Geochemical behaviour of palladium in soils and Pd/PdO model substances in the presence of the organic complexing agents L-methionine and citric acid
Fathi
Zereini
a,
Clare L. S.
Wiseman
*bc,
My
Vang
a,
Peter
Albers
d,
Wolfgang
Schneider
e,
Roland
Schindl
f and
Kerstin
Leopold
f
aInstitute for Atmospheric and Environmental Sciences, Department of Environmental Analytical Chemistry, Goethe University Frankfurt am Main, Frankfurt am Main, Germany
bSchool of the Environment, Earth Sciences Centre, University of Toronto, Rm. 1016V, 33 Willcocks St., Toronto, Ontario, Canada M5S 3E8. E-mail: clare.wiseman@utoronto.ca
cDalla Lana School of Public Health, University of Toronto, Toronto, Canada
dAQura GmbH, Hanau-Wolfgang, Germany
eUmicore AG & Co. KG, Hanau-Wolfgang, Germany
fInstitute for Analytical and Bioanalytical Chemistry, University of Ulm, Ulm, Germany
First published on 27th November 2015
Abstract
Risk assessments of platinum group metal (PGE) emissions, notably those of platinum (Pt), palladium (Pd) and rhodium (Rh), have been mostly based on data regarding the metallic forms used in vehicular exhaust converters, known to be virtually biologically inert and immobile. To adequately assess the potential impacts of PGE, however, data on the chemical behaviour of these metals under ambient conditions post-emission is needed. Complexing agents with a high affinity for metals in the environment are hypothesized to contribute to an increased bioaccessibility of PGE. The purpose of this study is to examine the modulating effects of the organic complexing agents, L-methionine and citric acid, on the geochemical behavior of Pd in soils and model substances (Pd black and PdO). Batch experimental tests were conducted with soils and model substances to examine the impacts of the concentration of complexing agents, pH and length of extraction period on Pd solubility and its chemical transformation. Particle surface chemistry was examined using X-ray photoelectron spectroscopy (XPS) on samples treated with solutions under various conditions, including low and high O2 levels. Pd was observed to be more soluble in the presence of organic complexing agents, compared to Pt and Rh. Pd in soils was more readily solubilized with organic complexing agents compared to the model substances. After 7 days of extraction, L-methionine (0.1 M) treated soil and Pd black samples, for instance, had mean soluble Pd fractions of 12.4 ± 5.9% and 0.554 ± 0.024%, respectively. Surface chemistry analyses (XPS) confirmed the oxidation of metallic Pd surfaces when treated with organic complexing agents. The type of organic complexing agent used for experimental purposes was observed to be the most important factor influencing solubility, followed by solution pH and time of extraction. The results demonstrate that metallic Pd can be transformed into more bioaccessible species in the presence of organic complexing agents which are ubiquitous in the environment.
Environmental impactEnvironmental concentrations of palladium (Pd) appear to be increasing at a greater rate relative to the other platinum group elements, platinum (Pt) and rhodium (Rh), used in the control of vehicular emissions. This study examines the effects of the organic complexing agents, L-methionine and citric acid, on the geochemical behavior of Pd in soils and model substances. The results confirm that Pd in soils readily complexes with organic chelating agents. The outer atomic surfaces of Pd model substances were also observed to be partially oxidized when treated with L-methionine and citric acid. The type of organic complexing agent used for experimental purposes was determined to be the most important factor influencing solubility, followed by solution pH and time of extraction. |
A Introduction
Environmental concentrations of platinum (Pt), palladium (Pd) and rhodium (Rh), which are used as exhaust catalysts to control exhaust emissions, have been steadily increasing since automotive catalytic converters came into widespread use in most industrialized countries in the 1970s and 1980s.1–10 Originally, Pt emissions drew the most attention, as it tended to occur in higher concentrations relative to the other platinum group elements (PGE). Since the 1990s, a shift in the use of Pd over Pt as the active catalyst in automotive catalytic converters has taken place, however, resulting in relative increases of this noble metal in the environment. In recent years, studies have demonstrated that Pd is now occurring at higher concentrations in environmental media such as soils and dust in various countries such as the US,11 Germany,2 Bulgaria,12 Brazil13 and Canada.14 Increased emissions of Pd as a function of time have also been demonstrated by Sievers and Schuster15 in their examination of tunnel dust. It is often assumed that PGE are likely to have a low solubility and mobility post-emission into the environment, as they are used in a metallic, inert form in exhaust converters. Several studies have observed, however, that PGE are soluble in environmental media and bioaccessible to a variety of terrestrial and aquatic organisms.12,16–21 In particular, Pd has been demonstrated to be more mobile and readily bioaccessible compared to the other PGE.12,22–25While gaps in knowledge continue to exist regarding the chemical behaviour of PGE post-emission, several studies have shown that PGE can be transformed into more reactive species in the presence of various ions and common organic complexing agents.26–40 For instance, Poprizki38 reported an increased solubility of Pd in the presence of Cl− compared to NO3− and SO42−. Zereini et al.40 confirmed the influence of these anionic species on Pt using a platinum/aluminium model substance. Pd has also been shown to form mobile complexes in various Cl− rich solutions and in the presence of O2.33,44 Bruder et al.39 measured a higher solubility for PGE associated with urban airborne PM (PM10) extracted with the organic complexing agents L-methionine and ethylenediaminetetraacetic acid (EDTA). Zereini et al.35 also observed that metallic Pd in a model substance can be partially oxidized and transformed to PdOx (x < 1) when treated with EDTA in solution, using a combination of X-ray Photoelectron Spectroscopy (XPS) and Transition Electron Microscopy/Energy Dispersive X-ray Spectrometry (TEM/EDX) techniques. Physiologically based extraction experiments provide further support for an increased solubility of PGE when exposed to simulated gastrointestinal and lung fluids.33,34,41,42
Overall, the results of various studies suggest that PGE are capable of being transformed into more mobile and bioaccessible species under ambient environmental conditions. Most studies have, however, conducted their experiments using commercially available model substances, standard reference materials and/or automotive catalyst material. Little data is available on the chemical behaviour of PGE post-emission under field conditions. As such, this highlights a need for experimental studies using field collected samples, to validate the existing findings of published studies. In light of this, the purpose of this study is to examine the chemical behaviour and transformation of Pd in field-collected soils treated with organic complexing agents, L-methionine and citric acid, employing isotope dilution (ID) ICP-Q-MS and XPS. L-Methionine is an essential amino acid and plays an important role in plant metabolism.39 Citric acid is a low molecular weight organic acid commonly present in plants and can be released in significant quantities in soils via root systems.43 For comparative purposes, chemical changes in the model substances Pd black and PdO are studied under the same experimental conditions. Palladium is the focus for two reasons: (1) available data on the chemical behaviour of this element in the environment is very limited, and (2) increases in the environmental concentrations of Pd have been shown to be greater relative to that of Pt in recent years.2,11–15
B Experimental
Materials
Soil samples (n = 6) were collected directly along the Autobahn A5 close to Frankfurt am Main, Germany, at depths of 0–3 cm. The A5 is an 8 lane high traffic volume road with ca. 110![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 to 131
000 to 131![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 vehicles per day (data from 2004, HSVV-Hessen). Two model Pd substances were used for experimental purposes: (1) Pd black, which is chemically similar to the bulk of the catalyst material used in exhaust converters, and (2) PdO, which is also present in catalytic converters. Pd black (99.36% metallic Pd powder (Umicore)) has a specific surface area of 20 m2 g−1 and a mean particle size ca. 22.23 μm (±31.25 μm).35 PdO (99.9% in powder form (Aldrich, 520748-5G)), is finer, with a mean particle size of 4.49 μm (±5.88 μm).35 Two organic complexing agents with a high affinity for metals, which are commonly present in the environment, were used for experimental purposes: (1) L-methionine (Merck), and (2) citric acid (neo Lab).
000 vehicles per day (data from 2004, HSVV-Hessen). Two model Pd substances were used for experimental purposes: (1) Pd black, which is chemically similar to the bulk of the catalyst material used in exhaust converters, and (2) PdO, which is also present in catalytic converters. Pd black (99.36% metallic Pd powder (Umicore)) has a specific surface area of 20 m2 g−1 and a mean particle size ca. 22.23 μm (±31.25 μm).35 PdO (99.9% in powder form (Aldrich, 520748-5G)), is finer, with a mean particle size of 4.49 μm (±5.88 μm).35 Two organic complexing agents with a high affinity for metals, which are commonly present in the environment, were used for experimental purposes: (1) L-methionine (Merck), and (2) citric acid (neo Lab).
Sample extraction and dissolution testing
Soil samples were air-dried, sieved to <2 mm and ground using an agate mill. About 1 g of sample material was transferred to dark brown, polyethylene bottles with 40 ml of 0.1 M L-methionine. The same was done with samples extracted with 0.1 M citric acid solutions. Extractions were done in triplicate with each respective solution. Samples were placed on a horizontal mixer (Laboshake RO 300/16 (Gerhardt)) and alternately shaken for 15 minutes followed by 30 minutes rest for a total of 6 days at room temperature (23–25 °C). Samples were then filtered using cellulose-acetate membrane filters (pore size 0.2 μm (Sartorius AG)) to separate the solid from the liquid phase. Solid phase soil samples were digested in a microwave (MARS Xpress of CEM GmbH) with aqua regia (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 ratio of HCl to HNO3 (Suprapur quality, Merck)) for the determination of total Pd concentrations.
3 ratio of HCl to HNO3 (Suprapur quality, Merck)) for the determination of total Pd concentrations.
All samples (solid and liquid phase) were co-precipitated with Te according to the German Institute for Standardization's method DIN 19741 to minimize molecular interferences in measuring Pd. Sample concentrations were measured using isotope dilution ICP-Q-MS (Varian 820-MS) in collision mode with He. Platinum and Rh concentrations were also measured in soil samples for comparison. Pd concentrations were calculated based on results for the isotope ratios 105Pd/106Pd, 105Pd/108Pd and 105Pd/110Pd. The isotope ratios 194Pt/198Pt, 195Pt/198Pt and 196Pt/198Pt were used to determine Pt levels, while Rh concentrations were calculated based on the 103Rh signal. The following instrumental set-up was used: plasma flow 17.7 L min−1, sheath gas flow 0.25 L min−1, auxiliary flow 1.68 L m−1, nebulizer flow 0.94 L min−1, ICP RF power 1.40 kW, He gas flow 120 ml min−1.
Dissolution tests were also conducted with Pd and PdO model substances and the same organic complexing agents, citric acid and L-methionine, in separate batch experiments to assess the potential influence of solution pH, reaction time and ionic concentration on Pd solubility. Three different batch experiments were carried out in parallel with the respective extract solutions under the following conditions: (1) samples treated over a 6 day period with L-methionine and citric acid solutions of variable concentration (0.001, 0.01 and 0.1 M) at a constant pH (5.9 ± 0.3 (L-methionine) and 2.2 ± 0.3 (citric acid)) and dissolved O2 concentration (8.4 ± 0.03 mg L−1 (L-methionine) and 8.3 ± 0.02 mg L−1 (citric acid)), (2) samples extracted for 6 days with the respective complexing agents at variable pH levels (pH 6, 7, 8 and 9 for L-methionine and pH 2, 5, 7 and 9 for citric acid, adjusted with the addition of NaOH) but at the same solution concentration of 0.1 M for both L-methionine and citric acid solutions, and (3) samples treated with a solution concentration of 0.1 M for both L-methionine and citric acid at pH of 7 until chemical equilibrium was reached. To determine this, sample aliquots were taken after the following time intervals: 6 hours and 7, 14, 21, 42, 72, 100, 147, 190, 208 and 251 days. In addition to the batch experiments with organic complexing agents, the model substances were extracted with distilled water and NaClO4 (0.1 M, pH 7) for comparison. Relatively high concentrations of L-methionine and citric acid were used for the extractions to effectively determine the effects of complexing agents on the solubility and chemical transformation of Pd. For each experiment, three parallel samples of the respective model substances (60 mg) were treated with 100 ml of solution at room temperature (23–25 °C). Samples were alternately shaken for 15 min followed by 30 min rest during the reaction period. Following the extraction periods, samples were left undisturbed for 18 hours to settle. Sample aliquots of 50 to 100 μl were then transferred to centrifuge tubes, diluted to 10 ml with 0.5% HNO3 and centrifuged for 15 min at 3500 RPM (Heraeus, Megafuge) prior to measurement.
The soluble Pd fraction was determined using isotope dilution ICP-Q-MS in collision mode with He using the same instrumental settings as described previously. It was not necessary to isolate and pre-enrich Pd in the sample extracts, as Pd is present at higher concentrations in the model substances and in a form with minimal matrix interferences.35
Particle surface chemistry analysis
Pd black samples (30 mg) were weighed and placed in capped, dark brown polyethylene bottles with 20 ml of the respective organic complexing agents, L-methionine solution and citric acid, at solution strengths of 0.1 M. Samples were alternately shaken on a horizontal mixer for 15 minutes, followed by 30 minutes rest, over two different time periods of 6 and 251 days.The solid (residue) phase was separated from the liquid phase of treated samples using a blue ribbon filter for surface chemistry analyses. Particle surface chemistry was analysed using XPS (ESCALAB 250 Xi (Thermo Fisher Scientific)) to detect chemical changes following the respective treatment periods. For this, samples were placed on a tantalum sheet, introduced into the high vacuum pre-chamber of the XPS spectrometer system and pumped down at room temperature prior to analysis. The outer ca. 3–4 atomic layers of each single sample were analysed using targeted monochromatic X-ray radiation (X-ray spot diameter: 900 μm) and measuring the kinetic energy of the photo-emitted electrons.35 Measurements were carried out at Aqura GmbH, Hanau, Germany. More detailed information regarding XPS analysis can be found in Reniers and Tewell45 and Powell & Jablonski.46
Parallel Pd black samples were also treated with L-methionine and citric acid solutions (0.1 M) under both O2-rich and O2-depleted conditions in a separate experiment prior analysis using XPS. This was done to assess differences in the chemical transformation of Pd under aerobic and anaerobic conditions in aquatic systems. For this, Pd black samples (30 mg) were placed in capped polyethylene bottles with 50 ml of each respective organic complexing solution. Two different experiments were conducted with the respective samples: (1) one where O2 was added to sample solutions (8.6 mg O2 per L), and (2) another with O2 depleted solutions (1.8 mg O2 per L), achieved by pumping N2 into the samples. Samples were shaken every 15 minutes, followed by 30 minutes rest, for 6 days. Following the reaction period, sample residues were isolated from solutions and analysed per XPS, as described above.
Samples treated under low O2 conditions were prepared for XPS measurement in a glove box with N2, while those prepared with higher O2 levels were examined under ambient conditions. Pd concentrations in sample solutions were determined using ID-ICP-Q-MS. Pd black in untreated, powder form was measured via XPS for control purposes.
Quality assurance and control
A number of steps were taken to ensure quality assurance and control of sample preparation and analysis, also described in Zereini et al.2,35 Briefly, lab ware was pre-cleaned in acid baths, followed by rinsing several times with purified water. All chemicals used were of the highest grade (i.e. Suprapur quality, Merck). The analytical methods employed are internationally recognized and have been validated by rigorous testing methods (e.g. Round Robin testing).2,34,47–50The following Pd isotopes were measured: 105Pd, 106Pd, 108Pd and 110Pd. The internal standards, 115In and 169Tm (Merck), were used for quality assurance and control.
Pd was measured in blanks of 0.5% HNO3 (mean concentration: 0.021 ± 0.012 μg L−1), 0.1 M citric acid (mean concentration: 0.007 ± 0.002 μg L−1) and 0.1 M L-methionine (mean concentration: 0.144 ± 0.042 μg L−1). Pd standard solutions (certified level: 5 μg L−1 Pd) were also measured to validate instrumental accuracy (measured mean levels: 4.9 ± 0.2 μg L−1). The limit of detection (LOD), determined as 3 times the standard deviation of blanks, was 0.036 μg L−1. This compares to the LOD given in the German Institute of Standardization's method, DIN 323645, of 0.028 μg L−1.50
XPS particle surface chemistry analyses were validated using reference data bases (certified values: Pd 3d5/2 (binding energy in eV): Pd (334.9 eV), PdO (336.3 eV), PdO2 (337.9 eV), PdSO4 (338.7 eV) and Pd(CN)2 (339.2 eV)). The binding energy scale for Au 4f7/2 (84.00 eV) was used for calibration.35 The LOD was 0.1 atom%.
C Results and discussion
Pd concentrations and behaviour in soils
The mean concentration of Pd in soil samples was 210 μg kg−1 and ranged between 101 and 290 μg kg−1. Mean levels of Pt and Rh were 14 and 4.4 μg kg−1, respectively (min/max: 2.7/37 μg Pt per kg and 2.5/6.4 μg Rh per kg). The results support recent studies which have observed a shift toward higher concentrations of Pd over Pt in various environmental media.2,12,14 Determined Pd concentrations are 20 times higher compared to levels measured in soils sampled in 1994 (mean: 8.9 μg kg−1) along the same stretch of Autobahn close to Frankfurt am Main.3 Soil samples also collected from this site in 2004 had mean Pd levels of 83 μg kg−1, representing an average 2.5-fold increase in Pd concentrations in the last 10 years.This trend of increasing concentrations of Pd in soils over time can be attributed to the greater use of this PGE in automotive catalytic converters, including that for diesel-run engines.51 Similar trends have also been reported by Sievers and Schuster15 in their examination of Pd in tunnel dust over time.
Relatively high soluble Pd fractions were measured for soil samples treated with organic complexing agents, most notably with L-methionine. Soils extracted with 0.1 M L-methionine had a measured mean soluble Pd fraction of 12.4 ± 5.9%. Pt and Rh solubility was lower, with means of 5.1 ± 4.5% for Pt and 1.4 ± 1.2% for Rh. Soils treated with citric acid had comparatively lower soluble fractions of PGE, with mean levels of 7.3 ± 4.3% for Pd, 3.5 ± 0.77% for Pt and 1.5 ± 1.2% for Rh (Fig. 1). Pd solubility was twice as high in samples treated with L-methionine, despite the lower pH of the citric acid solution (pH 2 vs. pH 6).
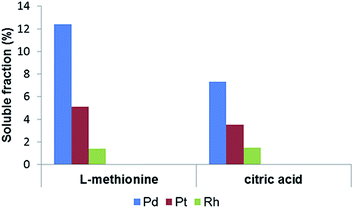 | ||
| Fig. 1 Mean Pd, Pt and Rh solubility (%) in soils treated with 0.1 M L-methionine (pH 6) and 0.1 M citric acid (pH 2) for 6 days. | ||
The higher solubility of Pd relative to Pt and Rh highlight a need to consider the human and environmental health implications of exposures to this metal, especially in consideration of recent findings demonstrating increases in the environmental concentrations of Pd.2,11–15
Pd concentrations and behaviour in model substances
The solubility of model substances treated with the organic complexing agents was found to be considerably lower compared to soils. For metallic Pd (Pd black) treated with 0.1 M L-methionine at pH 7, the mean solubility of Pd was 0.20% (min/max: 0.18/0.21%) after a 6 day extraction period. The soluble fraction in Pd black treated with 0.1 M citric acid averaged 0.01% (min/max: 0.005–0.007%), about 120 times lower than that determined for Pd in soils extracted with this organic complexing agent. The differences in measured soluble fractions between soils and Pd black could be partially due to the particle size of the media examined. Pd black has a mean particle size of ca. 22.23 μm (±13.25 μm).35 Pd emitted from exhaust converters can be emitted as particles of various sizes together with other PGE, including particles in the nano range which would be expected to be more soluble. A size-dependent solubility of Pd-containing particles has been confirmed elsewhere.33,34,39 Pd black is in metallic form, similar to the mostly insoluble catalyst material used in exhaust converters.52 In light of the results, the presence of a more reactive and soluble chemical Pd species in soils, formed post-emission in the environment, is likely. Pd in airborne PM collected in Vienna was also observed to be significantly more soluble than ground catalyst material using a physiologically-based extraction test,42 which lends support to the hypothesis that PGE are likely to be chemically transformed following emission.Pd solubility was also observed to be influenced by the concentration of organic solutions used, solution pH and time of extraction. At a constant pH of 5.9 ± 0.3, the solubility of Pd black increased with the concentration of the L-methionine and citric acid solutions used (0.01, 0.05 and 0.1 M) (Fig. 2). Mean soluble fractions were 0.280 ± 0.009%, 0.352 ± 0.005% and 0.554 ± 0.024% for 0.01, 0.05 and 0.1 M L-methionine solutions, respectively. For citric acid, mean Pd solubility was 0.032 ± 0.003% (0.01 M), 0.068 ± 0.005% (0.05 M) and 0.097 ± 0.004% (0.1 M).
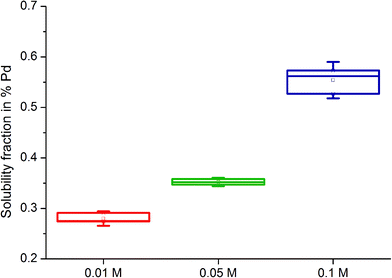 | ||
| Fig. 2 Box and whiskers plot of Pd black solubility (%) following treatment with L-methionine solutions (0.01 M, 0.05 M and 0.1 M) of pH 5.9 ± 0.3 (8.4 ± 0.03 mg O2 per L) after 21 days. | ||
To compare, Pd black treated with distilled water was determined to have a mean solubility of 0.0003 ± 0.0002% (for PdO: 0.00002 ± 0.000015%), which was comparatively much lower. This provides further support that the presence of organic complexing agents contributes to an increased solubility of metallic Pd in the model substance.
PdO was observed to have a lower solubility than Pd black under the various conditions examined in this study, with L-methionine and citric acid having little influence on PdO model substance solubility. This observation was similar to that observed by Zereini et al.35 in an earlier study employing EDTA solutions of variable strength. PdO samples treated with L-methionine solutions had measured soluble Pd fractions of 0.015 ± 0.001% (0.01 M), 0.017 ± 0.001% (0.05 M) and 0.020 ± 0.001% (0.1 M). Solubility was even less for citric acid solutions, with measured soluble fractions of <0.0002%.
The pH of organic solutions also influenced the solubility of model substances, with increasing amounts of Pd detected in solutions at lower pH levels. In Pd black samples treated with 0.1 M L-methionine, the highest soluble fraction was measured at pH 6, with a mean of 0.540 ± 0.055%. Solubility then decreased with increases in alkalinity, with means of 0.363 ± 0.023% (pH 7), 0.319 ± 0.014% (pH 8) and 0.286 ± 0.007% (pH 9) (Fig. 3). Similarly, PdO was slightly more soluble at a lower pH when treated with citric acid, with mean soluble fractions of 0.018 ± 0.001% (pH 6), 0.015 ± 0.001% (pH 7), 0.014 ± 0.002% (pH 8) and 0.008 ± 0.0012% (pH 9). Of particular relevance to Pd solubility in field soils are the results for samples treated with solutions having a neutral pH (pH 7), which falls in the typical pH range of most soils. The soils collected along the German Autobahn, for instance, had a pH of 6.7 ± 0.15.
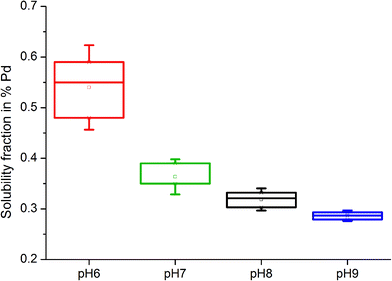 | ||
| Fig. 3 Box and whiskers plot of Pd black solubility (%) following treatment with 0.1 M L-methionine solutions of variable pH (6, 7, 8 and 9) after 21 days. | ||
In addition to the concentration and pH of organic solutions, length of extraction period was observed to modulate model substance solubility. Most notably, the solubility of Pd black treated with L-methionine (0.1 M, pH 7) in the presence of NaClO4 (0.1 M) steadily increased over time during the course of the extraction period. After 251 days, approximately 90% of the total metallic Pd present in Pd black was measured in the soluble fraction. L-Methionine was also more effective in solubilizing Pd black compared to EDTA solutions used in an earlier study with the same model substance.35 Pd black extracted with citric acid had a soluble fraction of 0.0012% after 6 hours. This increased to 0.0057% after 7 days. Pd black solubility stagnated somewhat thereafter but increased again after 64 days, suggesting a time-dependent biphasic trend in solubility with citric acid.
Similarly, PdO was not that soluble in citric acid, with a soluble fraction of about 0.002% after 7 days. With NaClO4 (0.1 M, pH 7, no complexing agents), Pd black and PdO had soluble fractions of 0.0013 ± 0.0006% and <0.0001%, respectively, after 7 days. This demonstrates that the organic complexing agents are more effective in inducing Pd solubility.
Metallic Pd black had the highest observed solubility in L-methionine, followed by treatment with EDTA35 and citric acid, in solutions with similar concentrations (0.1 M), pH levels (7.1 ± 0.15) and dissolved O2 contents (8.33 mg L−1 ± 0.14) (Fig. 4). Similarly, Poprizki38 also reported a low solubility (<0.001%) for metallic Pd when treated with ionic solutions containing anions of Cl−, NO3−, SO42−, and PO43−.
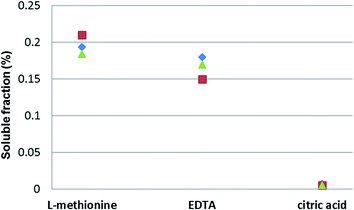 | ||
| Fig. 4 Solubility (%) of Pd black in 0.1 M L-methionine, 0.1 M ethylenediamine tetra acetic acid (EDTA)35 and 0.1 M citric acid solutions (pH 7) after 21 days (individual samples depicted). | ||
Pd black kinetics
The thermodynamics of the model substances were modelled using data from the long term solubility studies, similar to that done in the Zereini et al. study35 using data obtained from experimental studies with EDTA. Applying the slope of the linear regression, an initial reaction rate, r, of 8.08 ± 0.29 × 10−12 mol L−1 s−1 was calculated for a reaction period of 1 to 21 days for L-methionine. The dissolution rate, R, normalized to the mass (0.501 g) and specific surface area (20 m2 g−1) determined for Pd black35 was estimated to be 2.90 ± 0.11 nmol m−2 h−1. The R for citric acid, calculated in the same way as for L-methionine (reaction period of 1 to 24 days), was estimated to be 0.05 ± 0.01 nmol m−2 h−1, which is two orders of magnitude lower. Despite its lower pH, the R for citric acid is also two orders of magnitude lower than that calculated in earlier study for Pd black exposed to equimolar concentrations of EDTA (R = 2.01 ± 0.17 nmol m−2 h−1).35 Dahlheimer et al.32 estimated an R of 4.26 nmol m−2 h−1 for elemental Pd treated with a synthetic siderophore desferrioxamine-B (DFO-B). The results suggest that the type of organic complexing agent used is a more important modulating factor influencing elemental solubility than solution pH.Surface particle chemistry of model substances
The surface regions of untreated Pd black particles, examined using XPS, are comprised mainly of metallic Pd (49.0%, binding energy (BE): 334.8 eV) and Pd(II) (39.1%, BE: 336.0 eV).35 Pd is also present in a higher oxidized form but in comparatively small amounts, with 11.9% (BE: 336.8 eV). The outer atomic regions of Pd reference materials are dominated by Pd(0) (BE: 334.9 eV), Pd(II) (BE: 336.3 eV) and Pd(IV) (BE: 337.9 eV) (Pd(IV)).35The particle surface chemistry of Pd black changed little when treated with a solution consisting of distilled water only, with binding energies primarily measured in the range of 334.7 eV and 335.9 eV. In contrast, a weakly oxidized Pd species was detected on the surface of Pd black residues (solid phase) when treated with L-methionine (44.1 atom%, BE: 335.0 eV) (Table 1). Significant amounts of Pd(II) were also detected (33.8 atom%, BE: 336.3 eV). In addition, Pd(IV) was present in the amount of ca. 9.2 atom% (BE: 337.8 eV). In sample extracts (liquid phase) of Pd black treated with L-methionine, Pd was observed to have a higher oxidative status compared to that determined for sample residues. Pd in solution was determined to be primarily present as Pd(II) (54.7 atom%) and Pd(IV) (32.5 atom%), with binding energies of 336.0 eV and 337.1 eV, respectively.
| Element | Pd black (99.36% Pd) | Pd/L-methionine (R) (6 days) | Pd/L-methionine (S) (6 days) | Pd/L-methionine (R) (251 days) | Pd/L-methionine (S) (251 days) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| eV | Atom% | eV | Atom% | eV | Atom% | eV | Atom% | eV | Atom% | |
| C 1s | 284.1 | 57.1 | 283.2 | 5.6 | 283.1 | 13.2 | 284.2 | 48.7 | 283.4 | 6.7 |
| 285.4 | 19.1 | 284.1 | 28.8 | 284.0 | 25.8 | 285.3 | 35.3 | 284.2 | 34.7 | |
| 287.3 | 16.6 | 284.9 | 32.7 | 284.9 | 38.8 | 287.2 | 8.2 | 285.0 | 46.0 | |
| 289.3 | 7.2 | 285.9 | 16.6 | 286.4 | 14.2 | 288.2 | 7.7 | 286.2 | 10.1 | |
| 287.5 | 16.4 | 287.9 | 8.0 | 288.2 | 2.6 | |||||
| Cl 2p3/2 | — | — | 198.3 | 90.2 | — | — | 198.8 | 83.5 | ||
| 200.3 | 9.8 | 200.5 | 16.5 | |||||||
| N 1s | — | — | 395.2 | 4.7 | 398.0 | 10.8 | 398.4 | 13.3 | 297.9 | 9.9 |
| 397.5 | 20.1 | 398.4 | 43.1 | 399.4 | 45.0 | 399.1 | 49.2 | |||
| 399.1 | 65.9 | 399.7 | 39.2 | 400.4 | 26.6 | 400.2 | 34.0 | |||
| 400.6 | 9.3 | 401.2 | 6.8 | 401.5 | 15.0 | 401.6 | 6.9 | |||
| Na 1s | — | — | — | — | — | — | 1071.9 | 100 | — | — |
| — | — | |||||||||
| O 1s | 527.1 | 3.6 | 526.3 | 1.4 | 529.7 | 20.5 | 530.5 | 10.8 | 530.0 | 1.8 |
| 529.4 | 71.3 | 526.4 | 3.5 | 531.1 | 39.9 | 531.9 | 52.4 | 532.0 | 74.4 | |
| 531.6 | 25.2 | 530.0 | 95.1 | 532.4 | 39.7 | 533.4 | 36.8 | 533.0 | 23.7 | |
| Pd 3d5/2 | 334.8 | 49.0 | 334.4 | 12.8 | 334.6 | 12.7 | 335.9 | 12.8 | 336.1 | 9.0 |
| 336.0 | 39.1 | 335.0 | 44.1 | 336.0 | 54.7 | 337.4 | 70.7 | 337.1 | 35.4 | |
| 336.8 | 11.9 | 336.3 | 33.8 | 337.1 | 32.5 | 338.4 | 16.5 | 338.0 | 55.7 | |
| 337.8 | 9.2 | |||||||||
The degree of oxidation on metallic Pd black surfaces treated with L-methionine was observed to be time dependent, with higher amounts occurring at the end of the long term experiments (251 days). Oxidized forms of Pd species lie in the range between Pd(II) and Pd(IV) (Fig. 5). About 17 atom% of the Pd in Pd black residues (solid phase) was observed to have a BE higher than 338.4, suggestive of a higher oxidation status (Table 1). Most Pd in Pd black residues was present as Pd(IV), with 70.7 atom% (BE: 337.4 eV), after 251 days. Lower amounts of Pd(II) are present, with 12.8 atom% (BE: 335.9 eV). High binding energies were also detected in sample solutions (liquid phase) after 251 days, with BE's of 337.1 eV (35.4 atom%) and 338 eV (55.7 atom%). Clearly, the results show that longer time periods are conducive to the formation of Pd/L-methionine complexes.
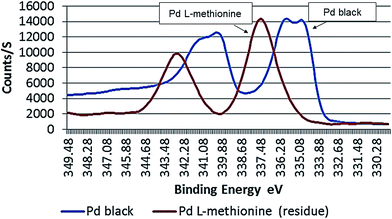 | ||
| Fig. 5 Binding energy (eV) of Pd black surfaces (outer 3–4 atomic layers) in untreated and L-methionine treated samples (long term test: 251 days). | ||
Compared to EDTA,35 treatment with L-methionine results in a greater shift in the binding energy of the outer 3–4 atomic shells of Pd in the model substance. A maximum signal on the Pd 3d5/2 peak of L-methionine treated Pd black was stronger compared to that determined for samples treated with EDTA (337.4 eV vs. 335.3 eV). The stronger signals detected in this range are suggestive of a greater degree of oxidation on the outer surfaces of Pd black. This may also reflect the formation of strong coordination complexes with ligands that serve as electron donors (e.g. S-containing ligands, amino acid and carbonyl groups).
In addition, the binding energy and concentration (atom%) of Cl (Cl 2p), C (C 1s), Na (Na 1s), N (N 1s) and O (O 1s) was examined on the particle surfaces of untreated and treated Pd black. These elements were examined in the residue (solid) and solution (liquid phase) of samples using XPS to obtain further information regarding the presence and formation of Pd/L-methionine complexes. The results yielded a broad spectrum for N 1s. Unprotonated amino functional N dominated (45 atom%), followed by ammonium-N (26.6 atom%) and a weakly oxidized N-species with 15 atom%. The determined binding energy of S indicated the presence of sulfane-S (69.3%), sulfide-S (17.1 atom%) and sulfate/sulfonate-S (13.6 atom%). Aliphatic C was mainly detected, with C–OOH functional groups, from the methionine (Table 1). Oxygen was present mainly as carbonyl and/or hydroxyl groups. The results for N2, O2, S and C were similar for sample solutions. The presence of these substances in both sample residues and solutions of Pd black treated with L-methionine is an indication that Pd/L-methionine complexes are formed. The importance of amino groups (NH2) and S in forming strong bonds with Pd was illustrated in an earlier study of the crystal structure of Pd(II) (L-methionine)Cl2-complexes.53 Another study of Pd(II) and methionine sulfoxide showed that bonds can be formed with Pd via carboxylate and amino groups.54 The production and application of various commercially produced Pd/methionine compounds for use in cancer chemotherapy are further examples of the importance of such complexes.31,55–58
Compared to L-methionine, citric acid was less effective in transforming the surface chemistry of metallic Pd black, with oxidation occurring only in minimal amounts. Following treatment with citric acid, about 35.3 atom% of Pd (residue) remained in metallic form (BE: 334.8). Circa 29 atom% of Pd (residue) was determined to be weakly oxidized (BE: 335.7 eV). An approximate 28 atom% of the total was present in an oxidized form with a higher binding energy in Pd residue. Similar results were observed for sample solutions (Table 2).
| Element | Pd/citric acid (R) | Pd/citric acid (S) | ||
|---|---|---|---|---|
| eV | Atom% | eV | Atom% | |
| C 1s | 284.4 | 30.5 | 284.1 | 14.8 |
| 285.5 | 17.3 | 284.8 | 33.0 | |
| 287.0 | 16.7 | 286.1 | 8.6 | |
| 288.5 | 34.5 | 286.9 | 8.3 | |
| 290.6 | 1.0 | 288.7 | 35.3 | |
| O 1s | 529.9 | 33.5 | 530.4 | 7.6 |
| 531.6 | 63.5 | 531.5 | 62.5 | |
| 532.7 | 29.8 | |||
| Pd 3d5/2 | 334.8 | 35.3 | 334.4 | 26.1 |
| 335.7 | 29.2 | 335.0 | 41.2 | |
| 336.9 | 28.1 | 336.3 | 20.9 | |
| 338.4 | 7.4 | 337.8 | 11.8 | |
Overall, the results demonstrate the L-methionine is highly effective in transforming/complexing metallic Pd to Pd(II) and Pd(IV), while citric acid does not appear to readily complex with Pd black. This supports the results for soil samples, which showed that Pd present in naturally-occurring soils is readily solubilized in the presence of L-methionine, forming new organic-Pd complexes that are likely to be more bio-accessible.
Pd solubility and transformation as a function of dissolved O2 content
A signal maximum of ca. 335 eV (44.1 atom%) in the outer atomic layers was detected for metallic Pd sample residues treated with O2-enriched L-methionine solutions. Surprisingly, a higher binding energy value was detected in the sample treated with a reduced O2 content (1.6 mg L−1) (BE: 335.9 eV) (50.3 atom%) (Fig. 6). Similar results were also seen for the liquid phase of treated samples (O2 enriched: 336 eV, 54.7 atom%; O2 depleted: 336.6 eV, 45.3 atom%) (Table 3).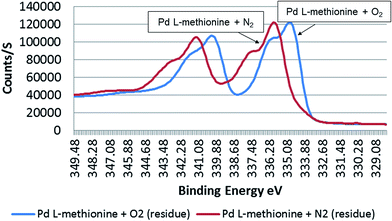 | ||
| Fig. 6 Binding energy (eV) of Pd black residue surfaces (outer 3–4 atomic layers) in 0.1 M L-methionine treated under O2-depleted (with N2) (1.6 mg L−1) and O2-enriched (8.6 mg L−1) conditions. | ||
| Element | Pd/L-methionine (R) | Pd/L-methionine (S) | Pd/L-methionine (R) | Pd/L-methionine (S) | ||||
|---|---|---|---|---|---|---|---|---|
| O2 concentration: 8.6 mg L−1 | O2 concentration: 1.6 mg L−1 | |||||||
| eV | Atom% | eV | Atom% | eV | Atom% | eV | Atom% | |
| O 1s | 526.3 | 1.4 | 529.7 | 20.5 | 528.0 | 0.8 | 530.8 | 68.5 |
| 526.4 | 3.5 | 531.1 | 39.9 | 530.4 | 69.3 | 532.2 | 31.5 | |
| 530.0 | 95.1 | 532.4 | 39.7 | 531.9 | 24.2 | |||
| 533.7 | 5.8 | |||||||
| Pd 3d5/2 | 334.4 | 12.8 | 334.6 | 12.7 | 334.5 | 9.2 | 333.3 | 8.1 |
| 335.0 | 44.1 | 336.0 | 54.7 | 335.9 | 50.3 | 335.1 | 17.1 | |
| 336.3 | 33.8 | 337.1 | 32.5 | 337.3 | 25.5 | 336.6 | 45.3 | |
| 337.8 | 9.2 | 338.4 | 15.0 | 337.4 | 29.5 | |||
Pd in sample solutions (both O2-enriched and O2-depleted) was found to be more oxidised compared to sample residues. Under low oxygen conditions, metallic Pd treated with L-methionine had O2 concentrations of 69.3 atom% (BE: 530.4 eV) and 68.5 atom% (BE: 530.8 eV) in the sample residue and solution, respectively (Table 3). On the atomic surfaces of Pd black treated in the presence of higher amounts of oxygen had O2 concentrations (95.1 atom%, BE: 530 eV (residue) and 39.9 atom%, BE 531.1 eV (solution)). Interestingly, the results indicate that metallic Pd is more easily transformed to Pd(II) and Pd(IV) under reduced O2 conditions, findings of relevance for sediments in aquatic systems. The Gaussian/Lorentzian curve results demonstrate the transformation behaviour for Pd (Table 3), with a higher binding energy of 335.9 eV for the O2 depleted sample (50.3 atom%). The O2-rich sample had a binding energy of 335.0 eV (44.1 atom%). The results for citric acid treated samples confirm that the surface chemistry of Pd changes little in the presence of this organic substance, under both O2-rich and O2-depleted conditions (Table 4). There was little difference in the concentration of Pd species in residues of the respective high and low oxygen citric acid treated samples. The results were, however, different for sample solutions. Specifically, it was observed that species of Pd(IV) dominate (46.9 atom%, BE: 337.0 eV) in solution with low levels of oxygen. In the oxygen rich sample solution, Pd with a binding energy of 335.2 was more prominent (41.2 atom%). There was some variability in the oxidation of Pd in sample solutions, with one O2-rich sample having a measured O2 concentration of 63.7 atom% (BE: 531.6 eV) vs. 66.8 atom% (BE: 531.3 eV) for the O2-depleted solution. This suggests that higher O2 concentrations both in solution and on the atomic surfaces of particles do not necessarily result in Pd species with a higher oxidation status. This is also confirmed by the results for Pd/L-methionine.
| Element | Pd/citric acid (R) | Pd/citric acid (S) | Pd/citric acid (R) | Pd/citric acid (S) | ||||
|---|---|---|---|---|---|---|---|---|
| O2 concentration: 8.6 mg L−1 | O2 concentration: 1.6 mg L−1 | |||||||
| eV | Atom% | eV | Atom% | eV | Atom% | eV | Atom% | |
| O 1s | 529.9 | 36.5 | 530.0 | 7.6 | 529.8 | 33.2 | 530.0 | 7.3 |
| 531.6 | 63.5 | 531.5 | 62.5 | 531.3 | 66.8 | 531.8 | 62.7 | |
| 532.7 | 29.8 | 533.0 | 30.0 | |||||
| Pd 3d5/2 | 334.8 | 35.3 | 334.4 | 26.1 | 334.8 | 33.8 | 334.5 | 9.6 |
| 335.7 | 29.1 | 335.2 | 41.2 | 335.8 | 27.8 | 335.9 | 32.6 | |
| 336.9 | 28.1 | 336.7 | 20.9 | 336.9 | 30.7 | 337.0 | 46.9 | |
| 338.4 | 7.4 | 338.0 | 11.8 | 338.4 | 7.8 | 338.1 | 11.0 | |
D Conclusions
Overall, the findings here demonstrate that the presence of organic complexing agents are likely to influence the solubility and bioaccessibility of emitted metallic Pd in the environment. Clearly, certain organic complexing agents, notably L-methionine, are more effective in transforming metallic Pd into more soluble species. This finding is supported by the results for both field collected soils and Pd model substances, which showed that L-methionine is highly effective in transforming Pd into a more soluble species. This also implies, however, that different soil materials can have strong influence on the solubility depending on the presence or absence of certain ligands. In addition to the type of organic complexing agent, other factors play a role in modulating Pd solubility such as pH and reaction time. The solubility of Pd in soils was demonstrated to be higher compared to that in model substances, which highlights a need to conduct studies employing field-collected samples to adequately assess PGE behaviour and potential toxicity.The results of this study support more recent results that show that metallic Pd is likely to readily complex with organic substances commonly present in the environment, contributing to an enhanced reactivity and mobility of this element under ambient conditions. The findings have important implications for exposed terrestrial and aquatic organisms. In light of the study results, documented increasing amounts of Pd relative to other PGE in the environment are a cause of concern that highlights the need for further monitoring.
Acknowledgements
This research was funded through the Deutsche Forschungsgemeinschaft (DFG project: GZ: ZE950/2-1 and ZE950/2-3) under the title “Experimental investigations into the influence of organic complexing agents and inorganic anions (Cl−, NO3−, SO42− and PO43−) on the transformation behaviour and the mobility of metallic palladium (Pd) and PdO”. We would like to thank the Deutsche Forschungsgemeinschaft (DFG) for funding support for this project.Notes and references
- H. Wichmann, G. Anquandah, C. Schmidt, D. Zachmann and M. Bahadir, Environ. Sci. Technol., 2007, 388, 121–127 CAS.
- F. Zereini, H. Alsenz, C. L. S. Wiseman, W. Püttmann, E. Reimer, R. Schleyer, E. Bieber and M. Wallasch, Sci. Total Environ., 2012, 416, 261–268 CrossRef CAS PubMed.
- F. Zereini, C. Wiseman and W. Püttmann, Environ. Sci. Technol., 2007, 41, 451–456 CrossRef CAS PubMed.
- K. Leopold, M. Maier, S. Weber and M. Schuster, Environ. Pollut., 2008, 156, 341–347 CrossRef CAS PubMed.
- M. D. Hays, S. H. Cho, R. Baldauf, J. J. Schauer and M. Shafer, Atmos. Environ., 2011, 45, 925–934 CrossRef CAS.
- A. P. Ribeiro, A. M. G. Figueiredo, J. E. S. Sarkis, M. A. Hortellani and B. Markert, Environ. Monit. Assess., 2012, 184, 7373–7382 CrossRef CAS PubMed.
- A. Bozlaker, N. J. Spada, M. P. Fraser and S. Chellam, Environ. Sci. Technol., 2014, 48, 54–62 CrossRef CAS PubMed.
- O. Morton-Bermea, E. Hernández-Álvarez, S. Ordoñez-Quiñonez, L. E. Beramendi-Orosco, J. Vega-Rodríguez and O. Amador-Muñoz, in Platinum Metals in the Environment, ed. F. Zereini and C. L. S. Wiseman, Springer-Verlag, 2015, pp. 257–264 Search PubMed.
- S. Rauch and B. Peucker-Ehrenbrink, in Platinum Metals in the Environment, ed. F. Zereini and C. L. S. Wiseman, Springer-Verlag, 2015, pp. 3–17 Search PubMed.
- Platinum Metals in the Environment, ed. F. Zereini and C. L. S. Wiseman, Springer Verlag, 2015, p. 492 Search PubMed.
- S. Chellam and A. Bozlaker, in Platinum Metals in the Environment, ed. F. Zereini and C. L. S. Wiseman, Springer-Verlag, 2015, pp. 199–242 Search PubMed.
- V. R. Lyubomirova and R. Djingova, in Platinum Metals in the Environment, ed. F. Zereini and C. L. S. Wiseman, Springer-Verlag, 2015, pp. 243–255 Search PubMed.
- A. M. G. Figueiredo and A. P. Ribeiro, in Platinum Metals in the Environment, ed. F. Zereini and C. L. S. Wiseman, Springer-Verlag, 2015, pp. 131–144 Search PubMed.
- C. L. S. Wiseman, Z. Hassan Pour and F. Zereini, Chemosphere, 2015 DOI:10.1016/j.chemosphere.2015.11.056 , in press.
- H. Sievers and M. Schuster, in Platinum Metals in the Environment, ed. F. Zereini and C. L. S. Wiseman, Springer-Verlag, 2015, pp. 187–198 Search PubMed.
- H. J. Ballach, in Emissionen von Platinmetallen, Analytik, Umwelt- and Gesundheitsrelevanz, ed. F. Zereini and F. Alt, Springer-Verlag, 1999, pp. 217–227 Search PubMed.
- N. Feichtmeier and K. Leopold, in Platinum Metals in the Environment, ed. F. Zereini and C. L. S. Wiseman, Springer-Verlag, 2015, pp. 311–338 Search PubMed.
- N. Ruchter, S. Zimmermann and B. Sures, in Platinum Metals in the Environment, ed. F. Zereini and C. L. S. Wiseman, Springer-Verlag, 2015, pp. 351–360 Search PubMed.
- B. Sures, N. Ruchter and S. Zimmermann, in Platinum Metals in the Environment, ed. F. Zereini and C. L. S. Wiseman, Springer-Verlag, 2015, pp. 383–399 Search PubMed.
- H. G. Zechmeister, S. Hann and G. Koellensperger, in Platinum Metals in the Environment, ed. F. Zereini and C. L. S. Wiseman, Springer-Verlag, 2015, pp. 339–349 Search PubMed.
- S. Zimmermann, B. Sures and N. Ruchter, in Platinum Metals in the Environment, ed. F. Zereini and C. L. S. Wiseman, Springer-Verlag, 2015, pp. 361–381 Search PubMed.
- J. D. Eckhardt, J. Schäfer, H. Puchelt and H. Stüben, in Anthropogenic Platinum Group Element Emissions, ed. F. Zereini and F. F. Alt, Springer-Verlag, Berlin, 2000, pp. 47–55 Search PubMed.
- S. Rauch and G. M. Morrison, Sci. Total Environ., 1999, 235, 261–268 CrossRef CAS PubMed.
- S. Zimmermann, C. M. Menzel, D. Stüben, H. Taraschewski and B. Sures, Environ. Pollut., 2003, 124, 1–5 CrossRef CAS PubMed.
- S. Zimmermann, U. Baumann, H. Taraschewski and B. Sures, Environ. Pollut., 2004, 27, 195–202 CrossRef.
- S. Wood, C. Tait, C. Vlassopoulos and D. Janecky, Geochim. Cosmochim. Acta, 1994, 58, 625–637 CrossRef CAS.
- F. Zereini, B. Skerstupp, F. Alt, E. Helmers and H. Urban, Sci. Total Environ., 1997, 206, 137–146 CrossRef CAS.
- S. Lustig, S. Zang, W. Beck and P. Schramel, Mikrochim. Acta, 1998, 129, 189–194 CrossRef CAS.
- K. Jarvis, S. Parry and M. Piper, Environ. Sci. Technol., 2001, 35, 1031–1036 CrossRef CAS PubMed.
- S. Wood and J. van Middlesworth, Can. Mineral., 2004, 42, 411–421 CrossRef CAS.
- G. Yang, C. Jin, J. Hong, Z. Guo and L. Zhu, Spectrochim. Acta, Part A, 2004, 60, 3187–3195 CrossRef PubMed.
- S. R. Dahlheimer, C. R. Neal and J. B. Fein, Environ. Sci. Technol., 2007, 41, 870–875 CrossRef CAS PubMed.
- C. Colombo, A. J. Monhemius and J. A. Plant, Sci. Total Environ., 2008, 389, 46–51 CrossRef CAS PubMed.
- F. Zereini, C. L. S. Wiseman and W. Püttmann, Environ. Sci. Technol., 2012, 46, 10326–10333 CAS.
- F. Zereini, C. L. S. Wiseman, M. Vang, P. Albers, W. Schneider, R. Schindl and K. Leopold, Environ. Sci.: Processes Impacts, 2015, 17, 915–921 CAS.
- Z. Ding, Q. Wang and X. Hu, Procedia Environ. Sci., 2013, 18, 679–685 CrossRef CAS.
- M. Vang, Master's Thesis, Goethe-Universität Frankfurt am Main, unpublished, 2013.
- J. Poprizki, Master's Thesis, Goethe-Universität Frankfurt am Main, unpublished, 2014.
- B. Bruder, C. L. S. Wiseman and F. Zereini, in Platinum Metals in the Environment, ed. F. Zereini and C. L. S. Wiseman, Springer-Verlag, 2015, pp. 265–275 Search PubMed.
- F. Zereini, I. Müller and C. L. S. Wiseman, in Platinum Metals in the Environment, ed. F. Zereini and C. L. S. Wiseman, Springer-Verlag, 2015, pp. 277–288 Search PubMed.
- A. Turner and S. Price, Environ. Sci. Technol., 2008, 42, 9443–9448 CrossRef CAS PubMed.
- C. Puls, A. Limbeck and S. Hann, Atmos. Environ., 2012, 55, 213–219 CrossRef CAS.
- A. R. Angumeenal and D. Venkappayya, LWT--Food Sci. Technol., 2013, 50, 367–370 CrossRef CAS.
- B. R. Tagirov, N. N. Baranova, A. V. Zotov, N. N. Akinfiev, N. A. Polotnyanko, N. D. Shikina, L. A. Koroleva, Y. V. Shvarov and E. N. Bastrakov, Geochim. Cosmochim. Acta, 2013, 117, 348–373 CrossRef CAS.
- F. Reniers and C. Tewell, J. Electron Spectrosc. Relat. Phenom., 2005, 142, 1–25 CrossRef CAS.
- C. J. Powell and A. Jablonski, J. Electron Spectrosc. Relat. Phenom., 2010, 178, 331–346 CrossRef.
- M. B. Gómez, M. M. Gómez and M. A. Palacios, J. Anal. At. Spectrom., 2003, 18, 80–83 RSC.
- J. Messerschmidt, A. von Bohlen, F. Alt and R. Klockenkämper, Analyst, 2000, 125, 397–399 RSC.
- H. Alsenz, F. Zereini, C. Wiseman and W. Püttmann, Anal. Bioanal. Chem., 2009, 395, 1919–1927 CrossRef CAS PubMed.
- DIN 19741, Bodenbeschaffenheit – Bestimmung der Gehalte von Platingruppenelementen (Platin, Palladium, Rhodium) in Böden, Bodenmaterialien and Schlämmen, 2012 Search PubMed.
- Johnson Matthey, Platinum 2013.
- R. Schlögl, G. Indlekofer and P. Oelhafen, Angew. Chem., 1987, 99, 312–322 CrossRef.
- A. Caubet, V. Moreno, E. Molins and C. Miravitlles, J. Inorg. Biochem., 1992, 48, 135–152 CrossRef CAS.
- P. Corbi, F. Cagnin, L. P. B. Sabeh, A. Massabni and C. M. Costa-Neto, Spectrochim. Acta, Part A, 2007, 66, 1171–1174 CrossRef PubMed.
- O. Vicol, N. Hurduc and I. A. Schneider, J. Inorg. Nucl. Chem., 1978, 41, 309–315 CrossRef.
- H. M. Marafie, N. Shuaib and S. El-Ezaby, Polyhedron, 1987, 6, 1391–1397 CrossRef CAS.
- M. Calaf, A. Caubet and V. Moreno, J. Inorg. Biochem., 1995, 59, 63–77 CrossRef CAS.
- B. Stypinski-Mis and G. Anderegg, Anal. Chim. Acta, 2000, 406, 325–332 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |
