Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Towards “bionic” proteins: replacement of continuous sequences from HIF-1α with proteomimetics to create functional p300 binding HIF-1α mimics†
George M.
Burslem‡
ab,
Hannah F.
Kyle
bc,
Alexander L.
Breeze
bcd,
Thomas A.
Edwards
bc,
Adam
Nelson
ab,
Stuart L.
Warriner
ab and
Andrew J.
Wilson
*ab
aSchool of Chemistry, University of Leeds, Woodhouse Lane, Leeds, LS29JT, UK
bAstbury Centre for Structural Molecular Biology, University of Leeds, Woodhouse Lane, Leeds, LS29JT, UK. E-mail: a.j.wilson@leeds.ac.uk
cSchool of Molecular and Cellular Biology, Faculty of Biological Sciences, University of Leeds, Woodhouse Lane, Leeds LS2 9JT, UK
dDiscovery Sciences, AstraZeneca R&D, Alderley Park, Cheshire, SK10 4TG, UK
First published on 24th March 2016
Abstract
Using the HIF-1α transcription factor as a model, this manuscript illustrates how an extended sequence of α-amino acids in a polypeptide can be replaced with a non-natural topographical mimic of an α-helix comprised from an aromatic oligoamide. The resultant hybrid is capable of reproducing the molecular recognition profile of the p300 binding sequence of HIF-1α from which it is derived.
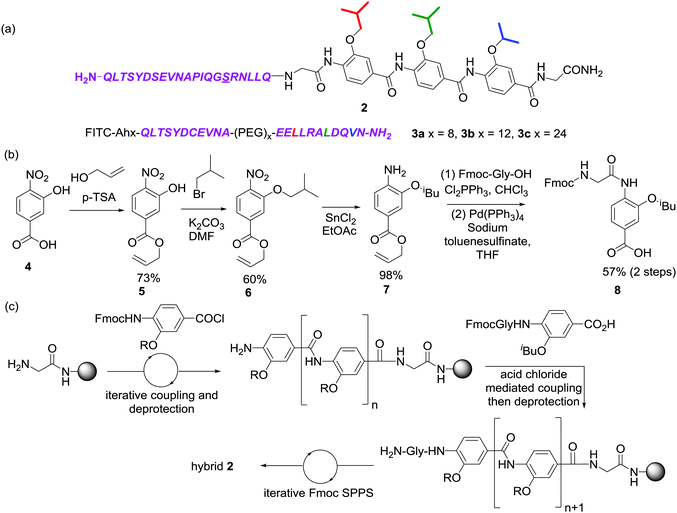
Nature uses a stunning array of architectures to carry out complex tasks including catalysis and cell signalling. Biopolymers are able to perform these feats because they self-organise and present functional motifs (e.g. an active site or binding surface) through precise 3-D orientation of primary structure. A long term goal in chemical and synthetic biology, therefore, is to expand on this diversity, and through the incorporation of non-natural functionality, elaborate bio-macromolecules with enhanced or orthogonal functionality and/or properties.1 Foldamers are oligomers that adopt well-defined conformations and either replicate structural and functional features of natural biomacromolecules or access, using non-natural building blocks, novel folds and functions not found in nature. An alternative approach to this bottom-up strategy, termed “protein-prosthesis”, lies at the cross-roads with strategies to chemically2–4 or genetically5 introduce non-natural function into proteins. In protein prosthesis,6,7 backbone engineering8 allows individual residues or sequences within proteins to be replaced with non-natural residues.9–13 Notable examples include the incorporation of β-amino acid residues in the B1 domain of Streptococcal protein G (GB1)14 and Betabellin-14,15 the re-engineering of a heterodimeric chorismate mutase enzyme16 using sequence based design, the replacement of loop regions in GB1 using PEG17 and the incorporation of an entire β-amino acid topological mimic of an α-helix into IL8.18 α-Helix mimetics19–23 employ a suitably functionalised generic scaffold to reproduce the spatial projection and composition of key side chains found at a helical interface between two proteins. Such α-helix mimetics have been shown by us24,25 and others26–28 to act as potent inhibitors of protein–protein interactions, but there are limited studies on their incorporation into higher-order structures.29 In this manuscript we illustrate the first steps towards this goal by replacement of an entire segment of the HIF-1α transactivation domain with an aromatic oligoamide (Fig. 1). In doing so, we provide the first example of a replacement of an extended peptide sequence with a topographical mimic.
 | ||
| Fig. 1 Approach for assembly of a “bionic” protein (a) NMR structure of the HIF-1α C-TAD in complex with p300; PDB ID: 18LC (p300 in green, helix 1 of HIF-1α in blue, key binding sequence comprising helices 2 and 3 of HIF-1α in purple and zinc as grey spheres), (b) structure of a proteomimetic inhibitor of HIF-1α/p300 based on helix 3 of HIF-1α (i, i + 4 and i + 7 mimicking side chains coloured red, green and blue respectively) (c) schematic depicting prior truncation studies on HIF-1α C-TAD together with conceptual structure of hybrids comprising peptide and proteomimetic or non-natural linkers. | ||
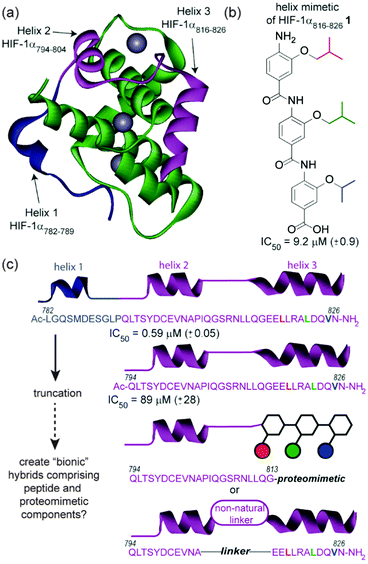
Our design focused upon a minimal region of the HIF-1α C-terminal transactivation domain (C-TAD) which interacts with the CREB-binding protein (CBP)/p300 co-activator to promote transcription (Fig. 1a).30,31 Previously our group illustrated that two helical domains (HIF-1α794–826, i.e. helix 2/3) within the 42 residue C-TAD peptide (HIF-1α782–826) were necessary for measurable binding to p300 (Fig. 1c).32 We had also identified a helix mimetic 1 based on our 3-O-alkylated aromatic oligoamide scaffold which was designed to mimic helix 3 of the HIF-1α C-TAD (HIF-1α816–826) and shown to act as a low μM (IC50 = 9.2 ± 0.9 μM) inhibitor of the HIF-1α/p300 interaction (Fig. 1b).33 In our design (hybrid 2), the helix 3 region (HIF-1α816–826) of HIF-1α (HIF-1α782–826) was replaced with the previously identified helix mimetic33 and the remainder of the sequence preserved (Scheme 1a). In parallel, we also designed a series of helix 2/3 conjugates 3a–c linked by PEG spacers (Scheme 1a); the purpose in designing these compounds was to ascertain the extent to which the p300 binding potency might be maintained when the linker region between key helical regions (HIF-1α794–808 and HIF-1α816–826) was replaced.
Previously developed solid-phase synthesis methods for the preparation of oligobenzamides facilitate rapid preparation of helix mimetic trimers34 but we found the method unsuitable for preparation of the target peptide-helix mimetic conjugate 2. To address the poor nucleophilicity of trimeric oligoamides with a terminal aniline, Fmoc-glycine was coupled to an isobutyl monomer 7 using dichlorotriphenylphosphorane (Scheme 1b). Noteworthy in this sequence was the use of an allyl ester, which facilitated ester cleavage in the presence of the base-labile Fmoc protecting group. Fischer esterification of nitro acid 4 with allyl alcohol gave ester 5 followed by alkylation with isobutyl bromide to give alkoxy nitro ester 6. The aryl nitro group was reduced to the aniline 7 using stannous chloride then acylated with Fmoc-glycine via in situ acid chloride formation with dichlorotriphenylphosphorane. Finally the acid was revealed with palladium(0) tetrakis triphenylphosphine and sodium toluenesulfinate scavenger35 to give the final building block 8 in good yield.
The desired peptide-helix mimetic hybrid 2 was subsequently obtained using Fmoc based solid-phase synthesis (Scheme 1c). O-Alkylated 2-hydroxybenzoic acid monomers were assembled on resin using the previously described microwave assisted approach until Fmoc-Gly-iBu dipeptide building block 8 had been coupled. Following deprotection, the oligomer was then extended using conventional Fmoc solid phase synthesis with HCTU-mediated couplings. The preparation of hybrid 2 was performed in a single run on an automated peptide synthesiser (CEM Liberty) followed by HPLC purification (see ESI† for additional details). PEG linked helix 2/3 conjugates 3a–c were all synthesized using conventional Fmoc based solid phase synthesis and labelled with fluorescein to permit direct binding analyses (Scheme S2a, see ESI,† for details of the synthesis).
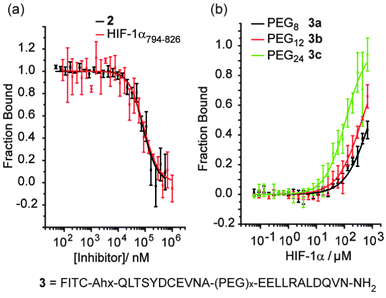
The hybrids 2 and 3a–c were then tested in a previously described fluorescence anisotropy p300/HIF-1α competition and p300 binding assays respectively (Fig. 2a).33 Hybrid 2 was shown to inhibit the interaction with comparable affinity to the HIF-1α794–826 peptide from which it was derived (IC50 values hybrid 2 = 83 ± 1.8 μM; HIF-1α794–826 = 89 ± 2.8 μM).32 Crucially, to avoid disulfide formation, the helix mimetic peptide 2 conjugate bears a cysteine to serine modification (underlined in Scheme 1), whereas the peptide does not. The mutation of this cysteine residue to a serine has been shown to reduce affinity by approximately 10-fold for the native sequence,36 which should be considered when comparing the affinities. It should also be noted that this affinity does not derive solely from the peptidic region, as a peptide comprising the same residues (HIF-1α794–815) shows no inhibitory activity (IC50 > 500 μM).32
The PEG linked hybrids 3a–c exhibited lower affinity for p300 – Kd3c = 144(±13) μM, 3a/b > 200 μM (Fig. 2c) – than FITC-HIF-1α794–826 (Kd = 6.74 ± 0.54 μM).32 This suggests either that the linker is not sufficiently long or that the side chains from the linker between helices 2 and 3 make productive non-covalent contacts with p300. A requirement for optimal linker length is supported by the fact that as the length of the PEG linker in 3a–c increases, so does binding affinity. In spite of the weaker binding affinities observed for 3a–c, the weak binding for HIF794–804 (helix 2) and HIF816–826 (helix 3) in isolation and lack of allosteric co-operativity between the two observed previously,32 indicates chelate co-operativity for 3a–c here and validates the approach.
At first the lower inhibitory potency of both HIF-1α794–826 and hybrid 2 in comparison to helix mimetic 1 (9.2(±0.9) μM) which we determined previously,33 might seem counterintuitive. One explanation that might account for this observation is that there may be a non-specific component to PPI inhibition associated with the aromatic oligoamide helix mimetic 1 (supported in part by a non-unity Hill co-efficient for the curve fitting, a property not observed for 2). Indeed, more hydrophobic compounds frequently bind with greater potency but poorer specificity and selectivity,37 whilst even for peptides, truncation can similarly lead to more potent but less specific binding.38 We therefore performed further biophysical analysis. Although we illustrated previously that inhibition of HIF-1α/p300 by 1 was specific to the scaffold and selective in comparison to the eIF4E/4G interaction,33 we expanded the range of assays here. Compound 1 exhibited inhibition of the p53/hDM2 interaction (IC50 = 16.4 ± 1.0 μM) when assessed in a fluorescence anisotropy competition assay (Fig. 3). In contrast, hybrid 2 was shown to exhibit minimal inhibition of this interaction (p53/hDM2 IC50 > 100 μM). Thus, by adding native amino acid residues from the HIF-1α sequence to the core helix mimetic 1 in hybrid 2, the specificity and selectivity of binding towards target interactions was improved.
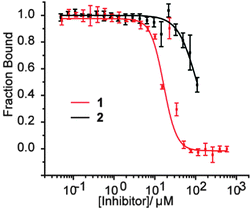 | ||
| Fig. 3 Biophysical screening of helix mimetic 1 and hybrid 2 to profile selectivity; FA competition data for inhibition of p53/hDM2. | ||
In conclusion, we have described the first example of a peptide-helix mimetic hybrid and in doing so conceptually illustrated that extended sequences from proteins can be replaced with molecules that topographically mimic such sequences. Our immediate efforts will focus on structural and biophysical studies on these hybrid mimetics with a view to optimizing the binding properties. Future efforts will focus on incorporating such secondary structure mimetics into longer protein sequences and exploring this replacement strategy for a broader array of protein functions. Application of this generic approach for preparation of functional peptide-helix mimetic hybrids, could allow assembly of protein-like objects with enhanced properties e.g. proteolytic and thermal stability, married with superior recognition properties (e.g. selectivity) when compared with simpler proteomimetics.
We thank AstraZeneca and EPSRC for PhD studentships (G. M. B. and H. F. K.) and the European Research Council [ERC-StG-240324, and ERC-PoC 632207] for support.
Notes and references
- K.-H. Altmann, B. Johannes, H. Kessler, F. Diederich, B. Krautler, S. Lippard, R. Liskamp, K. Muller, E. M. Nolan, B. Samori, G. Schneider, S. L. Schreiber, H. Schwalbe, C. Toniolo, C. A. A. van Boeckel, H. Waldmann and C. T. Walsh, ChemBioChem, 2009, 10, 16–29 CrossRef CAS PubMed.
- S. B. H. Kent, Chem. Soc. Rev., 2009, 38, 338–351 RSC.
- C. D. Spicer and B. G. Davis, Nat. Commun., 2014, 5, e4740 CrossRef PubMed.
- K. Lang and J. W. Chin, ACS Chem. Biol., 2014, 9, 16–20 CrossRef CAS PubMed.
- J. W. Chin, Reprogramming the genetic code, 2011.
- U. Arnold, M. P. Hinderaker, B. L. Nilsson, B. R. Huck, S. H. Gellman and R. T. Raines, J. Am. Chem. Soc., 2002, 124, 8522–8523 CrossRef CAS PubMed.
- U. Arnold, M. P. Hinderaker, J. Koditz, R. Golbik, R. Ulbrich-Hofmann and R. T. Raines, J. Am. Chem. Soc., 2003, 125, 7500–7501 CrossRef CAS PubMed.
- Z. E. Reinert and W. S. Horne, Org. Biomol. Chem., 2014, 12, 8796–8802 CAS.
- R. Kaul, A. R. Angeles, M. Jager, E. T. Powers and J. W. Kelly, J. Am. Chem. Soc., 2001, 123, 5206–5212 CrossRef CAS PubMed.
- A. Tam, U. Arnold, M. B. Soellner and R. T. Raines, J. Am. Chem. Soc., 2007, 129, 12670–12671 CrossRef CAS PubMed.
- A. A. Fuller, D. Du, F. Liu, J. E. Davoren, G. Bhabha, G. Kroon, D. A. Case, H. J. Dyson, E. T. Powers, P. Wipf, M. Gruebele and J. W. Kelly, Proc. Natl. Acad. Sci. U. S. A., 2009, 106, 11067–11072 CrossRef CAS PubMed.
- B. r. Eckhardt, W. Grosse, L.-O. Essen and A. Geyer, Proc. Natl. Acad. Sci. U. S. A., 2010, 107, 18336–18341 CrossRef CAS PubMed.
- B.-C. Lee and R. N. Zuckermann, ACS Chem. Biol., 2011, 6, 1367–1374 CrossRef CAS PubMed.
- Z. E. Reinert, G. A. Lengyel and W. S. Horne, J. Am. Chem. Soc., 2013, 135, 12528–12531 CrossRef CAS PubMed.
- G. Olajos, A. Hetényi, E. Wéber, L. J. Németh, Z. Szakonyi, F. Fülöp and T. A. Martinek, Chem. – Eur. J., 2015, 21, 6173–6180 CrossRef CAS PubMed.
- C. Mayer, M. M. Müller, S. H. Gellman and D. Hilvert, Angew. Chem., Int. Ed., 2014, 53, 6978–6981 CrossRef CAS PubMed.
- Z. E. Reinert, E. D. Musselman, A. H. Elcock and W. S. Horne, ChemBioChem, 2012, 13, 1107–1111 CrossRef CAS PubMed.
- R. David, R. Guünther, L. Baumann, T. Lühmann, D. Seebach, H.-J. R. Hofmann and A. G. Beck-Sickinger, J. Am. Chem. Soc., 2008, 130, 15311–15317 CrossRef CAS PubMed.
- C. G. Cummings and A. D. Hamilton, Curr. Opin. Chem. Biol., 2010, 14, 341–346 CrossRef CAS PubMed.
- V. Azzarito, K. Long, N. S. Murphy and A. J. Wilson, Nat. Chem., 2013, 5, 161–173 CrossRef CAS PubMed.
- H. Moon and H.-S. Lim, Curr. Opin. Chem. Biol., 2015, 24, 38–47 CrossRef CAS PubMed.
- M. Pelay-Gimeno, A. Glas, O. Koch and T. N. Grossmann, Angew. Chem., Int. Ed., 2015, 54, 8896–8927 CrossRef CAS PubMed.
- A. J. Wilson, Prog. Biophys. Mol. Biol., 2015, 119, 33–40 CrossRef CAS PubMed.
- A. Barnard, K. Long, H. L. Martin, J. A. Miles, T. A. Edwards, D. C. Tomlinson, A. Macdonald and A. J. Wilson, Angew. Chem., Int. Ed., 2015, 54, 2960–2965 CrossRef CAS PubMed.
- V. Azzarito, J. A. Miles, J. Fisher, T. A. Edwards, S. Warriner and A. Wilson, Chem. Sci., 2015, 6, 2434–2443 RSC.
- A. Kazi, J. Sun, K. Doi, S.-S. Sung, Y. Takahashi, H. Yin, J. M. Rodriguez, J. Becerril, N. Berndt, A. D. Hamilton, H.-G. Wang and S. d. M. Sebti, J. Biol. Chem., 2011, 286, 9382–9392 CrossRef CAS PubMed.
- M. Oh, J. H. Lee, W. Wang, H. S. Lee, W. S. Lee, C. Burlak, W. Im, Q. Q. Hoang and H.-S. Lim, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 11007–11012 CrossRef CAS PubMed.
- B. B. Lao, I. Grishagin, H. Mesallati, T. F. Brewer, B. Z. Olenyuk and P. S. Arora, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 7531–7536 CrossRef CAS PubMed.
- M. K. P. Jayatunga, S. Thompson and A. D. Hamilton, Bioorg. Med. Chem. Lett., 2014, 24, 717–724 CrossRef CAS PubMed.
- S. A. Dames, M. Martinez-Yamout, R. N. De Guzman, H. J. Dyson and P. E. Wright, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 5271–5276 CrossRef CAS PubMed.
- S. J. Freedman, Z.-Y. J. Sun, F. Poy, A. L. Kung, D. M. Livingston, G. Wagner and M. J. Eck, Proc. Natl. Acad. Sci. U. S. A., 2002, 99, 5367–5372 CrossRef CAS PubMed.
- H. F. Kyle, K. F. Wickson, J. Stott, G. M. Burslem, A. L. Breeze, C. Tiede, D. C. Tomlinson, S. L. Warriner, A. Nelson, A. J. Wilson and T. A. Edwards, Mol. BioSyst., 2015, 11, 2738–2749 RSC.
- G. M. Burslem, H. F. Kyle, A. L. Breeze, T. A. Edwards, A. Nelson, S. L. Warriner and A. J. Wilson, ChemBioChem, 2014, 15, 1083–1087 CrossRef CAS PubMed.
- N. Murphy, P. Prabhakaran, V. Azzarito, J. Plante, M. Hardie, C. Kilner, S. Warriner and A. Wilson, Chem. – Eur. J., 2013, 19, 5546–5550 CrossRef CAS PubMed.
- M. Honda, H. Morita and I. Nagakura, J. Org. Chem., 1997, 62, 8932–8936 CrossRef CAS.
- H. Cho, D.-R. Ahn, H. Park and E. G. Yang, FEBS Lett., 2007, 581, 1542–1548 CrossRef CAS PubMed.
- M. M. Hann, MedChemComm, 2011, 2, 349–355 RSC.
- C. García-Echeverría, P. Chène, M. J. J. Blommers and P. Furet, J. Med. Chem., 2000, 43, 3205–3208 CrossRef.
Footnotes |
| † Electronic supplementary information (ESI) available: Detailed experimental procedures and characterisation; additional biophysical data. See DOI: 10.1039/c6cc01812b |
| ‡ Current address: Yale University, New Haven, CT 06520, USA. |
| This journal is © The Royal Society of Chemistry 2016 |