Efficient and synergetic DNA delivery with pyridinium amphiphiles–gold nanoparticle composite systems having different packing parameters†
Adrian
Kizewski
a and
Marc A.
Ilies
*bc
aCollege of Science and Technology, Temple University, 1803 N Broad Street, Philadelphia, PA-19122, USA
bDepartment of Pharmaceutical Sciences and Moulder Center of Drug Discovery Research, Temple University School of Pharmacy, 3307 N Broad Street, Philadelphia, PA-19140, USA. E-mail: mailies@temple.edu; Fax: +1-215-707-5620; Tel: +1-215-707-1749
cTemple Materials Institute, 1803 N Broad Street, Philadelphia, PA-19122, USA
First published on 6th November 2015
Abstract
Mixtures of highly curved pyridinium-decorated Au nanoparticles and standard pyridinium cationic lipids efficiently and synergetically transfected DNA in vitro, while displaying an excellent cytotoxic profile.
Efficient delivery of nucleic acids for therapeutic purposes requires delivery systems able to compact the genetic material, maintain its structural integrity while overcoming various delivery barriers, and unload the genetic cargo to the nucleus (for DNA) or to the cytoplasm (for siRNA) of target cells/tissues.1–4 Synthetic transfection systems based on cationic lipids and other amphiphiles (lipoplexes)1,5,6 or cationic polymers (polyplexes)2 provide a safer alternative to viruses but their efficiency must be improved. In this context, it was shown by us and by others that pyridinium amphiphiles are particular well suited for overcoming the hurdles of compaction/release process.1,7–12 Extensive structure–activity relationships studies1,7–9,13 established pyridinium lipids SAINT-2, SPYRIT-7, Ole and gemini surfactants SPYRIT-68 as efficient, low toxic nucleic acid delivery systems in vitro and in vivo (Chart 1). Very recently our group proved that the properties of cationic lipids and gemini surfactants can be exploited in a synergistic manner.14 Thus, we showed that blending 5–10% (charge equivalent) pyridinium SPYRIT-68 into pyridinium cationic lipid SPYRIT-7 – based lipoplexes increases the fluidity of the lipoplex bilayer, reduces the size of lipoplexes, the minimum charge ratio +/− required to fully compact the nucleic acid and significantly increases the transfection efficiency without raising the cytotoxicity of the lipoplexes.14 These effects can be explained by the higher charge/mass ratio and lower packing parameter of pyridinium gemini surfactants as compared with corresponding lipids.1,7,8 These properties translate into a higher fluidity and charge density of the lipoplex bilayer, properties revealed by Safinya's group to be essential for ensuring efficient internalization via endocytosis and endosomal escape, with positive effects on transfection efficiency.15 We proved that addition of gemini surfactants into the lipoplex enhances transfection through temporary poration of external and internal membranes, thus efficiently overcoming two major internal delivery barriers.14
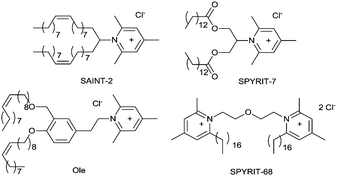 | ||
| Chart 1 Representative pyridinium amphiphiles with different packing parameters used in synthetic nucleic acid delivery systems. | ||
The high charge/mass ratio and controlled interfacial curvature of gemini surfactants can be mimicked by synthetic amphiphiles chemisorbed on spherical nanoparticles of well-defined size and curvature. Particularly interesting are gold nanoparticles, due to their biocompatibility, density, and special physicochemical, optical and surface properties.16,17 These properties recommended them for biological applications such as enzyme inhibitors and activators,18,19 drug and gene delivery systems.20–22 Gold compounds such as aurothioglucose, gold thiomalate, and auranofin are currently used in clinic for treatment of various forms of arthritis.16
Several synthetic methods are available for their generation, offering excellent control over colloidal size and surface ligands.16 In this study we decided to employ 20 nm round Au NPs, which have a curvature comparable with typical curvatures found in hydrated self-assembled gemini surfactants with hydrophilic linkers SPYRIT-68.7 This NP size was also found to be the smallest one that does not dramatically distort bilayer membranes, in related studies from our team.23 Thus, NP size and curvature impact their interaction with the cell membrane, where a very high curvature might induce a higher cytotoxic profile through membrane poration.24 It also controls the efficient blending with bilayer-forming cationic lipids such as Ole that display a cylindrical structure. From the methods available for the preparation of Au NPs we have selected the Frens method,25 further optimized by Grabar,26 which can generate highly homogeneous Au NPs in the desired size range with a very low polydispersity. The Au NPs generated this way have a surface decorated with citrate ligands, which are relatively easy to displace.16,25,26
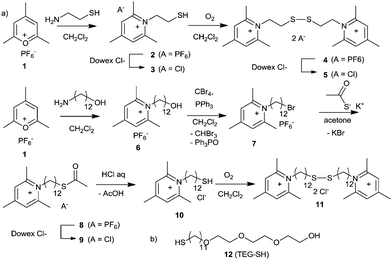
On the other hand, the high affinity of thiols for gold drives the displacement of weak ligands such as citrate from the surface of the metal, allowing facile interfacial modification of Au NPs.27 Thus, in order to mimic the properties of pyridinium gemini surfactants by pyridinium ligands supported by a curved Au NP, we designed and synthesized two pyridinium thiol ligands in which the two functional moieties are separated either via short (2C) or long (12C) linkers (Scheme 1). Chemisorption of fatty thiols on planar or curved gold surfaces is a well-established technique to produce self-assembled monolayers (SAMs), either planar27 or curved.20 The pyridinium moiety was generated in a single, high yield step via our established technology relying of the reaction of pyrylium salts with primary amines.9,28,29 This method allowed the direct access to short mercaptoethyl pyridinium ligand 2 (as PF6− salt), which was converted into the more biocompatible Cl−3via counterion exchange using Dowex Cl−.29,30 Since the Au NP surface decoration can be achieved with either thiols or disulfides, we converted the thiol 2 into the corresponding disulfide 4via oxidation with air in DCM, followed by PF6− exchange to Cl−, to yield bispyridinium disulfide 5. The pyridinium-dodecylthiol 10 was generated from the corresponding hydroxyl derivative 6, obtained from reaction of 12-aminododecanol with 2,4,6-trimethylpyrylium salt 1. The hydroxyl group of 6 was converted into bromide 7via CBr4/PPh3 in DCM, which was subsequently reacted with potassium thioacetate in acetone to yield the corresponding pyridinium-dodecylthioacetate 8. The counterion of 8 was changed to Cl−via Dowex Cl− yielding 9, which was transformed into the desired pyridinium-dodecylthiol 10 (as Cl−) with aqueous HCl under inert atmosphere. The disulfide 11 was also prepared via oxidation of 10 in DCM solution with air (Scheme S1, ESI†).
The direct decoration of Au NP with pyridinium mercapto derivatives 2–5, 10, 11 failed, irrespective of chain length or oxidation state of the thiol (SH or disulfide), due to fast nanoparticle aggregation. This was probably caused by the charge reversal on the Au surface upon decoration with positively charged mercapto ligands. During this process the nanoparticle surface potential shifts from negative to positive passing through a value of zero, where electrostatic repulsion is no longer present and particles can rapidly coalesce and precipitate.31,32
In order to sterically stabilize the Au NPs during the surface charge reversal we selected the amphiphilic triethyleneglycol mercaptan TEG-SH 1227 due to its amphiphilicity/wettability, excellent biocompatibility and cost. We successfully tested 12 for the surface decoration of Au NPs and we validated the stability of the resulting nanosystem. Displacement of citrate ligands by thiol 12 generated, as expected, a small increase in hydrodynamic radius of the decorated Au NP (from 21 to 25 nm) due to undecenyltriethyleneglycol-based corona, while the zeta potential remained almost unchanged (about −8 mV, see ESI†). Consequently, we performed the surface charge reversal of Au NPs by treating the citrate-decorated NPs with a blend of TEG-SH 12 with pyridinium mercapto derivatives 2–5, 10, or 11. Systematic assessment of different thiol/12 or disulfide/12 combinations revealed the importance of size match between the mercapto derivatives when performing the ligand exchange of Au NP surface. Only blends of TEG-SH 12 with either pyridinium thiol 10 or with pyridinium disulfide 11 generated stable, positively charged Au NPs (Fig. 1).
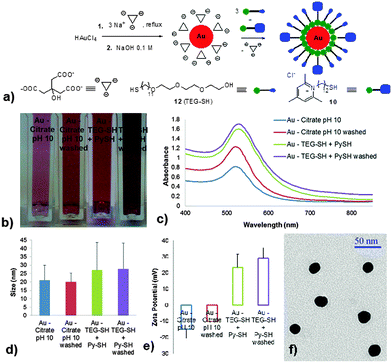 | ||
| Fig. 1 Synthesis and characterization of pyridinium-decorated Au nanoparticles: schematic representation of the synthesis and functionalization process (a) and physicochemical characterization throughout the stages of nanoparticle processing via color (b), surface plasmon spectrum (c), size (d), zeta potential (e) and TEM (f). See text and ESI† for details. | ||
The short mercaptans 2–5 failed to stabilize the AuNPs after ligand exchange despite the presence of 12, and coalescence of resulted NPs occurred in short time. Consequently, we focused our ligand exchange efforts on blends of TEG-SH 12 with pyridinium thiol Py-SH 10. Optimum ligand exchange performance and stability profile of resulting Py-SH/TEG-SH Au NPs were obtained using a molar ratio of thiols 12/10 of 3/1. These pyridinium-decorated Au NPs (Py–Au NPs) had a hydrodynamic size of about 27 nm and a zeta potential of about +30 mV after washing all released/unattached ligands. Lack of aggregation was confirmed by the color of the gold colloid, by the surface plasmon spectrum, and by TEM (Fig. 1).
We used these positively charged Py–Au NPs in subsequent DNA compaction, transfer and expression (transfection) experiments in conjunction with the pyridinium cationic lipid Ole (Chart 1) optimized by our group and transfection-efficient against many cell lines.13
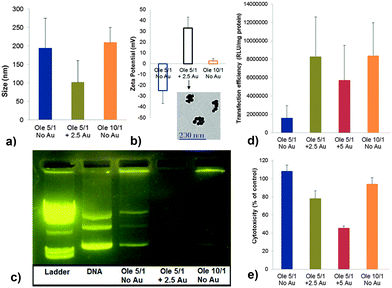
Through a combination of dynamic light scattering, zeta potential and electrophoretic mobility experiments we showed that Ole alone was able to fully compact plasmid DNA at a lipid/DNA charge ratio of 10, and generate lipoplexes about 200 nm in diameter, with a slightly positive (+4 mV) zeta potential (Fig. 2a–c). A lipid/DNA charge ratio of 5 was insufficient to compact the plasmid DNA, as shown by electrophoretic mobility of resulted complexes (Fig. 2c). However, adding only 2.5 equivalents of additional positive charge via blending Py–Au NPs in the Ole/DNA formulation (“Ole 5/1 + 2.5 Au”) fully compacted the DNA (Fig. 2c and Fig. S1, ESI,† with the DNA complex of purple color due to incorporation of Py–Au NPs).
Interestingly, the size of the hybrid lipoplexes decreased to about 150 nm and their zeta potential increased to about +35 mV, despite the (lower) amphiphile/DNA charge ratio of 7.5. These properties revealed the synergistic compaction effect of Py–Au NPs and Ole that allows the reduction of charge ratio while providing a better compaction of plasmid DNA (Fig. 2a and b, with Py–Au NPs clearly visible in the lipoplex structure). Moreover, transfection efficiency of these hybrid lipoplexes on NCI-H23 lung cancer cell line (at charge ratio of 7.5) was found to be similar to the efficiency of Ole lipoplexes having a lipid/DNA charge ratio of 10, and much higher than Ole lipoplexes formulated at a lipid/DNA charge ratio of 5 (Fig. 2d). Blending more Py–Au NPs in the Ole-based lipoplexes, for a total lipid/DNA charge ratio of 10, with 5 charge equivalents from Ole and 5 charge equivalents from Py–Au NPs, decreased the transfection efficiency and increased cytotoxicity of resulted DNA hybrid complexes. A similar effect was observed when blending (curved) pyridinium gemini surfactants SPYRIT-68 (Chart 1) into cationic lipid-based lipoplexes,14 reflecting a limited demand for a curved amphiphile in the lipoplex. One can consider that compaction of supercoiled plasmids involves regions of high curvature of backbone, easier to accommodate by blends of amphiphiles containing highly curved species as compared with simple lipid-only mixtures (compare lanes 4 and 5 in Fig. 2c).
The cytotoxicity of the hybrid complexes (“Ole 5/1 + 2.5 Au”) was slightly higher than the Ole-only based lipoplexes and increased when incorporating more Py–Au NPs (“Ole 5/1 + 5 Au”, Fig. 2e). This effect also mirrored the behavior of pyridinium lipid/gemini surfactant blends.14 In that case we could efficiently reduce the toxicity of the formulation, as well as the amount of cationic amphiphiles needed for full DNA compaction by adding co-lipids such as DOPE or cholesterol in the lipoplex formulations. Co-lipids can finely tune the packing parameter of the amphiphile blend and enhance the fluidity of the assembly and the hydrophobic effect, thus ensuring better compaction and delivery of DNA.1,7,8
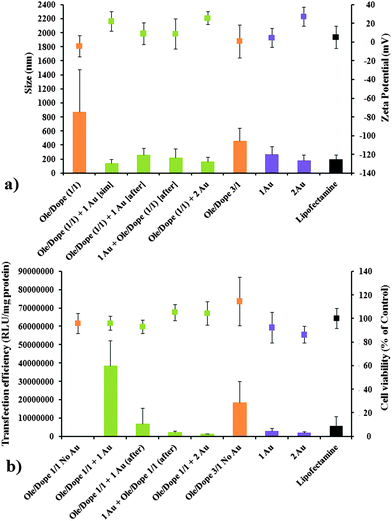
Therefore we subsequently investigated the effect of DOPE on transfection efficiency of Ole-based lipoplexes and Ole/Py–Au NPs hybrid DNA complexes (at different compositions). We assessed the physicochemical parameters of the complexes in parallel (Fig. 3). DNA complexes generated only from Py–Au NPs (at amphiphile/DNA charge ratios of 1 and 2, “1Au” and “2Au”) and from standard transfection system Lipofectamine® were included as reference.
Data from Fig. 3 revealed that Ole/DOPE (1/1 molar ratio) – based lipoplexes formulated at a cationic lipid/DNA charge ratio of 1 were relatively large, polydispersed and almost neutral in terms of zeta potential. As a consequence of the suboptimal physicochemical parameters, their transfection efficiency was very low. Increasing the cationic lipid/DNA charge ratio to 3 improved these physicochemical parameters, transfection efficiency and cytotoxic profile (Fig. 3a and b). The biological properties of these lipoplexes were superior to the ones displayed by DNA complexes generated from Py–Au NPs, irrespective of the charge ratio used (“1Au” and “2Au”), also surpassing Lipofectamine-based lipoplexes (Fig. 3b).
However, hybrid Ole/DOPE/Py–Au NPs DNA complexes having an Ole/DOPE 1/1 molar ratio and a total amphiphile/DNA charge ratio of 2, blended together at the same time with DNA (1 charge equivalent from Ole and 1 charge equivalent from Py–Au NPs, – “Ole/Dope (1/1) + 1 Au [sim]”) displayed smaller (∼175 nm) and more positively charged (∼+20 mV) lipoplexes. These hybrid DNA complexes showed a transfection efficiency superior to conventional Ole/Dope (1/1) lipoplexes made at a (higher) amphiphile to DNA charge ratio of 3, with an excellent cytotoxic profile, and greatly surpassing Lipofectamine-based lipoplexes. Increasing the amount of Py–Au NPs in the mix (“Ole/Dope (1/1) + 2 Au”) had a negative effect on physicochemical and biological properties (Fig. 3).
Synergetic action towards DNA compaction elicited by pyridinium cationic lipid Ole, colipid DOPE and Py–Au NPs was further proved when the plasmid DNA was treated the Ole/DOPE (1/1), followed by Py–Au NPs (“Ole/Dope (1/1) + 1 Au [after]”) or vice-versa, with Py–Au NPs, followed by Ole/DOPE (1/1) (“1 Au + Ole/Dope (1/1) [after]”, Fig. 3). These DNA complexes were inferior in both physicochemical and biological properties to the complexes formed by simultaneous addition of Ole/DOPE (1/1) and Py–Au NPs to DNA.
The superior physicochemical and biological properties of these hybrid nanosystems based on pyridinium amphiphiles constitute another direct proof of synergetic action towards nucleic acid delivery of different classes of amphiphiles with controlled packing parameters,1,7,8 across different types of colloidal materials.16,17,33–35
Financial support of NSF (CHE-0923077), Temple University Drug Discovery Initiative, TUSP Dean's Office and TU Undergraduate Research Program is gratefully acknowledged.
Notes and references
- B. Draghici and M. A. Ilies, J. Med. Chem., 2015, 58, 4091 CrossRef CAS PubMed.
- H. Yin, R. L. Kanasty, A. A. Eltoukhy, A. J. Vegas, J. R. Dorkin and D. G. Anderson, Nat. Rev. Genet., 2014, 15, 541 CrossRef CAS PubMed.
- W. Li and F. C. Szoka, Jr., Pharm. Res., 2007, 24, 438 CrossRef CAS PubMed.
- T. R. Pearce, K. Shroff and E. Kokkoli, Adv. Mater., 2012, 24, 3803 CrossRef CAS PubMed.
- T. Montier, T. Benvegnu, P. A. Jaffres, J. J. Yaouanc and P. Lehn, Curr. Gene Ther., 2008, 8, 296 CrossRef CAS PubMed.
- S. Bhattacharya and A. Bajaj, Chem. Commun., 2009, 4632 RSC.
- V. D. Sharma and M. A. Ilies, Med. Res. Rev., 2014, 34, 1 CrossRef PubMed.
- M. A. Ilies, T. V. Sommers, L. C. He, A. Kizewski and V. D. Sharma, in Amphiphiles: Molecular Assembly and Applications, ed. R. Nagarajan, ACS Symposium Series, Washington, DC, 2011, ch. 2, pp. 23–38 Search PubMed.
- M. A. Ilies, W. A. Seitz, B. H. Johnson, E. L. Ezell, A. L. Miller, E. B. Thompson and A. T. Balaban, J. Med. Chem., 2006, 49, 3872 CrossRef CAS PubMed.
- A. A. P. Meekel, A. Wagenaar, J. Šmisterová, J. E. Kroeze, P. Haadsma, B. Bosgraaf, M. C. A. Stuart, A. Brisson, M. H. J. Ruiters, D. Hoekstra and J. B. F. N. Engberts, Eur. J. Org. Chem., 2000, 665 CrossRef CAS.
- I. van der Woude, A. Wagenaar, A. A. Meekel, M. B. ter Beest, M. H. Ruiters, J. B. Engberts and D. Hoekstra, Proc. Natl. Acad. Sci. U. S. A., 1997, 94, 1160 CrossRef CAS.
- S. S. Le Corre, N. Belmadi, M. Berchel, T. Le Gall, J. P. Haelters, P. Lehn, T. Montier and P. A. Jaffres, Org. Biomol. Chem., 2015, 13, 1122 CAS.
- S. Savarala, E. Brailoiu, S. L. Wunder and M. A. Ilies, Biomacromolecules, 2013, 14, 2750 CrossRef CAS PubMed.
- V. D. Sharma, J. Lees, N. E. Hoffman, E. Brailoiu, M. Madesh, S. L. Wunder and M. A. Ilies, Mol. Pharmacol., 2014, 11, 545 CrossRef CAS PubMed.
- A. J. Lin, N. L. Slack, A. Ahmad, C. X. George, C. E. Samuel and C. R. Safinya, Biophys. J., 2003, 84, 3307 CrossRef CAS PubMed.
- E. C. Dreaden, A. M. Alkilany, X. Huang, C. J. Murphy and M. A. El-Sayed, Chem. Soc. Rev., 2012, 41, 2740 RSC.
- R. A. Sperling, P. Rivera Gil, F. Zhang, M. Zanella and W. J. Parak, Chem. Soc. Rev., 2008, 37, 1896 RSC.
- M. Stiti, A. Cecchi, M. Rami, M. Abdaoui, V. Barragan-Montero, A. Scozzafava, Y. Guari, J. Y. Winum and C. T. Supuran, J. Am. Chem. Soc., 2008, 130, 16130 CrossRef CAS PubMed.
- M. C. Saada, J. L. Montero, D. Vullo, A. Scozzafava, J. Y. Winum and C. T. Supuran, J. Med. Chem., 2011, 54, 1170 CrossRef CAS PubMed.
- Y. Ding, Z. Jiang, K. Saha, C. S. Kim, S. T. Kim, R. F. Landis and V. M. Rotello, Mol. Ther., 2014, 22, 1075 CrossRef CAS PubMed.
- P. Ghosh, G. Han, M. De, C. K. Kim and V. M. Rotello, Adv. Drug Delivery Rev., 2008, 60, 1307 CrossRef CAS PubMed.
- A. Fernandez-Barbero, I. J. Suarez, B. Sierra-Martín, A. Fernandez-Nieves, F. Javier de las Nieves, M. Marquez, J. Rubio-Retama and E. Lopez-Cabarcos, Adv. Colloid Interface Sci., 2009, 147–148, 88 CrossRef CAS PubMed.
- S. Savarala, S. Ahmed, M. A. Ilies and S. L. Wunder, Langmuir, 2010, 26, 12081 CrossRef CAS PubMed.
- L. Shang, K. Nienhaus and G. U. Nienhaus, J. Nanobiotechnol., 2014, 12, 1 CrossRef PubMed.
- G. Frens, Nature Phys. Sci., 1973, 241, 20 CrossRef CAS.
- K. C. Grabar, R. G. Freeman, M. B. Hommer and M. J. Natan, Anal. Chem., 1995, 67, 735 CrossRef CAS.
- J. C. Love, L. A. Estroff, J. K. Kriebel, R. G. Nuzzo and G. M. Whitesides, Chem. Rev., 2005, 105, 1103 CrossRef CAS PubMed.
- M. A. Ilies, W. A. Seitz, M. T. Caproiu, M. Wentz, R. E. Garfield and A. T. Balaban, Eur. J. Org. Chem., 2003, 2645 CrossRef CAS.
- M. A. Ilies, W. A. Seitz, I. Ghiviriga, B. H. Johnson, A. Miller, E. B. Thompson and A. T. Balaban, J. Med. Chem., 2004, 47, 3744 CrossRef CAS PubMed.
- V. D. Sharma, E. O. Aifuwa, P. A. Heiney and M. A. Ilies, Biomaterials, 2013, 34, 6906 CrossRef CAS PubMed.
- S. Savarala, F. Monson, M. A. Ilies and S. L. Wunder, Langmuir, 2011, 27, 5850 CrossRef CAS PubMed.
- S. Savarala, S. Ahmed, M. A. Ilies and S. L. Wunder, ACS Nano, 2011, 5, 2619 CrossRef CAS PubMed.
- J. O. Radler, I. Koltover, T. Salditt and C. R. Safinya, Science, 1997, 275, 810 CrossRef CAS PubMed.
- I. Koltover, T. Salditt, J. O. Radler and C. R. Safinya, Science, 1998, 281, 78 CrossRef CAS PubMed.
- E. Mahon, T. Aastrup and M. Barboiu, Top. Curr. Chem., 2012, 322, 139 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5cc05760d |
| This journal is © The Royal Society of Chemistry 2016 |