Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Cationic aluminum hydride complexes: reactions of carbene–alane adducts with trityl-borate†
Levy L.
Cao‡
,
Erika
Daley‡
,
Timothy C.
Johnstone
and
Douglas W.
Stephan
*
Department of Chemistry, University of Toronto, 80 St. George St., Toronto, Ontario M5S 3H6, Canada. E-mail: dstephan@chem.utoronto.ca
First published on 14th March 2016
Abstract
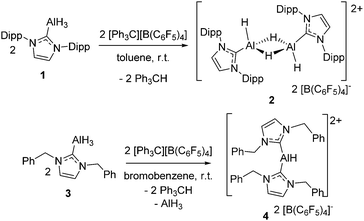
Reaction of (Idipp)AlH3 with [Ph3C][B(C6F5)4] in toluene affords the dimeric aluminum dication [((Idipp)AlH(μ-H))2][B(C6F5)4]22. In contrast, the reaction of (IBn)AlH3 with [Ph3C][B(C6F5)4] in bromobenzene gives a redistribution product, the salt of a monomeric dication [(IBn)2AlH][B(C6F5)4]24.
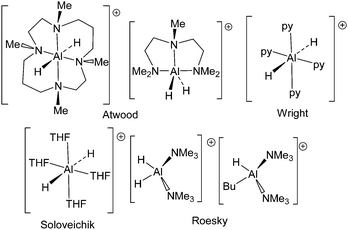
Aluminum hydrides are important reagents in organic, inorganic and materials chemistry. Indeed, such species are used in a variety of roles including reagents for reductions1–6 and in inorganic synthesis.7,8 Applications as components in materials chemistry and in particular, hydrogen storage materials9–11 have also garnered attention. From a coordination chemistry perspective, aluminum-hydride derivatives exhibit a range of geometries resulting from the low steric demand of hydride and the ability of aluminum to accommodate coordination numbers ranging from three to six. The majority of reported aluminum hydride species are either neutral or anionic while, in contrast, cationic aluminum-hydride species are less common. A matrix isolation study at 4 K described a [AlH]˙+ radical cation which was studied by EPR spectroscopy.12,13 The first fully characterized Al-hydride cations [H2Al((MeNCH2CH2N(Me)CH2CH2CH2)2)][AlH4] and [H2Al(MeN(CH2CH2NMe2)2)][AlH4] (Scheme 1) were prepared and reported by Atwood and coworkers in 1991.14,15 These species exhibited six- and five-coordinate aluminum centers, respectively. In 1994, Soloveichik and coworkers described the structure of the salt [AlH2(C4H8O)4][(C5H5)3Yb(Na)Yb(C5H5)3] (Scheme 1) which contained a six-coordinate aluminum-dihydride cation.16 In 2004, we employed an aluminum complex of a phosphinimine–amine ligand to generate salts of the aluminum-hydride cation [(iPr2C6H3N)C(Me)CHPPh2(NC6H3iPr2)AlH][B(C6F5)4].17 Roesky and coworkers exploited a bulky non-coordinating anion to isolate the salts [H2Al(NMe3)2]2[(AlH)8(CCH2tBu)6] and [H(nBu)Al(NMe3)2][(AlH)7(AlNMe3)(CCH2tBu)6] (Scheme 1) in 2005.18 These latter compounds are examples of four-coordinate aluminum hydride cations. Most recently, Wright and coworkers described the structure of [(1,4-H-pyrid-1-yl)4Al][(pyridine)4AlH2], which was formed from the reaction of (tBuO)AlH2 and pyridine.19 In this manuscript, we describe two reactions of carbene–alane adducts with trityl cation affording the first dimeric four-coordinate and monomeric three-coordinate aluminum hydride dications.
The known carbene–alane adduct (Idipp)AlH31, was prepared via literature methods.20 Reaction of species 1 with one equivalent of [Ph3C][B(C6F5)4] at room temperature in toluene resulted in the immediate precipitation/crystallization of a new species 2 (Scheme 2). The formation of 2 proceeds via hydride abstraction from 1, liberating Ph3CH, followed by subsequent dimerization and crystallization. Although this avenue of reactivity is not widely exploited in aluminum-hydride chemistry, it is known in the literature to generate both aluminum17 and transition metal alkyl cations.21,22 The formation of 2 represents, to our knowledge, the first dimeric aluminum-hydride dication salt.
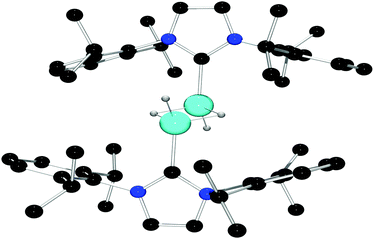
The complete insolubility of this product in all organic solvents in which it was stable precluded spectroscopic characterization, however compound 2 was characterized by single crystal X-ray diffraction (Fig. 1). Solution of the structure via direct methods revealed that the asymmetric unit contains a single Al center and a [B(C6F5)4]− anion, and structure refinement confirmed the molecular structure of 2 to be that of the centrosymmetric dimeric dication salt, [((Idipp)AlH(μ-H))2][B(C6F5)4]2. The Al center adopts a pseudo-tetrahedral geometry featuring coordinate bonds to the carbene, a terminal hydride, and two bridging hydrides. The angles about Al range from 102.1(8)° to 128.5(9)° and the Al–C bond length is 1.964(3) Å. The terminal Al-hydride bond length refined to 1.50(2) Å and the bridging Al–H distances were determined to be 1.66(2) Å and 1.71(2) Å. The resulting Al⋯Al distance in the cation of 2 is 2.584(2) Å, a value greater than the sum of the covalent radii of the two atoms.23 The structure of the cation 2 is reminiscent of the neutral dimeric Al(II) species reported by Jones and coworkers,24 which features an Al–Al single bond (2.6375(8) Å, and terminal Al–H distances of 1.56(2) Å and 1.53(2) Å). Similarly, the compound [(iPr2N(CNDipp)2AlH)]224 exhibited an Al⋯Al distance of 2.675(1) Å with terminal Al–H distances of 1.53(2) Å. The observation of a shorter Al⋯Al distance in 2 in comparison to those in Al(II) species is consistent with the higher oxidation state of the Al centers in 2 prompting shorter Al–Hbridge bonds thus drawing the Al atoms closer together.
To probe the impact of altering the carbene, 1,3-dibenzylimidazol-2-ylidene (IBn) was allowed to react with (EtMe2N)AlH3 in toluene, to readily afford (IBn)AlH3 (3) in 72% yield after recrystallization. This species gives rise to a broad singlet resonance in 1H NMR spectrum in C6D5Br at 4.38 ppm corresponding to the aluminum-bound hydride center. The 27Al NMR spectrum of 3 shows a broad singlet resonance at 108.7 ppm. These data are comparable to those seen for related NHC-alane adducts.25,26 An X-ray crystallographic study of 3 (see ESI,† Fig. S3) confirmed the formulation, revealing a Al–CNHC bond length of 2.059(2) Å, which falls within the range of typical Al–CNHC bonds (2.034–2.067 Å).25,26
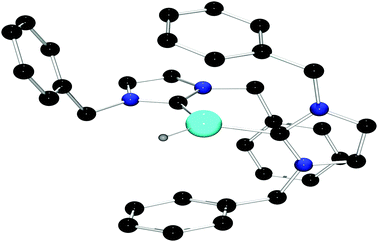
Treatment of 3 with a stoichiometric amount of [Ph3C][B(C6F5)4] in C6H5Br at room temperature results in the formation of Ph3CH and a new species 4 in 46% yield. The 1H NMR spectrum of 4 in C6D5Br shows resonances attributable to the IBn ligand but no signal was observed in the 27Al NMR spectrum. The IR spectrum of 4 featured a signal at 1963 cm−1, which is in the same region as observed for other cationic aluminum hydride species.18 The solid state structure of 4 was unambiguously confirmed by X-ray crystallography. Compound 4 proved to be the dicationic borate salt [(IBn)2AlH][B(C6F5)4]2. The cation of this salt is a planar monohydrido aluminum dication containing two IBn ligands (Fig. 2). The planes of the two carbenes are oriented approximately orthogonal to each other, allowing the benzyl substituents to envelop the aluminum center. Two of the pendent arenes are positioned above and below the pseudo-trigonal coordination plane of the aluminum center. The sum of the angles about aluminum is 359° and the C–Al–C′ angle is 113.6(2)°. The Al–C bond distances in 4 are 1.987(4) Å and 2.010(4) Å. The structural data also reveals that one of the benzyl substituents on one of the IBn ligands is oriented such that a C–C π-bond is positioned above with Al–C distances of 3.124(5) Å and 2.572(4) Å, respectively.
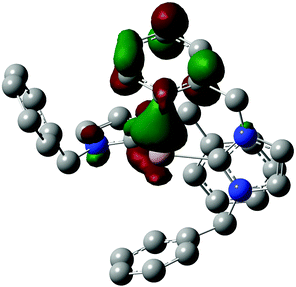
DFT calculations were performed at the B3LYP/6-311G(d,p) level of theory to gain further insight into the electronic structure of dication 4. The optimized geometry corresponded well with the crystallographically determined structure (non-hydrogen RMSD = 0.30 Å), and a frequency calculation confirmed that the IR active band at 1963 cm−1 arises from a normal mode comprising almost exclusively the Al–H stretch (see ESI†). Based on natural population analysis (NPA),27,28 the Al atom of compound 4 bears a natural atomic charge of +1.50 (see ESI,† Fig. S11 and Table S2). An NPA analysis reported for a similar dicationic hydrido boron complex prepared by Ong and co-workers29 suggested that their boron complex was stabilized by redistribution of positive charge from the boron center onto the carbodicarbene supporting ligands. The lack of such charge redistribution in 4 suggests that it is stabilized by other effects. The LUMO of the dication (Fig. 3) is mainly located on the Al atom but has significant contribution from the proximal arene ring. An electronic interaction between the Al center and the aromatic ring of the pendent benzyl group, inferred from the crystal structure of 4 in addition to the delocalized nature of the LUMO, was corroborated by the results of a natural bond orbital (NBO) analysis.30 Using second order perturbation theory, a donor acceptor interaction was identified between a bonding NBO on the pendent arene ring and an empty lone pair NBO on the Al center. The Al-based NBO has essentially pure 3pz character and the bonding NBO, localized between the two carbon atoms of the arene ring that are closest to the metal center, has π-symmetry and is oriented such that it overlaps with the Al-based NBO (see ESI,† Fig. S12 and Table S3). This interaction may impart stabilization on the complex, accounting for its unexpected stability. For instance, NMR spectroscopy revealed no evidence of degradation of a sample of 4 that had been stored in an inert atmosphere for over a month. The Al–arene interaction in 4 is reminiscent of the olefin–Al interaction described for CH2![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C(CH3)CH2CH2CH2Al(C6F5)231,32 and (C6H10)Al(C6F5)3.33 The equivalence between the benzyl groups of 4 observed in the solution state NMR spectrum suggest that this interaction is fluxional, at least on the NMR time scale. The transient nature of the interaction, coupled with the fact that the distance separating the Al center and the nearest carbon of the arene ring is greater than the sum of their van der Waals radii, leads us to formulate the species as a 3-coordinate aluminum complex.
C(CH3)CH2CH2CH2Al(C6F5)231,32 and (C6H10)Al(C6F5)3.33 The equivalence between the benzyl groups of 4 observed in the solution state NMR spectrum suggest that this interaction is fluxional, at least on the NMR time scale. The transient nature of the interaction, coupled with the fact that the distance separating the Al center and the nearest carbon of the arene ring is greater than the sum of their van der Waals radii, leads us to formulate the species as a 3-coordinate aluminum complex.
Although redistribution reactions are common in aluminum chemistry, the formation of 4 is a rare example of such a redistribution involving a carbene ligand. One can speculate that the reaction of 3 with [Ph3C][B(C6F5)4] proceeds to generate a dimeric analog of 2 but that the lesser steric demands of IBn facilitate the redistribution affording 4 and liberation of AlH3.
The above reactions of NHC–alane adducts with trityl borate were shown to generate the dimeric aluminum-hydride dicationic salt 2 as well as the monomeric three-coordinate aluminum-hydride salt 4. These observations represent the first dicationic aluminum-hydride salts to be characterized. The differing nature of these species illustrates the influence of carbene substituents on the aggregation of aluminum-hydrides.
Notes and references
- J. Powell, N. James and S. J. Smith, Synthesis, 1986, 338–340 CrossRef CAS
.
- B. Alcaide, C. Lopez-Mardomingo and J. Plumet, J. Chem. Res., Synop., 1987, 270–271 CAS
.
- E. V. Dehmlow and R. Cyrankiewicz, J. Chem. Res., Synop., 1990, 24 CAS
.
- H. Haubenstock, Top. Stereochem., 1983, 14, 231–300 CAS
.
- A. Boussonnière, R. Bénéteau, J. Lebreton and F. Dénès, Eur. J. Org. Chem., 2013, 7853–7866 CrossRef
.
- H. Cho, Tetrahedron, 2014, 70, 3527–3544 CrossRef CAS
.
- R. J. Wehmschulte and P. P. Power, Polyhedron, 2000, 19, 1649–1661 CrossRef CAS
.
- H. W. Roesky, Inorg. Chem., 2004, 43, 7284–7293 CrossRef CAS PubMed
.
- J. A. Jegier and W. L. Gladfelter, Coord. Chem. Rev., 2000, 206–207, 631–650 CrossRef CAS
.
- J. Graetz and B. C. Hauback, MRS Bull., 2013, 38, 473–479 CrossRef CAS
.
- S. Srinivasa Murthy and E. Anil Kumar, Appl. Therm. Eng., 2014, 72, 176–189 CrossRef CAS
.
- L. B. Knight, Jr., R. L. Martin and E. R. Davidson, J. Chem. Phys., 1979, 71, 3991–3995 CrossRef
.
- L. B. Knight, Jr., S. T. Cobranchi, B. W. Gregory and E. Earl, J. Chem. Phys., 1987, 86, 3143–3150 CrossRef
.
- J. L. Atwood, K. D. Robinson, C. Jones and C. L. Raston, J. Chem. Soc., Chem. Commun., 1991, 1697–1699 RSC
.
- D. A. Atwood, Coord. Chem. Rev., 1998, 176, 407–430 CrossRef CAS
.
- S. Ya. Knjazhansky, I. Yu. Nomerotsky, B. M. Bulychev, V. K. Belsky and G. L. Soloveichik, Organometallics, 1994, 13, 2075–2078 CrossRef CAS
.
- J. D. Masuda, D. M. Walsh, P. Wei and D. W. Stephan, Organometallics, 2004, 23, 1819–1824 CrossRef CAS
.
- A. Stasch, H. W. Roesky, M. Noltemeyer and H.-G. Schmidt, Inorg. Chem., 2005, 44, 5854–5857 CrossRef CAS PubMed
.
- R. J. Less, H. R. Simmonds and D. S. Wright, Dalton Trans., 2014, 43, 5785–5792 RSC
.
- R. J. Baker, A. J. Davies, C. Jones and M. Kloth, J. Organomet. Chem., 2002, 656, 203–210 CrossRef CAS
.
- V. C. Williams, G. J. Irvine, W. E. Piers, Z. Li, S. Collins, W. Clegg, M. R. J. Elsegood and T. B. Marder, Organometallics, 2000, 19, 1619–1621 CrossRef CAS
.
- S. Zhang and W. E. Piers, Organometallics, 2001, 20, 2088–2092 CrossRef CAS
.
- B. Cordero, V. Gómez, A. E. Platero-Prats, M. Revés, J. Echeverría, E. Cremades, F. Barragán and S. Alvarez, Dalton Trans., 2008, 2832–2838 RSC
.
- S. J. Bonyhady, D. Collis, G. Frenking, N. Holzmann, C. Jones and A. Stasch, Nat. Chem., 2010, 2, 865–869 CrossRef CAS PubMed
.
- T. K. Sen, S. C. Sau, A. Mukherjee, P. K. Hota, S. K. Mandal, B. Maity and D. Koley, Dalton Trans., 2013, 42, 14253–14260 RSC
.
- N. Holzmann, A. Stasch, C. Jones and G. Frenking, Chem. – Eur. J., 2011, 17, 13517–13525 CrossRef CAS PubMed
.
- A. E. Reed and F. Weinhold, J. Chem. Phys., 1983, 78, 4066–4073 CrossRef CAS
.
- A. E. Reed, R. B. Weinstock and F. Weinhold, J. Chem. Phys., 1985, 83, 735 CrossRef CAS
.
- W.-C. Chen, C.-Y. Lee, B.-C. Lin, Y.-C. Hsu, J.-S. Shen, C.-P. Hsu, G. P. A. Yap and T.-G. Ong, J. Am. Chem. Soc., 2014, 136, 914–917 CrossRef CAS PubMed
.
- J. P. Foster and F. Weinhold, J. Am. Chem. Soc., 1980, 102, 7211–7218 CrossRef CAS
.
- G. Ménard and D. W. Stephan, Angew. Chem., Int. Ed., 2012, 51, 4409–4412 CrossRef PubMed
.
- G. Ménard, L. Tran, J. S. J. McCahill, A. J. Lough and D. W. Stephan, Organometallics, 2013, 32, 6759–6763 CrossRef
.
- G. Ménard and D. W. Stephan, Angew. Chem., Int. Ed., 2012, 51, 8272–8275 CrossRef PubMed
.
Footnotes |
| † Electronic supplementary information (ESI) available. Experimental details and spectral data. CCDC 1453308–1453310. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6cc01585a |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2016 |