 items existing nowadays (Table 1)48,50–61 substantially cover the major informational potential of the examined glycome.8
With the still expanding stock of glycan motives available for screening, correct analysis and interpretation of the mined data may become difficult and time-consuming. In order to facilitate combing through the screening data, several software programmes have been developed, such as Outlier Motif Analysis,62 GlycoSearch software,63 Quantitative Structural Activity Relationship (QSAR),64 and GlycanMotifMiner.65 In principle, they are based on quantitative evaluation of how individual structural motives correspond to the GBP binding affinity, usually in the form of numerical scores of statistical calculations.
Importantly, the very structure of the glycan motif is just one parameter to consider. Other factors matter like the orientation and density of the glycans displayed, depending on the nature, valency and geometry of the scaffold, and even the type of glycan immobilisation.66 Therefore, in order to obtain reliable data, glycan probes must be optimised including the structure, geometry and density of the linker, carrier and labels.
4. Glycans on solid carriers – glycoarrays, nanoparticles, and quantum dots
The biomaterials containing a solid core of various types and shapes – planar, cluster-type or tubular67 – are covered in glycan ligands displayed on the surface in a 3D mode.4.1. Glyconanoparticles
Besides the scaffold function, the carrier solid brings in other practical features. Metal oxides such as Fe2O3 are excellent contrast agents in non-invasive magnetic resonance imaging (MRI) of human soft tissues due to their superparamagnetic properties. In the last two decades, many of these magnetic materials have appeared on the commercial market, such as Super-Paramagnetic Iron Oxide (SPIO) nanoparticles,68 Very Small Iron Oxide Particles (VSOP),69 Feraheme®,70 Primovist®,71 and others. Moreover, iron oxide glyconanoparticles exhibit exothermic behaviour in alternating current magnetic field, directly applicable in, e.g., hyperthermia therapy of tumours.72 Their nontoxic and biodegradable nature are significant advantages in biomedical applications, compared to heavy-metal-containing quantum dots; for example in drug delivery, detection of altered (cancer) cells, magnetic resonance imaging, and in vivo thermotherapy (see section 7 for details).Gold nanoparticles exhibit an excellent response in colorimetric bioassays;73 the reason is the extremely high extinction coefficient of gold (106–109 M−1 cm−1 depending on the size and shape of the nanoparticle and its ligands)74 and also good self-assembling potential typical of gold in colloidal form. The colour change from red to violet, induced by aggregation upon binding of glycopeptide gold nanoparticles to the lectin ligand (e.g., ConA), is easy to observe even with the naked eye.75 In another set-up, a gold nanocluster with glycoproteins from chicken egg-white was prepared that showed significant red photoluminescence properties, which were attributed to the presence of Cys and Tyr amino acids in the glycoprotein.76
Tethered with specific glycans, gold glyconanoparticles are ideal for efficient binding of lectins of choice, such as in the case of PA-IL (LecA) adhesive lectin of Pseudomonas aeruginosa as shown by Reynolds et al.77 The multivalent presentation of Gal ligands in the nanoparticle arrangement caused an immense 3000-fold increase in lectin affinity (Kd per Gal ligand = 50 nM) over the monovalent counterpart, which corresponds to the strongest PA-IL inhibitor found to date. This model is promising for designing anti-adhesives that could prevent pathogen invasion in vivo. An example of a potent anticancer therapeutic platform was reported by Biswas et al.78 Gold nanoparticles coated with Thomsen–Friedenreich antigen, a disaccharide specific for many carcinoma cells, efficiently bound galectin-3, and, as a result, inhibited tumour cell growth (Fig. 3).
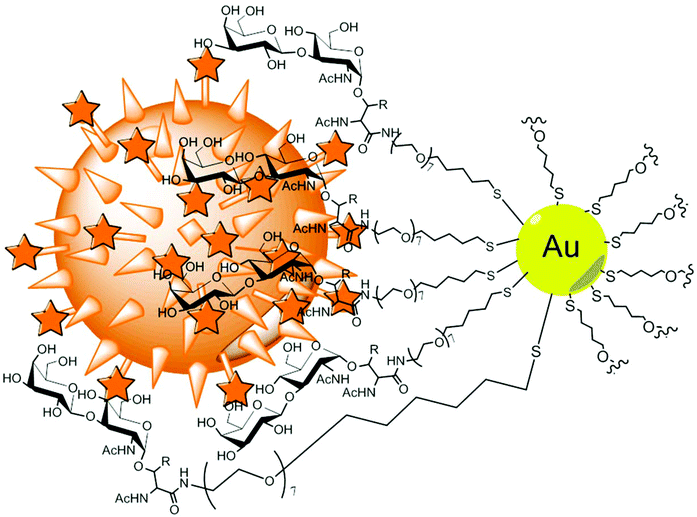 | ||
| Fig. 3 Gold nanoparticles tethered with Thomsen–Friedenreich antigens. The epitope is O-linked via a threonine alkane/PEG linker. The binding to Gal-3 positive breast cancer cells (orange) efficiently induced apoptosis.78 | ||
4.2. Quantum dots
The term “quantum dots”, coined by Prof. Mark A. Reed,79 denotes semiconductor nanocrystals, typically containing binary or ternary compounds of heavy metals such as Cd or Pb with characteristic fluorescent properties. Similar to classical nanoparticles, quantum dots are abundantly used especially as inexpensive and efficient analytical and diagnostic tools. With suitable glycan coating, they can label selected lectins even in complex mixtures. Again, the binding potency is immensely multiplied thanks to the multivalent glycan presentation, as shown in selective labelling of FimH lectin of E. coli flagella80 or of galectin-3 tumour marker.81 Quantum dots and nanoparticles may even be used together in one bioprobe, as shown by Hu et al.82 Thus, beneficial features of both systems, namely super-paramagnetic and fluorescent properties, are elegantly combined, enabling a time-saving simultaneous detection of multiple components in one pot. This approach promises to show new possibilities in high-throughput assay and screening techniques. A major drawback of quantum dots, their considerable toxicity, is conveniently decreased in the presence of a glycopolymer envelope, thanks to covalent conjugation.83 Pei et al.84 prepared quantum dots coated with a star-shaped glycopolymer hull and showed their use as targeted fluorescent probes. The hybrid quantum dots were shown to bind ConA in vitro and enter human carcinoma cells by endocytosis. Upon internalisation by Hep G2 cells, green fluorescence was emitted.4.3. Glycoarrays
Glycoarrays were invented in 200285,86 as followers of DNA and protein arrays, and filled the achingly perceived gap in high-throughput methodologies. Since then, the number of publications regarding the design and biological applications of glycoarrays have grown exponentially and the topic has been reviewed.87,88 The main advantages of the glycoarray set-up are simultaneous monitoring of numerous samples in the high-throughput mode, and minute (pg amounts) consumption of analytes.There are numerous methods of immobilisation of glycan ligands on the array surface.88 Besides noncovalent strategies based on adhesion, the most demanded way is site-specific covalent immobilisation of glycans on the array surface, preferably without changing their natural orientation and conformation. To accomplish this, the glycans in question must be equipped with a suitable functional group (thiol, amine, etc.) to react with the solid support. The immobilisation of underivatised reducing oligosaccharides still remains the major challenge. Beckmann et al.89 immobilised a range of unprotected reducing sugars on functionalised glass slides by means of Diels–Alder ligation with inverse electron demand. Binding assays were performed with fluorescently labelled lectins. Another gentle immobilisation method90 that fully preserves the original glycan structure including conformation comprises cheap cyanuric chloride as a linker. The intact structure of fifteen model saccharide ligands was confirmed by LC-MS and NMR and binding was tested with standard lectins. A novel bifunctional spacer, 2-amino-N-(2-aminoethyl)benzamide, by Song et al.51 was specially designed for immobilisation and fluorescent detection of underivatised natural glycans. It is directly conjugated to the sugars via its arylamino group by reductive amination. Thus, over 200 glycans from various sources were immobilised on functionalised glass slides and tested for binding to galectin-1 and -3. This approach should facilitate the preparation of natural glycan microarrays, consisting of naturally occurring glycans directly isolated in bulk from target cells/tissues. Immobilisation becomes more complicated if the array contains sensitive glycan derivatives such as glycosphingolipids, present in all eukaryotic membranes. In this case, special attention must be paid to the intactness of both the hydrophilic glycan and the hydrophobic ceramide moiety; if only the glycan part is analysed, the assay may give incomplete or misleading information. Arigi et al.91 solved this problem by cleaving the fatty-N-acyl moiety of the ceramide aglycone with sphingolipid N-deacylase and derivatising the free amide with a fluorescent tag. In contrast, Song et al.92 performed ozonolysis of the sphingosine moiety and derivatisation of the originated aldehyde. Tagged fluorescent glycosphingolipids were chromatographically separated, quantified and covalently coupled to glass slides. The microarrays were then assayed with biological samples of patients with Lyme disease in order to identify relevant glycosphingolipids, prospective for further structural identification. Thus, time-consuming structural analysis was to be performed solely with pre-selected target ligands and not with the whole glycome; this approach was termed “shotgun glycomics”. Analogously, this approach was shown with O- and N-glycans released from glycoproteins.51,93
Elling and coworkers94–96 presented an elegant green one-pot preparation of a library of poly(N-acetyllactosamine) polymers of varying lengths as ligands for fungal CGL2 galectin from Coprinus cinereus. The defined mix of poly(LacNAc) units was prepared by a combined action of human β1,4-galactosyltransferase-1 and Helicobacter pylori β1,3-N-acetylglucosaminyltransferase in one reaction step and the glycans were covalently attached to functionalised microtiter plates. The glycan–lectin interaction was measured by ELLA assay.
The density of glycan coating on the array surface is critical for an efficient multivalent interaction with analysed lectins. In a two-dimensional arrangement, the ligand density reaches saturation at some point. Therefore, efforts were made to further increase the ligand density by fabricating the glycan coating in a 3D mode (Fig. 4). This may be accomplished by, e.g., conjugating the array to a polymer scaffold decorated with pendant glycans97 or by constructing arrays coated in multivalent dendrimers instead of monovalent glycan units.98 The response increase due to the multivalency effect largely depends on how the distances within the polymer/dendrimer “brush” fit the lectin ligand morphology. Another extension of classical arrays that largely increases the array multivalency are microarrays based on glyconanoparticles or quantum dots printed on a polymer matrix (Fig. 4). Thus, the threshold of saturation of ligand density is much shifted to higher levels. The presence of a flexible polymer base film on the wafers is imperative in order to ensure the adaptation to conformational requirements of nanoparticle solids. Tong et al.99 prepared such a hybrid microarray using gold glyconanoparticles and poly(allyl amine) perfluorophenyl azide by means of photocoupling chemistry. Notably, the hybrid array unproportionally amplified the response to high-affinity ligands compared to the low-affinity ones, in contrast to the standard array printed with free sugar ligands.
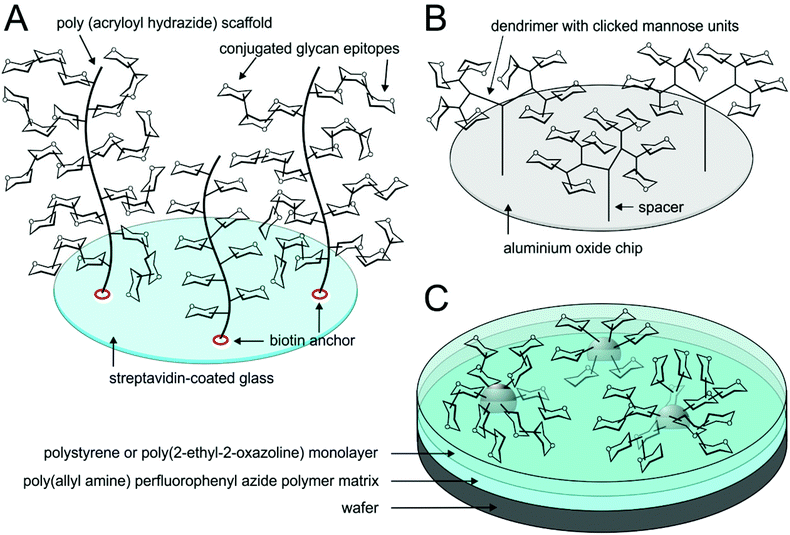 | ||
| Fig. 4 Glycoarrays in a 3D mode. Three-dimensional glycan coating is realised in the form of (A) glycopolymer brushes,97 (B) branched dendrimers98 or (C) glyconanoparticles printed on the array.99 | ||
Functionalisation of inert solid materials with carbohydrates helps to increase their biocompatibility, in vivo tolerability and functionality, and to minimise undesirable side effects like formation of blood clots on the implant material when in the body, its potential cancerogenic and/or allergenic effects. For example, stainless steel suitable for all sorts of bioimplants may be conjugated with a nanomolar layer of passivation silica coating and functionalised with N-acetyl-D-glucosamine or D-galactose by means of alkoxysilane chemistry.100 Inorganic material like hydroxyapatite is suitable as a matrix for bone tissue regeneration; however, interaction and communication with the extracellular matrix must be ensured by adding biological cues. Russo et al.101 presented innovative nanostructured hydroxyapatite decorated with α-glucosides via Huisgen cycloaddition and its binding to ConA. Nanoporous gold, useful as a matrix for the formation of self-assembled monolayers, for separation techniques or for immobilisation of biomolecules, was derivatised with α-mannoside and tested for lectin interaction.102
5. Glycodendrimers and glycoclusters
Biomaterials containing organic or biological dendrimer scaffolds decorated with glycan chains may be based on, e.g., resorcinarene, calixarene, fullerene, aromatic cores, or neoglycopeptides, as well as on saccharide scaffolds such as cyclodextrins, branched oligo- and polysaccharides (Fig. 5).103–109 There are some recent reviews on this topic.110,111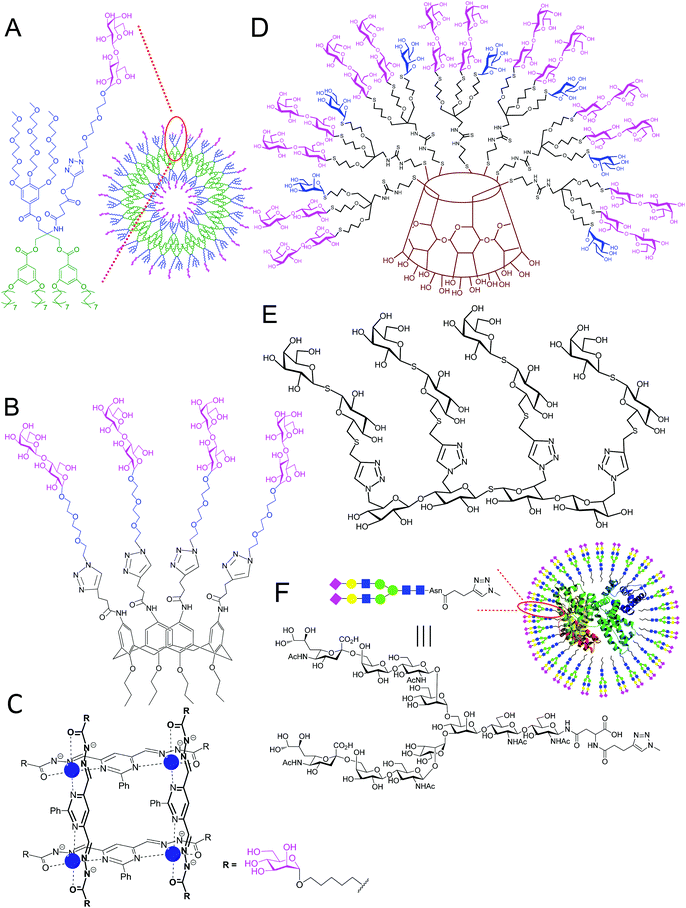 | ||
| Fig. 5 Examples of glycodendrimer structures used for lectin binding. (A) glycodendrimersome;103 (B) lactosyl calix[4]arene;104 (C) α-mannosylated tetranuclear [2 × 2] grid with Zn2+ cations (blue);105 (D) α-mannosyl β-lactosyl β-cyclodextrin glycocluster;106 (E) tetravalent thiogalactoside glycomimetic;107 and (F) neoglycoconjugate with human serum albumin.108,109 | ||
5.1. Glycodendrimersomes
In analogy to polymersomes described in section 7.2, Zhang et al.103 constructed eighteen amphiphilic glycodendrimers of a novel design with three different glycosylation patterns – so-called glycodendrimersomes – that self-assemble into stable vesicle-like structures (Fig. 5A). The most efficient binding of lectins from various sources was shown in glycodendrimersomes with heterogenic display of glycans, compared to those with ligands of only one type. The supramolecular multivalency of glycodendrimersomes mimics natural biomembranes and it is universally applicable in many areas of nanomedicine. Glycodendrimersomes were used as diagnostic tools in a study with artificial lectin ligands, in order to reveal diverse aspects of protein–glycan multivalent interactions.112 Importantly, the surface pattern of displayed ligands was programmable in terms of topology and local density. The programming of surface glycans was realised through self-assembly of selected monomeric building blocks of Janus dendrimers. In another study, Zhang et al.113 used glycodendrimersomes in a structure–activity relationship study with native forms of galectin-8.In general, the idea of ligand heterogeneity is much closer to the real situation than the presentation of just one type of glycans. This is because the naturally occurring cells usually tune the composition of the surface glycan envelope to modify their affinity and selectivity. Ponader et al.114 showed another implementation of the heterogeneous multivalent concept. In this case, the ligands were displayed at a defined sequence and positions along the oligomer backbone, which originated through solid-phase synthesis from defined functionalised building blocks.
5.2. Glycocalixarenes
Glycocalixarene dendrimers are among the most popular scaffolds in glycodendrimer chemistry.115 Calixarenes (or resorcinols) are cyclic oligomers originated from condensation of phenols (or resorcinols) and aldehydes. They are especially valuable for their ability to accommodate guest molecules and transport them to particular destinations. The calixarene macrocycles may vary in size; the even-numbered conjugates (n = 4, 6, 8) are cheap and readily chemically and commercially available, contrary to their odd-numbered counterparts.Calixarenes tethered with carbohydrates at the upper and/or lower rim were reported as strong ligands of a variety of lectins, some of them are of pathogenic nature. For instance, calix[4]arene116 and calix[5]arene117 derivatives acted as good inhibitors of cholera toxin (the lowest IC50 fell into the picomolar range). Adhesive lectins of the Pseudomonas aeruginosa opportunistic bacterium were inhibited by galactosyl calixarenes of diverse conformations118 as well as by resorcin[4]arene, tetragalactosylated at the lower rim.119 The former substance reported by Cecioni et al.120 was patented as a base for a pharmaceutical composition.
For potential clinical applications, the cone conformers of lactosyl calixarenes with a thioureido linker were shown to efficiently bind tandem-repeat type galectin-4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 121 whereas its counterparts containing a triazole linker prepared by click chemistry showed high affinity to galectin-3 (Fig. 5B).104
121 whereas its counterparts containing a triazole linker prepared by click chemistry showed high affinity to galectin-3 (Fig. 5B).104
5.3. Glycosylated aromatic scaffolds
Aromatic cores are a basic structural element of organic dendrimer scaffolds. They often dispose of additional favourable properties such as luminescence in the case of tetraphenylethylenes.122 Faint luminophores per se, they enhance the emitted photoluminescence intensity by up to three orders of magnitude upon aggregation of the glycodendrimer with target lectins – exhibiting the so-called aggregation-induced emission (AIE). The reversible character of ligand–dendrimer aggregation ensures the turn-on/turn-off character of biosensors constructed on this principle. An example is 6′-sialyllactosyl tetraphenylethylene fluorescent probe, used by Kato et al.123 for detecting influenza virus.Aromatic scaffolds based on pyridine or pyrimidine aldehydes by Chmielewski et al.105 self-assemble into supramolecular grid-shaped tetranuclear complexes of the [2 × 2] type, bearing a coordinated zinc cation and eight glycan residues (Fig. 5C). The programmed formation is reversibly dependent on pH and dilution. The complexes strongly interact with tetravalent ConA upon assembly into polymeric networks leading to almost quantitative precipitation of aggregates from the solution. Another supramolecular structure based on [2]rotaxane aims at LecA and LecB bacterial lectins.124 André et al.125 presented a symmetrical tetravalent aromatic dendri-inhibitor that efficiently blocked binding of the human macrophage galactose-binding C-type lectin (MGL) to cells and the matrix at the nM concentration.
5.4. Glycocyclodextrins
Heterogeneous display of ligands was demonstrated using a cyclodextrin core.106 The described synthetic procedure based on a modular convergent strategy enables the preparation of conjugates with defined density and orientation of ligands; in this case, α-mannosyl and β-lactosyl moieties (Fig. 5D). The heterogeneous display of ligands results in the so-called “heterocluster effect” – the binding affinity of a glycan ligand is synergically increased in the presence of another sugar non-ligand.Hydrophobic self-assembly of cyclodextrin-containing building blocks reported by Grünstein et al.126 yielded heptamannosylated cyclodextrin scaffolds with a fluorescent ruthenium(II) core. The resulting homogeneously glycosylated multivalent cyclodextrin sensors exhibited strong binding to Man-specific receptors of E. coli. Self-assembling functionalised cyclodextrin dendrimers yielded a promising platform for preparing bilayer vesicles and membrane mimics.127,128 In this case, the hydrophobic cyclodextrin cavity accommodates a guest molecule of a suitable size and shape, such as adamantane (Ka = 4.104 M−1), conjugated with selected sugar(s). They can interact with certain lectin ligands to simulate behaviour in an artificial glycocalyx (Fig. 6). It seems that in order to reach maximum agglutination with lectin ligands, the density of glycans on the cyclodextrin surface must fall into certain borders to correspond to the binding requirements of lectin ligands; the best result was reached with bivalent guest molecules, each carrying two sugar units. The cyclodextrin pocket itself may serve for drug delivery purposes as described in section 7.2.
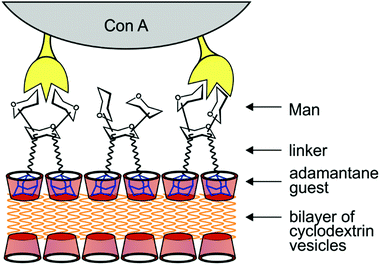 | ||
| Fig. 6 Schematic depiction of an artificial glycocalyx. Surface display of mannose is based on noncovalent internalisation of adamantane guest molecules (blue) in cyclodextrin vesicles (red) set up in a bilayer.127 | ||
5.5. Oligo- and polysaccharidic glycodendrimers
Analogous to cyclodextrins, uniform cyclic glycosyl scaffolds may be prepared in various sizes and with various types of sugar moieties, such as di-, tri-, and tetra-glucosamine cycles with glycosyl linker arms conjugated through the sugar amino groups.129 They were tested as inhibitors of Pseudomonas aeruginosa LecA lectin; the best results were reached with a tetravalent structure containing aromatic linkers (Kd = 79 nM). A tetrasaccharide glycomimetic invented by Magnani et al.130 served for diagnosis and therapeutic intervention against Pseudomonas bacteria. Our group prepared bivalent glycomimetics with linear spacers of different lengths for studying the binding to various lectins, such as galectin-1 and -3.131,132 François-Heude et al.133 presented veracious mimics of high-mannose oligosaccharides, in which some mannose moieties were substituted by triazole rings. Thus, with the help of click chemistry, substantial reduction of synthetic cost was accomplished. In bioaffinity tests with ConA and human macrophage mannose receptor, these mimics copied structural preferences of natural high mannose oligosaccharides, which shows how close their design and properties are to the original structures. Paulson et al.134 patented an original oligosaccharide motif containing sialic acid that binds Siglecs. This motif may be attached to a variety of carriers including nanoparticles, chromatographic matrices, polymers, dendrimers and even therapeutic agents. Besides analysis and separation, it serves for targeted intracellular delivery of agents into Siglec-overexpressing cancer cells. Carbohydrate polymers such as chitin may also be used as matrices for the construction of glycodendrimers. For instance, Zhou et al.135 tagged chitin nanocrystals with a fluorescent dye and conjugated them with miscellaneous glycan ligands. The fluorescent label facilitates the optical monitoring of glycan–lectin interaction. Functionalised chitin nanocrystals were used, e.g., for imaging of E. coli. Oligosaccharide clusters may also be exploited as inert scaffolds for target glycosylation. To ensure their inertness against enzyme-induced hydrolysis, they contain unnatural bonds such as in thio-107 (Fig. 5E) or selenoglycosides.1365.6. Other glycodendrimers
A variety of other dendrimer scaffolds have been proved to be useful in promoting lectin–carbohydrate interactions, for example glycosyl fullerenes,137 described also in section 7.2. If branched peptides are used as a matrix, the results are impressive “apple trees” of several dozens of amino acids with various topologies, of molecular weights approaching even 5 kDa. They show interesting biological activities, such as inhibition of biofilm formation, anticancerogenic and antimicrobial activities.138 They may also exhibit self-assembling properties leading to micelle/cluster formation as described in section 6. Cyclopeptides provide defined compact scaffolds that may be used for the construction of a range of dendrimers, from low-molecular structures to second- and third-generation conjugates with up to 64 glycans.139 Glycoprotein-based dendrimer ligands employed by Wang et al.109 (Fig. 5F) efficiently blocked binding of galectin-3 to prostate and lung cancer cells, with potential application as antimetastatic agents. The presented elegant conjugation of a functionalised BSA matrix and egg-yolk N-glycans by click chemistry designs a pathway to a new type of neoglycoproteins. Functionalised organic dendrons were used, e.g., for obtaining high resolution crystal structural data in the complex with lectins, such as in the case of human galectin-7 with three- and hexaglycosylated branched long-armed dendrimers prepared by means of click-chemistry.140 These studies help to reveal the details of interaction and crosslinking between lectins and multivalent glyco-ligands. Lactose-decorated (polyamidoamine; PAMAM) dendrimers were able to modulate Gal-3-mediated aggregation in three different cancer lines. The PAMAM dendrimer with fifteen lactose units was found to be the most efficient inhibitor of Gal-3-induced cell aggregation.1416. Glycosylated polymer scaffolds
6.1. Glycopolymer synthesis
Various synthetic approaches are of choice for the preparation of synthetic glycopolymers with one or more types of glycan units;142 for example, ionic polymerisation, ring-opening metathesis, click chemistry or radical polymerisation. The latter approach involves the techniques of Nitroxide-Mediated Radical Polymerisation (NMP),143 Reversible Addition Fragmentation Transfer (RAFT) polymerisation,144 and Atom Transfer Radical Polymerisation (ATRP).145Great interest is currently laid on the control of the sequence of incorporated monomers in the glycopolymer chain, ideally including the influence on folding and formation of tertiary structures.146 To this aim, Ponader et al.147 employed solid-phase synthesis for the preparation of defined glycopolymer segments, which were clicked on the poly(amidoamine) backbone. The main drawbacks of this approach are low isolated yields and numerous reaction steps. More promising results have been reached with the method of “single electron transfer living radical polymerisation” (SET-LRP) that enables to build multiblock glycopolymers from small sugar monomers.148
A high degree of control over the chain and linker length as well as over the glycan density may be exercised using a tandem post-polymerisation modification strategy149 (Fig. 7). These parameters are impossible to control in glycopolymers prepared by conventional approaches, such as chain-growth or step-growth polymerisation. In this case, binding preferences of the B subunit of cholera toxin were studied with a series of galactose glycopolymers of varying defined linker lengths. A clear preference for longer linkers was revealed, probably due to the deep binding pocket in the toxin, in contrast to peanut agglutinin control. Thus, the structure–activity relationship of the lectin binding process could be studied.
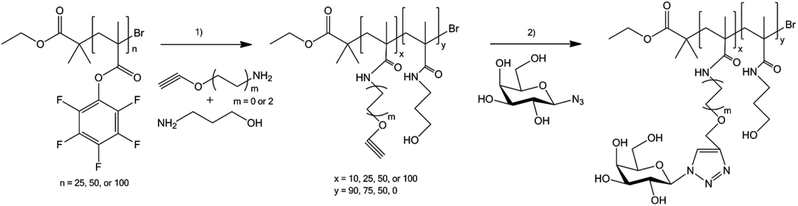 | ||
| Fig. 7 Synthesis of glycopolymers by using a tandem post-polymerisation modification strategy.149 (1) Amine (variable amounts)/triethylamine (1 eq.)/dimethylformamide, 5 h; (2) β-D-galactopyranosyl azide (1.5 eq.)/CuBr/tris(benzyltriazolylmethyl)amine, dimethyl sulfoxide. | ||
Preparation of sequence-defined glycopolymers according to Lutz and coworkers150 consists of placing reactive maleimides with various N-substitutions at defined locations in a bioinert polystyrene chain. This is accomplished in a particular kinetic regime when donor and acceptor co-monomers are successively added into the reaction mixture under precise time-control.151 Subsequent selective deprotection, derivatisation and substitution with selected hexoses afforded a single-chain glycopolymer with exact positioning of hexoses, useful in therapeutic or biomedicinal applications, such as trapping of bacteria and viruses.
6.2. Three-dimensional organisation of glycopolymers
A much valued property of glycopolymers is their ability to form three-dimensional nano-sized clusters in the shape of micelles, vesicles or rods. The most used protocol is based on a non-covalent self-assembly of amphiphilic block copolymers. Its main disadvantage, however, is the dynamic nature, which may result in insufficient stability. Particularly spherical micelles are perfectly suitable as substance carriers, targeted through the display of apt glycans on the particle surface.152 The glycans are recognised by lectins on target structures, such as tumour tissue. The transported substance is then taken up and accumulated at the tumour site, penetrating through the leaky surface with the typical pores of ca. 200 nm; this phenomenon is known as enhanced permeability and retention effect (EPR).153 Ideally, a controlled nanocluster size (hydrodynamic diameter larger than ca. 5 nm) should be sufficient in order to ensure a prolonged blood circulation time and delayed renal clearance.154 The targeted transporting function of glycopolymer nanoclusters (“polymersomes”) is described in section 7.2.The cluster size is also tunable depending on the method of preparation from the same starting components: for instance, nanoprecipitation from solution or formation through an aerosol flow reactor of either pre-glycosylated or post-glycosylated particles resulted in quite different particle diameters (97, 357 or 197 nm, respectively).161
Solid polymer nanoparticles represent an alternative that combines the advantages of a solid inorganic particle and the synthetic variability of polymers. Hybrid fluorescent poly(styrene)–poly(amido acid) copolymers reported by Jacobs et al.162 showed strong binding to chinese hamster ovary cells and outlined a promising route to bioimaging agents. The fluorescent dye – Nile Red – was encapsulated in the particle core. Polystyrene particles grafted with S-linked glycans by Kohri et al.163 were resistant to chemical and enzymatic hydrolysis.
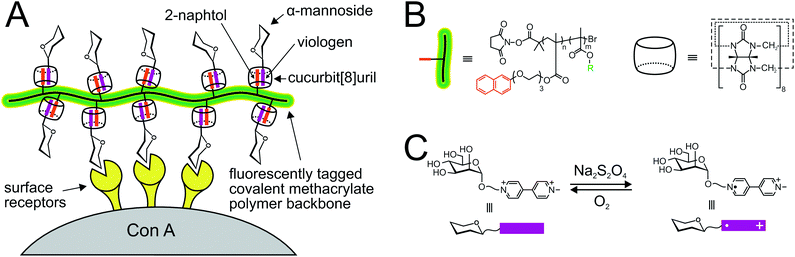 | ||
| Fig. 8 Self-assembling linear glycopolymers based on the strategy of non-covalent “supramolecular handcuffs”.164 The circular molecule of cucurbit[8]uril accommodates the 2-naphthol “pegs” of the methacrylate polymer backbone as well as α-mannosyl viologen, resulting in a “brush-like” supramolecular ternary glycopolymer complex that binds to ConA. The polymer scaffold is tagged with fluorescent rhodamine B. (B), structures of the polymer backbone and cucurbit[8]uril; (C), noncovalent reversible assembly and disassembly of the glycan/cucurbit[8]uril/polymer complex, depending on the conditions in the system (addition of Na2S2O4 or O2). | ||
Elegant glycosylation of polymer structures may be well utilised in enhancing the biocompatibility and adhesive parameters of non-glycosylated polymers for use in tissue engineering, wound healing, cell growth or cartilage repair. Silk fibroin protein, a product of Bombyx mori silk worm, is a matrix of eminent interest due to its excellent mechanical properties. Its click conjugation with synthetic glycopeptides yielded a hybrid water-soluble brush-like polymer with outstanding affinity towards ConA, in a water-soluble vine-format or as an insoluble biofilm.165 Russo et al.166 performed multiple glycosylation of collagen without affecting its morphology. Lactosylation was realised through reductive amination at lysine side chains.
An example of a glycopolymer-coated membrane was constructed by Yang and Ulbricht.167 The poly(ethylene terephthalate) membrane surface was grafted with either linear or comb-like galactose glycopolymers. Efficient specific binding of peanut agglutinin was observed especially in the comb-like set-up during convective flow through the membranes. The glycopolymer functionalised membranes are promising particularly for protein separation, capture of viruses or bacteria.
7. Top applications of biomaterials
The most important applications of lectin-binding biomaterials comprise especially biosensors and bioprobes for analytic/diagnostic uses, targeted delivery agents for medication or therapy168 as well as specific inhibition and bioimaging agents for magnetic resonance techniques.7.1. Biosensors and imaging methods
Biosensors rapidly and efficiently release a detectable signal upon interaction with the target substance. In general, they serve for measuring the kinetics of glycan–lectin interactions, for determining the specificity of binding in an array design or as diagnostic tools for detecting and quantifying tiny concentrations of lectin analytes in complex mixtures. For example, bio-functionalised nanostructures based on polydiacetylene make excellent membrane mimics and, at the same time, exhibit rapid colorimetric transition (blue-red) when they come into contact with the biological target. An example of such biosensors is glycoliposomes7 constructed by photopolymerisation and subsequent click glycosylation of resulting polydiacetylene vesicles. Tethered with relevant carbohydrates, they may aim at any sort of lectin, e.g., C. botulinum or E. coli toxins. In the past, their versatility was shown in the search of new antimicrobial peptides169 or in the detection of bacteria.170A low-cost, robust, fast and sensitive biosensor system is based on optical detection of a noncovalent complex of boronic acid tagged with a fluorescent dye, and glyco-gold nanoparticles. When binding to the target lectins occurs, the complex is released and fluorescence is turned on. This system is able to detect as little as the nM concentration of ConA.171
Biosensors based on magnetic nanoparticles are predestined for in vivo imaging through magnetic resonance (MRI) since their super-paramagnetic properties save the need for further derivatisation. In a nanoparticle array set up presented by El-Boubbou et al.172 it was possible to clearly differentiate between normal, malignant and potentially metastatic cells on the basis of their different MRI responses. Thus, complete information could be gathered on the glycan specificity of tumour cells, which is directly applicable, e.g., in the development of anti-adhesive agents. A gold biosensor with immobilised neoglycopeptides was able to detect pM amounts of Ricinus communis agglutinin and Gal-1 using surface plasmon resonance,173 which approaches the concentrations of Gal-1 as a marker in the sera of cancerogenic patients. A biosensor chip with attached glycopolymer brushes prepared through RAFT and click chemistry dramatically lowered the sensor detection limit by enhancing the lectin binding affinity.174
7.2. Biomedical applications
Besides their super-paramagnetic nature that predestines them to be quality contrast agents (see section 7.1), glyconanoparticles with an iron oxide core (Fe3O4) have optimum heat dissipation properties. As a result, they are used in clinical practice for targeted hyperthermia treatment of cancerogenic tissues. Lartigue et al.72 evaluated the magnetic, relaxometric and heat transfer properties of glyconanoparticles of varying sizes to find the optimum inorganic core size to be 16–18 nm. In order to enhance the biodegradability, water dispersibility and tolerability of glyconanoparticles in vivo, the solid metal core may be conveniently enveloped with glycopolymer coating. These glycopolymer-topped hybrid nanoparticles represent new-generation nanoparticles with optimised properties for in vivo usage. The presence of glycans on the particle surface ensures good dispersion and controlled size of aggregates in aqueous medium and enables endocytosis into cells. Muñoz-Bonilla et al.10 constructed hybrid glycopolymer nanoparticles with covalently attached glycopolymer coating originated through radical polymerisation at the particle interface. They exhibited good heat dissipation properties suitable for hyperthermia treatment. Fluorescent hybrid nanoparticles by Pfaff et al.9 contained an additional thin silica shell between magnetic particles and the glycopolymer coating. They were shown to enter lung cancer cells and be targeted to their nuclei, presumably by means of interaction with galectins. It was proved that decoration of nanoparticles with glycopolymer chains of varying lengths dramatically increases the efficiency of multivalent binding due to improved ligand mobility.175Vesicles formed from self-assembled amphiphilic glycopolymers (so-called polymersomes) have recently attracted increased attention as directed drug delivery agents.176 In the hollow spherical cavity, they can accommodate any compounds of a suitable size and shape and transport them to the destination marked by respective lectin ligands. Eissa et al.177 constructed large polymersomes of 25–50 μm diameter coated with various sugars. They could internalise a fluorescent dye and show binding to ConA (Fig. 9A). Recently it was found178 that gold nanoparticles functionalised with amphiphilic glycopolymers self-assembled into spherical aggregates or vesicles of a tunable size with hydrophilic glycan coating that could also be potentially used as targeted transport agents while maintaining the beneficial properties of both nanoparticles and glycopolymers. Besides the transport in a polymer envelope, drug delivery may also be accomplished by other methods, as shown by Aykaç et al.179 Gold nanoparticles decorated with lactose ligands and β-cyclodextrin vesicles on flexible linkers were designed to serve as efficient delivery agents of anticancer drugs like methotrexate to cancer tissue, marked by the presence of galectin-3 (Fig. 9B). Advantageously, the transported drug was unmodified and noncovalently loaded in the cyclodextrin pocket.
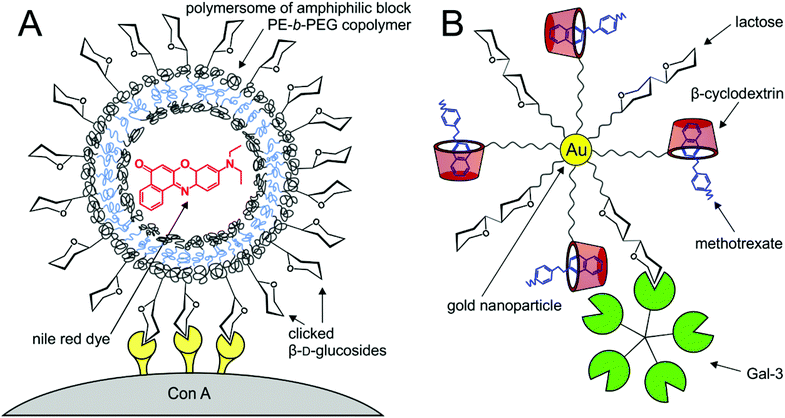 | ||
| Fig. 9 Lectin-targeted delivery systems. (A) β-D-Glucosylated click polyethylene–poly(ethylene glycol) polymersomes carrying a hydrophobic fluorescent Nile red dye (red) conjugated with tetrameric ConA;177 (B) gold nanoparticles tethered with a lactose epitope and β-cyclodextrin carrier (red) bound Gal-3 and was able to carry the methotrexate drug load (blue).179 | ||
A high degree of polyvalent glycosylation on carriers that faithfully mimic natural systems in size and shape may efficiently block binding of pathogens in vivo. For example, DC-SIGN (dendritic cell specific intercellular adhesion molecule-3-grabbing nonintegrin)40 is an important pathogen-recognising surface receptor of the C-type lectin family, found on dendritic cells as well as macrophages. Through binding to this receptor, some pathogens are able to evade the normal degradation processes involving antigen-presenting cells. Thus, blocking of binding of pathogens to this receptor is a promising strategy for new antiviral agents. A crucial parameter is the optimum ligand structure that should fit the 20 nm distance of carbohydrate-recognition domains of DC-SIGN.180 Using this strategy, Ribeiro-Viana et al.181 constructed a unique glyco-dendri-protein-nanoparticle with the highest degree of glycosylation constructed to date (1620 glycans in a “nested” design), which efficiently inhibited pseudotyped Ebola viral infection of mammalian cells in the nM-pM range (Fig. 10).
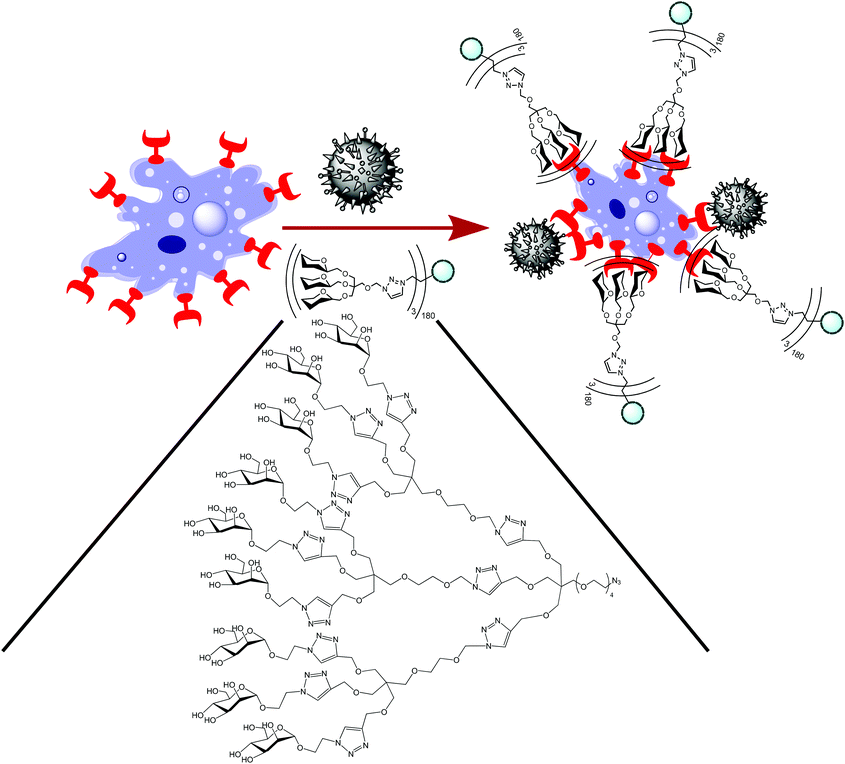 | ||
| Fig. 10 Glyco-dendri-protein nanoparticles featuring “nested” 1620 glycans.181 The azido-functionalised multivalent glycodendron was clicked to the L-homopropargylglycine tag on the protein cluster to yield the glyco-dendri-protein nanoparticle. The particles bind to DC-SIGN receptors (red) on T-lymphocytes (blue), same as pseudotyped Ebola virus (black). | ||
Pseudotyped Ebola viral particles were also used by Luczkowiak et al.182 with C60 glycodendrofullerene inhibitors. Tethering long spacers with twelve Man units brought the inhibition constant to a promising nM range. Very recently, C60 glycofullerene “superballs” tethered with 120 glycan units proved the inhibitory potency of pseudo-Ebola virus in a sub-nanomolar range.183 Another popular target virus, which may be fought by using this strategy, is HIV, such as in the case of a tetravalent mannoside dendrimer184 or mannose-containing glycopolymers.185 Other dendrimer-based inhibitors of medically important lectin targets were recently described,186 including Shiga-like toxin, enterotoxin, cholera toxin, and LecA, the virulence factor of P. aeruginosa.
8. Conclusions
A deep understanding of lectin–carbohydrate interactions opens immense and yet unexplored possibilities in many areas of biology and medicine. Biomaterials with tailored lectin affinities are applicable in targeted delivery of bioimaging agents and therapeuticals in vivo, inhibition of pathogen adhesion and breakage into cells, construction of organism-friendly bioimplants and tissue substitutes, specific and exact analysis and separation of complex biological samples, efficient screening for specific GBPs in a high-throughput set-up, production of artificial biomembranes and many other uses. Intensive research is currently devoted to fine-tuning of biomaterial properties, such as in sequence-controlled polymers or in hybrid polymer-layered nanoparticles, with the involvement of modern technologies like genetical engineering. The current trend envisages perfectly biotolerable materials with properties specified for individual applications, decorated with tailored carbohydrate structures in a controlled pattern and density. Such materials have good potential to step out of the proof-of-principle routine and enter everyday practice.Acknowledgements
This study was supported by the project no. 15-02578J of the Czech Science Foundation and by COST Action Chemistry CM1102 “MultiGlycoNano”. We would like to thank Dr Christopher S. Chambers, Inst. Microbiol., Prague, for language revision.References
- S. Cecioni, A. Imberty and S. Vidal, Chem. Rev., 2015, 115, 525 CrossRef CAS PubMed.
- D. Deniaud, K. Julienne and S. G. Gouin, Org. Biomol. Chem., 2011, 9, 966 CAS.
- N. Hao, K. Neranon, O. Ramström and M. Yan, Biosens. Bioelectron., 2016, 76, 113 CrossRef CAS PubMed.
- B. E. Collins and J. C. Paulson, Curr. Opin. Chem. Biol., 2004, 8, 617 CrossRef CAS PubMed.
- J. J. Lundquist and E. J. Toone, Chem. Rev., 2002, 102, 555 CrossRef CAS PubMed.
- M. Mammen, S. K. Choi and G. M. Whitesides, Angew. Chem., Int. Ed., 1998, 37, 2755 CrossRef CAS.
- M. P. Leal, M. Assali, I. Fernández and N. Khiar, Chem. – Eur. J., 2011, 17, 1828 CrossRef CAS PubMed.
- C. D. Rillahan and J. C. Paulson, Annu. Rev. Biochem., 2011, 80, 797 CrossRef CAS PubMed.
- A. Pfaff, A. Schallon, T. M. Ruhland, A. P. Majewski, H. Schmalz, R. Freitag and A. H. Müller, Biomacromolecules, 2011, 12, 3805 CrossRef CAS PubMed.
- A. Muñoz-Bonilla, G. Marcelo, C. Casado, F. J. Teran and M. Fernández-García, J. Polym. Sci., Part A: Polym. Chem., 2012, 50, 5087 CrossRef.
- W. C. Boyd, in The Proteins, ed. H. Neurath and K. Bailey, Academic Press, New York, 1954, vol. 2, pp. 756–844 Search PubMed.
- S. H. Barondes, Trends Biochem. Sci., 1988, 13, 480 CrossRef CAS PubMed.
- H. J. Gabius, S. André, J. Jiménez-Barbero, A. Romero and D. Solís, Trends Biochem. Sci., 2011, 36, 298 CrossRef CAS PubMed.
- T. Yau, X. Dan, C. C. W. Ng and T. B. Ng, Molecules, 2015, 20, 3791 CrossRef CAS PubMed.
- Y. van Kooyk and G. A. Rabinovich, Nat. Immunol., 2008, 9, 593 CrossRef CAS PubMed.
- H. Blanchard, K. Bum-Erdene and M. W. Hogo, Aust. J. Chem., 2014, 67, 1763 CrossRef CAS.
- F. T. Liu and G. A. Rabinovich, Nat. Rev. Cancer, 2005, 5, 29 CrossRef CAS PubMed.
- F. T. Liu, R. Y. Yang and D. K. Hsu, Ann. N.Y. Acad. Sci., 2012, 1253, 80 CrossRef CAS PubMed.
- G. R. Vasta, Nat. Rev. Microbiol., 2009, 7, 424 CrossRef CAS PubMed.
- G. A. Rabinovich and D. O. Croci, Immunity, 2012, 36, 322 CrossRef CAS PubMed.
- G. A. Rabinovich and M. A. Toscano, Nat. Rev. Immunol., 2009, 9, 338 CrossRef CAS PubMed.
- J. Hirabayashi and K. Kasai, Glycobiology, 1993, 3, 297 CrossRef CAS PubMed.
- M. Nagae and Y. Yamaguchi, Int. J. Mol. Sci., 2014, 15, 3768 CrossRef CAS PubMed.
- R. Y. Yang, G. A. Rabinovich and F. T. Liu, Expert Rev. Mol. Med., 2008, 10, e17 CrossRef PubMed.
- I. Camby, M. Le Mercier, F. Lefranc and R. Robert Kiss, Glycobiology, 2006, 16, 137R CrossRef CAS PubMed.
- L. Astorgues-Xerri, M. E. Riveiro, A. Tijeras-Raballand, M. Serova, C. Neuzillet, S. Albert, E. Raymond and S. Faivre, Cancer Treat. Rev., 2014, 40, 307 CrossRef CAS PubMed.
- L. Song, J. W. Tang, L. Owusu, M. Z. Sun, J. Wu and J. Zhang, Clin. Chim. Acta, 2014, 431, 185 CrossRef CAS PubMed.
- G. Pugliese, C. Iacobini, C. M. Pesce and S. Menini, Glycobiology, 2015, 25, 136 CrossRef CAS PubMed.
- N. C. Henderson, A. C. Mackinnon, S. L. Farnworth, F. Poirier, F. P. Russo, J. P. Iredale, C. Haslett, K. J. Simpson and T. Sethi, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 5060 CrossRef CAS PubMed.
- N. Ahmad, H. J. Gabius, S. André, H. Kaltner, S. Sabesan, R. Roy, B. Liu, F. Macaluso and C. F. Brewer, J. Biol. Chem., 2004, 279, 10841 CrossRef CAS PubMed.
- K. Ohtsubo, S. Takamatsu, M. T. Minowa, A. Yoshida, M. Takeuchi and J. D. Marth, Cell, 2005, 123, 1307 CrossRef CAS PubMed.
- P. Madsen, H. H. Rasmussen, T. Flint, P. Gromov, T. A. Kruse, B. Honore, H. Vorum and J. E. Celis, J. Biol. Chem., 1995, 270, 5823 CrossRef CAS PubMed.
- S. Saussez and R. Kiss, Cell. Mol. Life Sci., 2006, 63, 686 CrossRef CAS PubMed.
- W. I. Weis, M. E. Taylor and K. Drickamer, Immunol. Rev., 1998, 163, 19 CrossRef CAS PubMed.
- K. Drickamer and M. E. Taylor, Curr. Opin. Struct. Biol., 2015, 34, 26 CrossRef CAS PubMed.
- I. M. Dambuza and G. D. Brown, Curr. Opin. Immunol., 2015, 32, 21 CrossRef CAS PubMed.
- A. Cambi, M. Koopman and C. G. Figdor, Cell. Microbiol., 2005, 7, 481 CrossRef CAS PubMed.
- A. Engering, T. B. Geijtenbeek and Y. van Kooyk, Trends Immunol., 2002, 23, 480 CrossRef CAS PubMed.
- T. B. H. Geijtenbeek, S. J. van Vliet, A. Engering, B. A. Hart and Y. van Kooyk, Annu. Rev. Immunol., 2004, 22, 33 CrossRef CAS PubMed.
- T. B. H. Geijtenbeek, R. Torensma, S. J. van Vliet, G. C. F. van Duijnhoven, G. J. Adema, Y. van Kooyk and C. G. Figdor, Cell, 2000, 100, 575 CrossRef CAS PubMed.
- C. Napoletano, I. G. Zizzari, A. Rughetti, H. Rahimi, T. Irimura, H. Clausen, H. H. Wandall, F. Belleudi, F. Bellati, L. Pierelli, L. Frati and M. Nuti, Eur. J. Immunol., 2012, 42, 936 CrossRef CAS PubMed.
- A. Varki and T. Angata, Glycobiology, 2006, 16, 1R CrossRef CAS PubMed.
- P. R. Crocker, J. C. Paulson and A. Varki, Nat. Rev. Immunol., 2007, 7, 255 CrossRef CAS PubMed.
- O. J. Plante and P. H. Seeberger, Curr. Opin. Drug Discovery Dev., 2003, 6, 521 CAS.
- P. Sears and C. H. Wong, Science, 2001, 291, 2344 CrossRef CAS PubMed.
- P. Bojarová, R. R. Rosencrantz, L. Elling and V. Křen, Chem. Soc. Rev., 2013, 42, 4774 RSC.
- X. Song, J. Heimburg-Molinaro, R. D. Cummings and D. F. Smith, Curr. Opin. Chem. Biol., 2014, 18, 70 CrossRef CAS PubMed.
- E. Çelik, A. A. Ollis, Y. Lasanajak, A. C. Fisher, G. Gür, D. F. Smith and M. P. DeLisa, Biotechnol. J., 2015, 10, 199 CrossRef PubMed.
- D. F. Smith, X. Song and R. D. Cummings, Methods Enzymol., 2010, 480, 417 CAS.
- Y. Liu, A. S. Palma and T. Feizi, Biol. Chem., 2009, 390, 647 CAS.
- X. Song, B. Xia, S. R. Stowell, Y. Lasanajak, D. F. Smith and R. D. Cummings, Chem. Biol., 2009, 16, 36 CrossRef CAS PubMed.
- S. Ng, E. Lin, P. I. Kitov, K. F. Tjhung, O. O. Gerlits, L. Deng, B. Kasper, A. Sood, B. M. Paschal, P. Zhang, C. C. Ling, J. S. Klasssen, C. J. Noren, L. K. Mahal, R. J. Woods, L. Coates and R. Derda, J. Am. Chem. Soc., 2015, 137, 5248 CrossRef CAS PubMed.
- M. Maglinao, M. Eriksson, M. K. Schlegel, S. Zimmermann, T. Johannssen, S. Götze, P. H. Seeberger and B. Lepenies, J. Controlled Release, 2014, 175, 36 CrossRef CAS PubMed.
- V. Percec, P. Leowanawat, H. J. Sun, O. Kulikov, C. D. Nusbaum, T. M. Tran, A. Bertin, D. A. Wilson, M. Peterca, S. Zhang, N. P. Kamat, K. Vargo, D. Moock, E. D. Johnston, D. A. Hammer, D. J. Pochan, Y. Chen, Y. M. Chabre, T. C. Shiao, M. Bergeron-Brlek, S. André, R. Roy, H. J. Gabius and P. A. Heiney, J. Am. Chem. Soc., 2013, 135, 9055 CrossRef CAS PubMed.
- K. T. Huang, K. Gorska, S. Alvarez, S. Barluenga and N. Winssinger, ChemBioChem, 2011, 12, 56 CrossRef CAS PubMed.
- A. Novoa, T. Machida, S. Barluenga, A. Imberty and N. Winssinger, ChemBioChem, 2014, 15, 2058 CrossRef CAS PubMed.
- O. Blixt, S. Han, L. Liao, Y. Zeng, J. Hoffmann, S. Futakawa and J. C. Paulson, J. Am. Chem. Soc., 2008, 130, 6680 CrossRef CAS PubMed.
- X. Song, Y. Lasanajak, L. J. Olson, M. Boonen, N. M. Dahms, S. Kornfeld, R. D. Cummings and D. F. Smith, J. Biol. Chem., 2009, 284, 35201 CrossRef CAS PubMed.
- C. M. Nycholat, W. Peng, R. McBride, A. Antonopoulos, R. P. de Vries, Z. Polonskaya, M. G. Finn, A. Dell, S. M. Haslam and J. C. Paulson, J. Am. Chem. Soc., 2013, 135, 18280 CrossRef CAS PubMed.
- C. C. Wang, J. R. Chen, Y. C. Tseng, C. H. Hsu, Y. F. Hung, S. W. Chen, C. M. Chen, K. H. Khoo, T. J. Cheng, Y. S. Cheng, J. Jan, C. Y. Wu, C. Ma and C. H. Wong, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 18137 CrossRef CAS PubMed.
- Z. Wang, Z. S. Chinoy, S. G. Ambre, W. Peng, R. McBride, R. P. de Vries, J. Glushka, J. C. Paulson and G. I. Boons, Science, 2013, 341, 379 CrossRef CAS PubMed.
- K. A. Maupin, D. Liden and B. B. Haab, Glycobiology, 2011, 22, 160 CrossRef PubMed.
- D. Kletter, Z. Cao, M. Bern and B. Haab, Curr. Protoc. Chem. Biol., 2013, 5, 1 Search PubMed.
- P. Xuan, Y. Zhang, T. R. Tzeng, X. F. Wan and F. Luo, Glycobiology, 2011, 22, 552 CrossRef PubMed.
- S. R. Cholleti, S. Agravat, T. Morris, J. H. Saltz, X. Song, R. D. Cummings and D. F. Smith, OMICS: J. Integr. Biol., 2012, 16, 497 CAS.
- O. Oyelaran and J. C. Gildersleeve, Curr. Opin. Chem. Biol., 2009, 13, 406 CrossRef CAS PubMed.
- B. K. Gorityala, J. Ma, X. Wang, P. Chen and X. W. Liu, Chem. Soc. Rev., 2010, 3, 2925 RSC.
- S. Conolly and C. Cunningham, US Pat, 20050261575A1, 2005 Search PubMed.
- E. Schellenberger, S. Wagner and J. Schnorr, Eur. Pat, 2022508A1, 2009 Search PubMed.
- E. V. Groman, K. G. Paul, T. B. Frigo, H. Bengele and J. M. Lewis, US Pat, 8501158B2, 2013 Search PubMed.
- L. Blomqvist, A. Nordell, E. Jonas and H. Nilsson, Can. Pat, 2731642A1, 2009 Search PubMed.
- L. Lartigue, C. Innocenti, T. Kalaivani, A. Awwad, M. Sanchez Duque Mdel, Y. Guari, J. Larionova, C. Guérin, J. L. Montero, V. Barragan-Montero, P. Arosio, A. Lascialfari, D. Gatteschi and C. Sangregorio, J. Am. Chem. Soc., 2011, 133, 10459 CrossRef CAS PubMed.
- S. Watanabe, K. Yoshida, K. Shinkawa, D. Kumagawa and H. Seguchi, Colloids Surf., B, 2010, 81, 570 CrossRef CAS PubMed.
- X. Liu, M. Atwater, J. Wang and Q. Huo, Colloids Surf., B, 2007, 58, 3 CrossRef CAS PubMed.
- H. Tsutsumi, H. Ohkusa, H. Park, T. Takahashi, H. Yuasa and H. Mihara, Bioorg. Med. Chem. Lett., 2012, 22, 6825 CrossRef CAS PubMed.
- K. Selvaprakash and Y. C. Chen, Biosens. Bioelectron., 2014, 61, 88 CrossRef CAS PubMed.
- M. Reynolds, M. Marradi, A. Imberty, S. Penadés and S. Pérez, Chemistry, 2012, 18, 4264 CrossRef CAS PubMed.
- S. Biswas, S. H. Medina and J. J. Barchi Jr., Carbohydr. Res., 2015, 405, 93 CrossRef CAS PubMed.
- M. A. Reed, J. N. Randall, R. J. Aggarwal, R. J. Matyi, T. M. Moore and A. E. Wetsel, Phys. Rev. Lett., 1988, 60, 535 CrossRef CAS PubMed.
- C. I. Weng, H. T. Chang, C. H. Lin, Y. W. Shen, B. Unnikrishnan, Y. J. Li and C. C. Huang, Biosens. Bioelectron., 2015, 68, 1 CrossRef CAS PubMed.
- Y. Yang, X. C. Xue, X. F. Jin, L. J. Wang, Y. L. Sha and Z. J. Li, Tetrahedron, 2012, 68, 7148 CrossRef CAS.
- J. Hu, M. Xie, C. Y. Wen, Z. L. Zhang, H. Y. Xie, Y. Y. Chen, S. M. Zhou and D. W. Pang, Biomaterials, 2011, 32, 1177 CrossRef CAS PubMed.
- X. Jiang, M. Ahmed, Z. Deng and R. Narain, Bioconjugate Chem., 2009, 20, 994 CrossRef CAS PubMed.
- D. Pei, Y. Li, Q. Huang, Q. Ren, F. Li and T. Shi, Colloids Surf., B, 2015, 127, 130 CrossRef CAS PubMed.
- S. Fukui, T. Feizi, C. Galustian, A. M. Lawson and W. Chai, Nat. Biotechnol., 2002, 20, 1011 CrossRef CAS PubMed.
- D. Wang, S. Liu, B. J. Trummer, C. Deng and A. Wang, Nat. Biotechnol., 2002, 20, 275 CrossRef CAS PubMed.
- G. Huang, X. Chen and F. Xiao, Curr. Med. Chem., 2014, 21, 288 CrossRef CAS PubMed.
- S. Park, J. C. Gildersleeve, O. Blixt and I. Shin, Chem. Soc. Rev., 2013, 42, 4310 RSC.
- H. S. Beckmann, A. Niederwieser, M. Wiessler and V. Wittmann, Chemistry, 2012, 18, 6548 CrossRef CAS PubMed.
- K. Liang and Y. Chen, Bioconjugate Chem., 2012, 23, 1300 CrossRef CAS PubMed.
- E. Arigi, O. Blixt, K. Buschard, H. Clausen and S. B. Levery, Glycoconjugate J., 2012, 29, 1 CrossRef CAS PubMed.
- X. Song, Y. Lasanajak, B. Xia, J. Heimburg-Molinaro, J. M. Rhea, H. Ju, C. Zhao, R. J. Molinaro, R. D. Cummings and D. F. Smith, Nat. Methods, 2011, 8, 85 CrossRef CAS PubMed.
- X. Song, Y. Lasanajak, B. Xia, D. F. Smith and R. D. Cummings, ACS Chem. Biol., 2009, 4, 741 CrossRef CAS PubMed.
- K. Adamiak, T. Anders, M. Henze, H. Keul, M. Möller and L. Elling, J. Mol. Catal. B: Enzym., 2012, 84, 108 CrossRef CAS.
- C. Rech, R. R. Rosencrantz, K. Křenek, H. Pelantová, P. Bojarová, C. E. Römer, F. G. Hanisch, V. Křen and L. Elling, Adv. Synth. Catal., 2011, 353, 2492 CrossRef CAS.
- B. Sauerzapfe, K. Křenek, J. Schmiedel, W. W. Wakarchuk, H. Pelantová, V. Křen and L. Elling, Glycoconjugate J., 2009, 26, 141 CrossRef CAS PubMed.
- K. Godula and C. R. Bertozzi, J. Am. Chem. Soc., 2010, 132, 9963 CrossRef CAS PubMed.
- H. M. Branderhorst, R. Ruijtenbeek, R. M. Liskamp and R. J. Pieters, ChemBioChem, 2008, 9, 1836 CrossRef CAS PubMed.
- Q. Tong, X. Wang, H. Wang, T. Kubo and M. Yan, Anal. Chem., 2012, 84, 3049 CrossRef CAS PubMed.
- A. M. Slaney, V. A. Wright, P. J. Meloncelli, K. D. Harris, L. J. West, T. L. Lowary and J. M. Buriak, ACS Appl. Mater. Interfaces, 2011, 3, 1601 CAS.
- L. Russo, E. Landi, A. Tampieri, A. Natalello, S. M. Doglia, L. Gabrielli, L. Cipolla and F. Nicotra, Carbohydr. Res., 2011, 346, 1564 CrossRef CAS PubMed.
- Y. H. Tan, K. Fujikawa, P. Pornsuriyasak, A. J. Alla, N. V. Ganesh, A. V. Demchenko and K. J. Stine, New J. Chem., 2013, 37, 2150 RSC.
- S. Zhang, R. O. Moussodia, H. J. Sun and V. Percec, Angew. Chem., Int. Ed., 2014, 53, 10899 CrossRef CAS PubMed.
- S. Bernardi, P. Fezzardi, G. Rispoli, S. E. Sestito, F. Peri, F. Sansone and A. Casnati, Beilstein J. Org. Chem., 2014, 10, 1672 CrossRef PubMed.
- M. J. Chmielewski, E. Buhler, J. Candau and J. M. Lehn, Chem. – Eur. J., 2014, 20, 6960 CrossRef CAS PubMed.
- M. Gómez-García, J. M. Benito, A. P. Butera, C. Ortiz Mellet, J. M. García Fernández and J. L. Jiménez Blanco, J. Org. Chem., 2012, 77, 1273 CrossRef PubMed.
- A. J. Cagnoni, J. Kovensky and M. L. Uhrig, J. Org. Chem., 2014, 79, 6456 CrossRef CAS PubMed.
- S. Sugio, A. Kashima, S. Mochizuki, M. Noda and K. Kobayashi, Protein Eng., 1999, 12, 439 CrossRef CAS PubMed.
- H. Wang, W. Huang, J. Orwenyo, A. Banerjee, G. R. Vasta and L. X. Wang, Bioorg. Med. Chem., 2013, 21, 2037 CrossRef CAS PubMed.
- Y. M. Chabre and R. Roy, Chem. Soc. Rev., 2013, 42, 4657 RSC.
- H. Mrázek, L. Weignerová, P. Bojarová, P. Novák, O. Vaněk and K. Bezouška, Biotechnol. Adv., 2013, 31, 17 CrossRef PubMed.
- S. Zhang, R. O. Moussodia, C. Murzeau, H. J. Sun, M. L. Klein, S. Vértesy, S. André, R. Roy, H. J. Gabius and V. Percec, Angew. Chem., Int. Ed., 2015, 54, 4036 CrossRef CAS PubMed.
- S. Zhang, Q. Xiao, S. E. Sherman, A. Muncan, A. D. Ramos Vicente, Z. Wang, D. A. Hammer, D. Williams, Y. Chen, D. J. Pochan, S. Vértesy, S. André, M. L. Klein, H. J. Gabius and V. Percec, J. Am. Chem. Soc., 2015, 137, 13334 CrossRef CAS PubMed.
- D. Ponader, P. Maffre, J. Aretz, D. Pussak, N. M. Ninnemann, S. Schmidt, P. H. Seeberger, C. Rademacher, G. U. Nienhaus and L. Hartmann, J. Am. Chem. Soc., 2014, 136, 2008 CrossRef CAS PubMed.
- F. Sansone and A. Casnati, Chem. Soc. Rev., 2013, 42, 4623 RSC.
- D. Arosio, M. Fontanella, L. Baldini, L. Mauri, A. Bernardi, A. Casnati, F. Sansone and R. Ungaro, J. Am. Chem. Soc., 2005, 127, 3660 CrossRef CAS PubMed.
- J. Garcia-Hartjes, S. Bernardi, C. A. Weijers, T. Wennekes, M. Gilbert, F. Sansone, A. Casnati and H. Zuilhof, Org. Biomol. Chem., 2013, 11, 4340 CAS.
- S. Cecioni, R. Lalor, B. Blanchard, J. P. Praly, A. Imberty, S. E. Matthews and S. Vidal, Chem. – Eur. J., 2009, 15, 13232 CrossRef CAS PubMed.
- Z. H. Soomro, S. Cecioni, H. Blanchard, J. P. Praly, A. Imberty, S. Vidal and S. E. Mathews, Org. Biomol. Chem., 2011, 9, 6587 CAS.
- S. Cecioni, K. Faure, B. Guery, A. Imberty, S. Matthews and S. Vidal, World Pat, 2012076934A1, 2012 Search PubMed.
- S. André, F. Sansone, H. Kaltner, A. Casnati, J. Kopitz, H. J. Gabius and R. Ungaro, ChemBioChem, 2008, 9, 1649 CrossRef PubMed.
- J. X. Wang, Q. Chen, N. Bian, F. Yang, J. Sun, A. D. Qi, C. G. Yan and B. H. Han, Org. Biomol. Chem., 2011, 9, 2219 CAS.
- T. Kato, A. Kawaguchi, K. Nagata and K. Hatanaka, Biochem. Biophys. Res. Commun., 2010, 394, 200 CrossRef CAS PubMed.
- S. P. Vincent, K. Buffet, I. Nierengarten, A. Imberty and J.-F. Nierengarten, Chem. – Eur. J., 2016, 22, 88 CrossRef CAS PubMed.
- S. André, S. O'Sullivan, C. Koller, P. V. Murphy and H. J. Gabius, Org. Biomol. Chem., 2015, 13, 4190 Search PubMed.
- D. Grünstein, M. Magliano, R. Kikkeri, M. Collot, K. Barylyuk, B. Lepenies, F. Kamena, R. Zenobi and P. H. Seeberger, J. Am. Chem. Soc., 2011, 133, 13957 CrossRef PubMed.
- U. Kauscher and B. J. Ravoo, Beilstein J. Org. Chem., 2012, 8, 1543 CrossRef CAS PubMed.
- J. Voskuhl, M. C. Stuart and B. J. Ravoo, Chemistry, 2010, 16, 2790 CrossRef CAS PubMed.
- M. L. Gening, D. V. Titov, S. Cecioni, A. Audfray, A. G. Gerbst, Y. E. Tsvetkov, V. B. Krylov, A. Imberty, N. E. Nifantiev and S. Vidal, Chem. – Eur. J., 2013, 19, 9272 CrossRef CAS PubMed.
- J. L. Magnani, J. T. Patton and A. K. Sarkar, U.S. Pat, 20100041626A1, 2010 Search PubMed.
- A. Drozdová, P. Bojarová, K. Křenek, L. Weignerová, B. Henssen, L. Elling, H. Christensen, H. H. Jensen, H. Pelantová, M. Kuzma, K. Bezouška, M. Krupová, D. Adámek, K. Slámová and V. Křen, Carbohydr. Res., 2011, 346, 1599 CrossRef PubMed.
- A. Šimonová, C. E. Kupper, S. Böcker, A. Müller, K. Hofbauerová, H. Pelantová, L. Elling, V. Křen and P. Bojarová, J. Mol. Catal. B: Enzym., 2014, 101, 47 CrossRef.
- M. François-Heude, A. Méndez-Ardoy, V. Cendret, P. Lafite, R. Daniellou, C. Ortiz Mellet, J. M. García Fernández, V. Moreau and F. Djedaïni-Pilard, Chem. – Eur. J., 2015, 21, 1978 CrossRef PubMed.
- J. Paulson, B. Collins and S. Han, World Pat, 2007056525A2, 2007 Search PubMed.
- J. Zhou, N. Butchosa, H. S. Jayawardena, Q. Zhou, M. Yan and O. Ramström, Bioconjugate Chem., 2014, 25, 640 CrossRef CAS PubMed.
- S. André, K. E. Kövér, H. J. Gabius and L. Szilágyi, Bioorg. Med. Chem. Lett., 2015, 25, 931 CrossRef PubMed.
- I. Nierengarten and J. F. Nierengarten, Chem. – Asian J., 2014, 9, 1436 CrossRef CAS PubMed.
- J. L. Reymond and T. Darbre, Org. Biomol. Chem., 2012, 1, 1483 Search PubMed.
- G. C. Daskhan, N. Berthet, B. Thomas, M. Fiore and O. Renaudet, Carbohydr. Res., 2015, 405, 13 CrossRef CAS PubMed.
- S. Ramaswamy, M. H. Sleiman, G. Masuyer, C. Arbez-Gindre, M. Micha-Screttas, T. Calogeropoulou, B. R. Steele and K. R. Acharya, FEBS J., 2015, 282, 372 CrossRef CAS PubMed.
- A. K. Michel, P. Nangia-Makker, A. Raz and M. J. Cloninger, ChemBioChem, 2014, 15, 2106 CrossRef CAS PubMed.
- A. M. Eissa and N. R. Cameron, Adv. Polym. Sci., 2013, 253, 71 CrossRef CAS.
- V. Vázquez-Dorbatt, J. Lee, E. W. Lin and H. D. Maynard, ChemBioChem, 2012, 13, 2478 CrossRef PubMed.
- A. B. Lowe, B. S. Sumerlin and C. L. McCormick, Polymer, 2003, 44, 6761 CrossRef CAS.
- K. Ohno, Y. Tsujii and T. Fukuda, J. Polym. Sci., Part A: Polym. Chem., 1998, 36, 2473 CrossRef CAS.
- G. Yilmaz and C. R. Becer, Eur. Polym. J., 2013, 49, 3046 CrossRef CAS.
- D. Ponader, F. Wojcik, F. Beceren-Braun, J. Dernedde and L. Hartmann, Biomacromolecules, 2012, 13, 1845 CrossRef CAS PubMed.
- Q. Zhang, J. Collins, A. Anastasaki, R. Wallis, D. A. Mitchell, C. R. Becer and D. M. Haddleton, Angew. Chem., Int. Ed., 2013, 52, 4435 CrossRef CAS PubMed.
- S. J. Richards, M. W. Jones, M. Hunaban, D. M. Haddleton and M. I. Gibson, Angew. Chem., Int. Ed., 2012, 51, 7812 CrossRef CAS PubMed.
- N. Baradel, S. Fort, S. Halila, N. Badi and J. F. Lutz, Angew. Chem., Int. Ed., 2013, 52, 2335 CrossRef CAS PubMed.
- L. Zamfir and J. F. Lutz, Nat. Commun., 2012, 3, 1138 CrossRef.
- S. Kawakami and M. Hashida, J. Controlled Release, 2014, 190, 542 CrossRef CAS PubMed.
- H. Maeda, J. Controlled Release, 2012, 164, 138 CrossRef CAS PubMed.
- H. S. Choi, W. Liu, P. Misra, E. Tanaka, J. P. Zimmer, B. Itty Ipe, M. G. Bawendi and J. V. Frangioni, Nat. Biotechnol., 2007, 25, 1165 CrossRef CAS PubMed.
- W. T. Liau, C. Bonduelle, M. Brochet, S. Lecommandoux and A. M. Kasko, Biomacromolecules, 2015, 16, 284 CrossRef CAS PubMed.
- D. Pati, N. Kalva, S. Das, G. Kumaraswamy, S. Sen Gupta and A. V. Ambade, J. Am. Chem. Soc., 2012, 134, 7796 CrossRef CAS PubMed.
- C. Bonduelle, S. Mazzaferro, J. Huang, O. Lambert, A. Heise and S. Lecommandoux, Faraday Discuss., 2013, 166, 137 RSC.
- J. Kumar, A. Bousquet and M. H. Stenzel, Macromol. Rapid Commun., 2011, 32, 1620 CrossRef CAS PubMed.
- A. G. Dal Bó, V. Soldi, F. C. Giacomelli, C. Travelet, B. Jean, I. Pignot-Paintrand, R. Borsali and S. Fort, Langmuir, 2012, 28, 1418 CrossRef PubMed.
- A. G. Dal Bó, V. Soldi, F. C. Giacomelli, C. Travelet, R. Borsali and S. Fort, Carbohydr. Res., 2014, 397, 31 CrossRef PubMed.
- L. Valtola, A. Rahikkala, J. Raula, E. I. Kauppinen, S. Hietala and H. Tenhu, Eur. Polym. J., 2014, 59, 282 CrossRef CAS.
- J. Jacobs, A. Byrne, N. Gathergood, T. E. Keyes, J. P. A. Heuts and A. Heise, Macromolecules, 2014, 47, 7303 CrossRef CAS.
- M. Kohri, M. Sato, F. Abo, T. Inada, M. Kasuya, T. Taniguchi and T. Nakahira, Eur. Polym. J., 2011, 47, 2351 CrossRef CAS.
- J. Geng, F. Biedermann, J. M. Zayed, F. Tian and O. A. Scherman, Macromolecules, 2011, 44, 4276 CrossRef CAS.
- S. Das, D. Pati, N. Tiwari, A. Nisal and S. Sen Gupta, Biomacromolecules, 2012, 13, 3695 CrossRef CAS PubMed.
- L. Russo, A. Gautieri, M. Raspanti, F. Taraballi, F. Nicotra, S. Vesentini and L. Cipolla, Carbohydr. Res., 2014, 389, 12 CrossRef CAS PubMed.
- Q. Yang and M. Ulbricht, Macromolecules, 2011, 44, 1303 CrossRef CAS.
- E. Bouffard, K. El Cheikh, A. Gallud, A. Da Silva, M. Maynadier, I. Basile, M. Gary-Bobo, A. Morere and M. Garcia, Curr. Med. Chem., 2015, 22, 3014 CrossRef CAS PubMed.
- S. Kolusheva, L. Boyer and R. Jelinek, Nat. Biotechnol., 2000, 18, 225 CrossRef CAS PubMed.
- M. Rangin and A. Basu, J. Am. Chem. Soc., 2004, 126, 5038 CrossRef CAS PubMed.
- B. K. Gorityala, Z. Lu, M. L. Leow, J. Ma and X. W. Liu, J. Am. Chem. Soc., 2012, 134, 15229 CrossRef CAS PubMed.
- K. El-Boubbou, D. C. Zhu, C. Vasileiou, B. Borhan, D. Prosperi, W. Li and X. Huang, J. Am. Chem. Soc., 2010, 132, 4490 CrossRef CAS PubMed.
- C. E. Maljaars, A. C. de Souza, K. M. Halkes, P. J. Upton, S. M. Reeman, S. André, H. J. Gabius, M. B. McDonnell and J. P. Kamerling, Biosens. Bioelectron., 2008, 24, 60 CrossRef CAS PubMed.
- Y. Jin, K. H. Wong and A. M. Granville, J. Colloid Interface Sci., 2016, 462, 19 CrossRef CAS PubMed.
- T. Ishii, K. Miyata, Y. Anraku, M. Naito, Y. Yi, T. Jinbo, S. Takae, Y. Fukusato, M. Hori, K. Osada and K. Kataoka, Chem. Commun., 2016, 52, 1517 RSC.
- J. Liao, C. Wang, Y. Wang, F. Luo and Z. Qian, Curr. Pharm. Des., 2012, 18, 3432 CrossRef CAS PubMed.
- A. M. Eissa, M. J. P. Smith, A. Kubilis, J. A. Mosely and N. R. Cameron, J. Polym. Sci., Part A: Polym. Chem., 2013, 51, 5184 CrossRef CAS.
- D. Pei, Y. Li, Q. Huang, Q. Ren, F. Li and T. Shi, Colloids Surf., B, 2015, 126, 367 CrossRef CAS PubMed.
- A. Aykaç, M. C. Martos-Maldonado, J. M. Casas-Solvas, I. Quesada-Soriano, F. García-Maroto, L. García-Fuentes and A. Vargas-Berenguel, Langmuir, 2014, 30, 234 CrossRef PubMed.
- J. J. García-Vallejo, M. Ambrosini, A. Overbeek, W. E. van Riel, K. Bloem, W. W. Unger, F. Chiodo, J. G. Bolscher, K. Nazmi, H. Kalay and Y. van Kooyk, Mol. Immunol., 2013, 53, 387 CrossRef PubMed.
- R. Ribeiro-Viana, M. Sánchez-Navarro, J. Luczkowiak, J. R. Koeppe, R. Delgado, J. Rojo and B. G. Davis, Nat. Commun., 2012, 3, 1303 CrossRef PubMed.
- J. Luczkowiak, A. Muñoz, M. Sánchez-Navarro, R. Ribeiro-Viana, A. Ginieis, B. M. Illescas, N. Martín, R. Delgado and J. Rojo, Biomacromolecules, 2013, 14, 431 CrossRef CAS PubMed.
- A. Muñoz, D. Sigwalt, B. M. Illescas, J. Luczkowiak, L. Rodríguez-Pérez, I. Nierengarten, M. Holler, J.-S. Remy, K. Buffet, S. P. Vincent, J. Rojo, R. Delgado, J.-F. Nierengarten and N. Martín, Nat. Chem., 2016, 8, 50 CrossRef.
- S. Sattin, A. Daghetti, M. Thépaut, A. Berzi, M. Sánchez-Navarro, G. Tabarani, J. Rojo, F. Fieschi, M. Clerici and A. Bernardi, ACS Chem. Biol., 2010, 5, 301 CrossRef CAS PubMed.
- C. R. Becer, M. I. Gibson, J. Geng, R. Ilyas, R. Wallis, D. A. Mitchell and D. M. Haddleton, J. Am. Chem. Soc., 2010, 132, 15130 CrossRef CAS PubMed.
- V. Wittmann and R. J. Pieters, Chem. Soc. Rev., 2013, 42, 4492 RSC.
Footnote |
| † This work is dedicated to the memory of our dear friend and tutor Dr. Gerben Visser, Utrecht University, The Netherlands, who passed away in April 2015. |
| This journal is © The Royal Society of Chemistry 2016 |


