Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Effect of sewage sludge type on the partitioning behaviour of pharmaceuticals: a meta-analysis†
L.
Berthod
ab,
G.
Roberts
a,
A.
Sharpe‡
a,
D. C.
Whitley
b,
R.
Greenwood
c and
G. A.
Mills
*b
aAstraZeneca Brixham Environmental Laboratory, Freshwater Quarry, Brixham, Devon TQ5 8BA, UK
bSchool of Pharmacy and Biomedical Sciences, University of Portsmouth, St Michael's Building, White Swan Road, Portsmouth, Hampshire PO1 2DT, UK. E-mail: graham.mills@port.ac.uk
cSchool of Biological Sciences, University of Portsmouth, King Henry Building, King Henry I Street, Portsmouth, Hampshire PO1 2DY, UK
First published on 26th October 2015
Abstract
Assessment of the fate of pharmaceutical residues in the environment involves the measurement or prediction of their sewage sludge partition coefficient (Kd). Sewage sludge can be classified into four types: primary, activated, secondary and digested, each one with different physical and chemical properties. Published studies have measured Kd for pharmaceuticals in a variety of sludge types. This paper discusses the variability of reported Kd values of pharmaceuticals in different types of sewage sludge, using a dataset generated from the literature. Using a meta-analysis approach, it was shown that the measured Kd values depend on the type of sludge used in the test. Recommendations are given for the type of sludge to be used when studying the partitioning behaviour of pharmaceuticals in waste water treatment plants. Activated sludge is preferred due to its more homogenous nature and the ease of collection of consistent samples at a plant. Weak statistical relationships were found between Kd values for activated and secondary sludge, and for activated and digested sludge. Pooling of Kd values for these sludge types is not recommended for preliminary fate and risk assessments. In contrast, statistical analyses found stronger similarities between Kd values reported for the same pharmaceutical in primary and activated sludges. This allows the pooling of experimental values for these two sludge types to obtain a larger dataset for modelling purposes.
Water impactActive pharmaceutical ingredients (API) in waste water treatment plants partition between aqueous and sludge phases. Understanding this behaviour is important for regulatory purposes. A partition coefficient (Kd) describes how chemicals distribute between these phases and can be measured experimentally using specific tests. A number of Kd values have been published for APIs in a range of sludge types. This paper undertakes a meta-analysis of these Kd values to investigate how the partitioning is affected by the different sludge types and if there are correlations between the datasets. This information is useful to make initial predictions of the fate of an API during treatment processes and may reduce the need to undertake time-consuming OECD tests in preliminary environmental risk assessments. |
Introduction
Over the past decade the environmental fate of active pharmaceutical ingredients (APIs) and personal care products and their potential effects on organisms have been investigated.1 Municipal and hospital waste effluents are the most important sources of APIs entering the aquatic environment.2,3 Although some APIs are removed by waste water treatment plants (WWTPs), many substances are not fully biodegraded during treatment processes.4 In this case, the partitioning behaviour of the API between sludge and aqueous phases at WWTPs is the key indicator of their subsequent environmental fate. The distribution of APIs between these two phases is described by the partition coefficient Kd. It is defined as the dimensionless ratio of the equilibrium concentrations (expressed as L kg−1) of the substance in the sludge and aqueous phases:| Kd = [API]sludge/[API]aqueous | (1) |
Values of Kd can be measured experimentally or estimated by mathematical models. Usually, experimental measurements use specific guidelines, e.g. Organisation for Economic Co-operation and Development (OECD) 106,19 OECD 121 (ref. 20) or the US Environmental Protection Agency's Office of Prevention, Pesticides and Toxic Substances (OPPTS) guideline 835.1110.21 The OPPTS 835.1110 guideline is currently the only one applicable to the measurement of partitioning behaviour in sewage sludge. In this guideline, a series of experiments are performed using different aqueous concentrations of sludge and the linear section of the slope of the resultant isotherm is used to calculate Kd. The guideline recommends the use of activated sewage sludge, even though this matrix may not exhibit all of the different types of partitioning mechanism that can potentially occur in a WWTP.
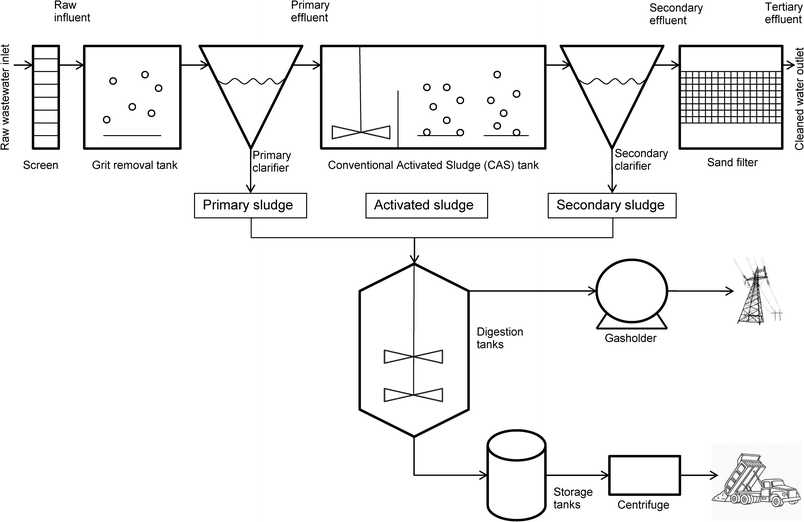
Within a WWTP different types of sewage sludge are present, each with varying physico-chemical properties (Table 1). Typically, four types of sludge (primary, activated, secondary and anaerobically digested) can be found within a plant (Fig. 1). All of these have been used in reported laboratory-based sorption experiments to measure Kd (Table 2). The matrix used has been shown to have an effect on the experimental values obtained. For example, comparisons have been made between primary and secondary,6,16 activated and primary8,13,17 and activated and digested.11 Activated sludge was found to be comparable to primary sludge.13,17 Primary sludge behaved differently to secondary sludge. This was attributed to differences in pH6 and surface properties of the material.16 The relationship between activated sludge and digested sludge was less clear.11
| Sludge type | Primary | Activated | Secondary | Digested |
|---|---|---|---|---|
| a Tchobanoglous et al. 2002 (ref. 22). b Wick et al. 2011 (ref. 27). c Wick et al. 2009 (ref. 14). d Carballa et al. 2007 (ref. 28). e Ternes et al. 2004 (ref. 6). f Radjenović et al. 2009 (ref. 13). g TSS: total suspended solids. h TOC: total organic carbon. i VSS: volatile suspended solids. j COD: chemical oxygen demand. k P/TSS: phosphorus per TSS. l Fe(III)/TSS: iron(III) per TSS. m N/TSS: nitrogen per TSS. | ||||
| pH | 5.0–8.0a | 6.8b | 6.8c | 7.7–8.6a |
| TSSg | 50–125d g L−1 | 10–35d g L−1 | 9c g L−1 | 27–42a g L−1 |
| TOCh | 35%e | 29b–49% | 25c–34e% | 25% |
| VSSi | 25–70d g L−1 (50–80%a) | 10–30d g L−1 (87f–100%) | 60%e | 12–20d g L−1 (44–48%) |
| CODj | 45–120d g L−1 | 10–50d g L−1 | — | 15–32d g L−1 |
| COD/TSS | 90–96%, 146%e | 100–143% | 110%e | 56–76% |
| P/TSSk | 0.8a–3.0%e | 2.8–11.0%a | 3.0%e | 1.5–4.0%a |
| Fe(III)/TSSl | <1.0%e | — | 4.0%e | 3.0–8.0%a |
| N/TSSm | 1.5–4.0%a | 2.4–5.0%a | 5.8%e | 1.6–3.0%a |
| Compound | K d activated | K d primary | K d secondary | K d digested |
|---|---|---|---|---|
| a Stevens-Garmon et al. 2011 (ref. 17). b Radjenović et al. 2009 (ref. 13). c Hörsing et al. 2011 (ref. 16). d Lajeunesse et al. 2012 (ref. 18). e Barron et al. 2009 (ref. 12). f Wick et al. 2009 (ref. 14). g Urase and Kikuta 2005 (ref. 9). h Suárez et al. 2008 (ref. 11). i Joss et al. 2005 (ref. 8). j Ternes et al. 2004 (ref. 6). k Andersen et al. 2005 (ref. 29). l Carballa et al. 2008 (ref. 10). m Stuer-Lauridsen et al. 2000 (ref. 5). n Göbel et al. 2005 (ref. 7). | ||||
| Acetaminophen | 595.0 (134.3)a,b | 18.0 (89.8)a,b | — | — |
| Alfuzosin | — | 1800.0c | 1200.0c | — |
| Amitriptyline | 4555.0a | 4897.0 (23.0)a,c | 5020.0 (120.6)c,d | 1049.0e |
| Androstenedione | 156.0a | 174.0a | — | — |
| Androsterone | 579.0a | 534.0a | — | — |
| Atenolol | 44.0 (40.4)a,b,f | 200.3 (112.9)a,b,c | 2800.0c | 11.0e |
| Atorvastatin | 198.0a | 216.0a | — | — |
| Atracurium | — | 350.0c | 1600.0c | — |
| Azelastine | — | 6400.0c | 470.0c | — |
| Biperiden | — | 820.0c | 750.0c | — |
| Bisoprolol | 40.0f | — | 110.0c | — |
| Bupropion | — | 85.0c | 140.0c | — |
| Caffeine | 30.0a | 30.0a | — | 14.0e |
| Carbamazapine | 53.8 (97.0)a,b,f,g,h | 102.3 (154.9)a,b,h,i | 120.6 (140.0)d,i,j | 39.5 (9.7)e,h,j |
| Chlorprothixene | — | 38![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000.0c 000.0c |
20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000.0c 000.0c |
— |
| Citalopram | — | 540.0c | 2105.0 (127.3)c,d | 282.0e |
| Clofibric Acid | 25.5g | — | 4.8j | 5.0e |
| Clomipramine | — | 17![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000.0c 000.0c |
6700.0c | — |
| Clotrimazol | — | 32![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000.0c 000.0c |
34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000.0c 000.0c |
8128.0e |
| Clozapine | 1642.0a | 1730.0a | — | — |
| Cyclophosphamide | — | 55.0j | 2.4j | — |
| Cyproheptadine | — | 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000.0c 000.0c |
3600.0c | — |
| Desloratadine | — | 3700.0c | 2900.0c | — |
| Diazapam | 91.8 (109.7)a,f,h | 125.0 (115.0)a,h,j | 21.0i | — |
| Diclofenac | 55.3 (104.2)b,g,h | 384.7 (43.3)b,h,i,j | 16.0i,j | 77.5 (50.2)e,h |
| Dicycloverine | — | 1400.0c | 1700.0c | — |
| Dilantin | 81.0a | 45.0a | — | — |
| Donepezil | — | 3600.0c | 970.0c | — |
| Duloxetine | — | 13![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000.0c 000.0c |
2900.0c | — |
| Erythromycin | 116.0 (51.2)b,h | 309.0b | — | 190.0e |
| Estradiol (E2) | 787.8 (64.1)a,g,h,k | 560.0a | — | 375.5 (69.7)l,h |
| Estriol | 63.0a | 58.0a | — | — |
| Estrone (E1) | 424.2 (42.8)a,g,h,k | 636.0a | — | 352.1 (62.7)l,h |
| Ethinylestradiol (EE2) | 763.0 (69.2)a,g,h,k | 515.3 (84.3)a,h,j | 349.0j | 414.1 (100.4)l |
| Ezetimibe | — | 2300.0c | 3000.0c | — |
| Fexofenadine | — | 2700.0c | 360.0c | — |
| Fluoxetine | — | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000.0c 000.0c |
7400 (26.8)c,d | — |
| Flutamide | — | 1500.0c | 750.0c | — |
| Gemfibrozil | 54.8 (75.3)a,b,g | 34.0 (45.8)a,b | — | — |
| Glibenclamide | 239.0b | 1941.0 (120.9)b,c | 1300.0c | — |
| Glimepiride | — | 2100.0c | 960.0c | — |
| Haloperidol | — | 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000.0c 000.0c |
2900.0c | — |
| Hydrochlorothiazide | 20.2b | 25.8b | — | — |
| Hydroxyzine | 819.0a | 989.0 (30.2)a,c | 720.0c | — |
| Ibuprofen | 32.4 (127.3)g,h,m | 14.8 (50.3)b,h,i | 183.6 (136.0)c,i,j | 31.4 (28.8)h,j |
| Ifosfamide | — | 22.0j | 1.4j | — |
| Indomethacin | 39.0g | — | — | 214.0e |
| Iopromide | 10.0h | 10.0i | 11.0j | 10.0 (43.4)h,j |
| Irbesartan | — | 700.0c | 940.0c | — |
| Ketoconazole | — | 9700.0c | 8500.0c | — |
| Ketoprofen | 22.5 (40.9)b,g | 226.0b | — | — |
| Loperamide | — | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000.0c 000.0c |
5500.0c | — |
| Loratidine | 3321.0b | 2336.0b | — | — |
| Maprotiline | — | 6700.0c | 4500.0c | — |
| Mefenamic acid | 434.0b | 294.0b | — | — |
| Meprobamate | 30.0a | 42.0a | — | — |
| Metoprolol | 65.0f | — | — | 18.0e |
| Mianserin | — | 3000.0c | 910.0c | — |
| Naproxen | 24.0g | 217.0i | 217.0i | 29.0e,h |
| Nefazodone | — | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000.0c 000.0c |
8300.0c | — |
| Nortriptyline | — | — | 6200.0k | 600.0e |
| Omeprazole | 107.0a | 130.0a | — | — |
| Oxazepam | 13.0f | 790.0c | 1100.0c | — |
| Paroxetine | — | 14![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000.0c 000.0c |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 650.0 (40.7)c,d 650.0 (40.7)c,d |
— |
| Phenylphenol | 347.0a | 652.0a | — | — |
| Pizotifen | — | 4700.0c | 3100.0c | — |
| Primidone | 18.5 (87.9)a,f | 45.0a | — | — |
| Progesterone | — | 750.0c | 1100.0c | — |
| Propanolol | 354.5 (10.2)b,f | 641.0b | — | 331.0e |
| Repaglinide | — | 170.0c | 210.0c | — |
| Risperidone | 861.0a | 1432.0c | 650.0c | — |
| Roxithromycin | 282.0h | 400.0i | 170.0i | 49.0 (70.8)h,l |
| Sertraline | — | 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000.0c 000.0c |
24![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000.0 (41.2)c,d 000.0 (41.2)c,d |
1883.0e |
| Sotalol | 18.0f | — | 360.0c | — |
| Sulfamethoxazole | 205.0 (54.4)b,h,n | 161.6 (129.9)b,c | 370.0c | 24.7 (103)e,h,l |
| Testosterone | 157.0a | 178.0a | — | — |
| Tramadol | 447.0f | 110.0c | 190.0c | — |
| Trimethoprim | 195.0 (28.6)a,b,h,n | 356.0 (26.1)a,b,c | 420.0c | 68.0e |
| Verapamil | 1501.0a | 1722.0 (6.4)a,c | 400.0c | — |
The location of the sampling point for the sludge within the WWTP is important but there is often inconsistency in different studies. For example, primary sludge can be sampled before the first clarifier6,17 or after it (Fig. 1).13 In one study primary sludge was sampled from a primary clarifier that had no secondary sludge recirculation.16 Comparison of sludge samples obtained from two different types of digesters, mesophilic and thermophilic, showed that sorption was not influenced by the operational conditions of the digester as long as the pH remained between 5.4 and 6.9.10 A study of the effect of sludge retention time on sorption, comparing the sorption behaviour of APIs with secondary sludge of different ages, found that sorption was lower with short retention times (2–3 days), than with longer retention times (10 days).16
Sewage sludge is a complex matrix. The physico-chemical properties of the sludge vary from plant to plant as well as between different locations within a WWTP. This contributes to the variability of the Kd values reported in the literature for APIs and related compounds. These inconsistencies could lead to incorrect environmental risk assessments (ERA) being made for some APIs. For example, Kd values are frequently measured using activated sludge as the solid phase. However, if assessing the potential of leaching of an API to soil when waste material is subsequently applied to land, it may be more appropriate to use digested sludge as the solid matrix in laboratory measurements.
This paper undertakes a meta-analysis of the Kd values of APIs published in the literature. It aims to identify the possible effects that the type of sewage sludge used in sorption experiments may have on the measured Kd values, and to investigate possible associations between the sorption behaviour of these matrices. This information may be useful in guiding the disposal of sewage sludge, aiding preliminary ERA and generating larger datasets for use in developing predictive mathematical models that describe the partitioning behaviour of APIs.
Materials and methods
Data collection
A literature search was undertaken initially using the major scientific journal databases (Google Scholar, ScienceDirect, Springer, Web of Science, Wiley Online Library) using the combined search terms: partition coefficient, pharmaceutical and sewage sludge. This identified 21 papers satisfying these search criteria. For the purpose of this meta-analysis, it was essential that the type of sludge within the WWTP used for the measurement of Kd for the APIs was specified. Seven papers were rejected on these grounds. Data for this study were collected from the remaining fourteen published papers (Table 2).5–18 These covered a wide range of APIs (antibiotics, anticancers, anti-inflammatories, cardiovasculars, central nervous system drugs and hormones). The authors reported Kd values in four types of sewage sludge (primary, activated, secondary or digested) and their classifications were used directly in the meta-analysis. This type of analysis does not take account of any small variations in experimental conditions used in the studies to ascertain the Kd values.Type of sludge
Primary sludge was collected just after the grit removal stage and before the first clarifier in some papers, or just after the primary clarifier in others (Fig. 1). In some papers the primary clarifier had no secondary sludge recirculation; while in others it did.6,9,16,17 Activated sludge was always sampled in the activation tank in the nitrification zone.11,13,17 Secondary sludge was collected in the secondary clarifier. However, there were differences in sludge age and secondary clarifier volume.6,16 Anaerobically digested sludge was sampled from the digester. Only one paper reported the type of digester (mesophilic or thermophilic) and sludge age.10The key physico-chemical properties (pH, total suspended solids (TSS), total organic carbon (TOC), volatile suspended solids (VSS) and chemical oxygen demand (COD)) of the four sludge types, where available, are detailed in Table 1.
Sorption data
All the experimentally derived Kd values were calculated using a linear isotherm. Due to the relatively small number of studies the data were grouped by sludge type regardless of any sampling differences. When an API had more than one Kd value for the same type of sludge, the values were averaged and the mean was used. APIs were only included if Kd values were available for more than one type of sludge. This resulted in a set of 79 APIs associated with a set of 196 Kd values (Table 2). Few of these APIs had Kd values available in the literature for all sludge types.Statistical analysis
To assess any similarities in sorption behaviour between sludge types several statistical tests were performed. Some of these tests require the data to have a normal distribution, and a Box–Cox plot revealed that a log transformation was required to normalise the data and stabilise the variation. The effectiveness of the transformation was confirmed for the transformed data using normal probability plots. First, the correlations between the measured ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd values of the APIs on different pairs of the four sludge types available in the literature were calculated (Table 3). Pearson correlation coefficients were considered significant if their associated p-value was below 0.05. Initially, a multiple comparison between the ln
Kd values of the APIs on different pairs of the four sludge types available in the literature were calculated (Table 3). Pearson correlation coefficients were considered significant if their associated p-value was below 0.05. Initially, a multiple comparison between the ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd values for the different sludge types was performed using a Tukey test. However, the results of this test were adversely affected by the sparseness of the data set. For example, there are several large values of Kd > 103 for primary sludge that have no corresponding measurement for some of the other sludge types. Differences in the mean Kd values thus reflect the coverage of reported values rather than intrinsic differences between the sludge types. Therefore, for each pair of sludge types a paired sample t-test was performed to assess their similarity, using only cases where Kd values were available for both sludge types. A 95% confidence level was applied, together with a Bonferroni correction to reduce the risk of a Type I error due to multiple comparisons (Table 4). Finally, linear regression was used to quantify the relationships between the ln
Kd values for the different sludge types was performed using a Tukey test. However, the results of this test were adversely affected by the sparseness of the data set. For example, there are several large values of Kd > 103 for primary sludge that have no corresponding measurement for some of the other sludge types. Differences in the mean Kd values thus reflect the coverage of reported values rather than intrinsic differences between the sludge types. Therefore, for each pair of sludge types a paired sample t-test was performed to assess their similarity, using only cases where Kd values were available for both sludge types. A 95% confidence level was applied, together with a Bonferroni correction to reduce the risk of a Type I error due to multiple comparisons (Table 4). Finally, linear regression was used to quantify the relationships between the ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd values obtained on the four different sludge types. As regression of the ln
Kd values obtained on the four different sludge types. As regression of the ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd values of one sludge type onto another involves errors in both variables, orthogonal, or total least squares, regression was used, rather than the usual ordinary least squares regression. The results of these three statistical tests were used in combination to assess the feasibility of inferring the ln
Kd values of one sludge type onto another involves errors in both variables, orthogonal, or total least squares, regression was used, rather than the usual ordinary least squares regression. The results of these three statistical tests were used in combination to assess the feasibility of inferring the ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd value of an API on one sludge type from its measured value on another sludge type.
Kd value of an API on one sludge type from its measured value on another sludge type.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd values for active pharmaceutical ingredients with different types of sewage sludge
Kd values for active pharmaceutical ingredients with different types of sewage sludge
| K d activated | K d primary | K d secondary | K d digested | ||
|---|---|---|---|---|---|
| K d activated | Correlation coefficient | 1 | |||
| p-value | |||||
| n | 45 | ||||
| K d primary | Correlation coefficient | 0.72 | 1 | ||
| p-value | <0.001 | ||||
| n | 40 | 73 | |||
| K d secondary | Correlation coefficient | 0.48 | 0.85 | 1 | |
| p-value | 0.027 | <0.001 | |||
| n | 21 | 51 | 55 | ||
| K d digested | Correlation coefficient | 0.82 | 0.88 | 0.80 | 1 |
| p-value | <0.001 | <0.001 | <0.001 | ||
| n | 19 | 19 | 16 | 23 | |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd values for active pharmaceutical ingredients with different types of sewage sludge
Kd values for active pharmaceutical ingredients with different types of sewage sludge
| Paired differences | |||||||
|---|---|---|---|---|---|---|---|
| n | Mean | Standard deviation | 95% confidence interval of the difference | t | p | ||
| Lower | Upper | ||||||
| t = Student's t-statistic. p = p-value associated with the t-test. | |||||||
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd activated–ln Kd activated–ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd primary Kd primary |
40 | −0.33 | 1.16 | −0.70 | 0.04 | −1.79 | 0.082 |
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd activated–ln Kd activated–ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd secondary Kd secondary |
21 | −0.57 | 1.78 | −1.38 | 0.24 | −1.47 | 0.157 |
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd activated–ln Kd activated–ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd digested Kd digested |
19 | 0.58 | 0.93 | 0.13 | 1.03 | 2.73 | 0.014 |
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd primary–ln Kd primary–ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd secondary Kd secondary |
51 | 0.42 | 1.16 | 0.09 | 0.75 | 2.58 | 0.013 |
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd primary–ln Kd primary–ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd digested Kd digested |
19 | 1.18 | 0.99 | 0.70 | 1.65 | 5.19 | <0.001 |
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd secondary–ln Kd secondary–ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd digested Kd digested |
16 | 1.52 | 1.57 | 0.69 | 2.36 | 3.88 | 0.001 |
Results and discussion
The literature search identified 14 papers satisfying the criteria for the meta-analysis. This relatively small number of publications reflects the fact that the study of the fate of APIs in the environment is relatively new, especially compared with many other classes of regulated pollutants (e.g. certain pesticides) that have been extensively investigated for many decades. As further work on the sorption behaviour of APIs within a WWTP is undertaken, the resultant data can be used to augment and strengthen the comparisons presented here.Activated and primary sludge
The correlation coefficient between the ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd values of activated and primary sludge was 0.72 with a p-value <0.001 (Table 3). This indicated that the sorption behaviour of the APIs in the dataset for these sludge types was similar. The paired sample t-test for the ln
Kd values of activated and primary sludge was 0.72 with a p-value <0.001 (Table 3). This indicated that the sorption behaviour of the APIs in the dataset for these sludge types was similar. The paired sample t-test for the ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd activated–ln
Kd activated–ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd primary sludge pair gave a p-value of 0.08 (Table 4). This was above the Bonferroni-corrected threshold value of p = 0.05/6 = 0.0083, indicating that these two sludge types behave similarly. The confidence interval associated with the t-test included zero, also showing that there was no significant difference between the sorption behaviour of these two sludges.
Kd primary sludge pair gave a p-value of 0.08 (Table 4). This was above the Bonferroni-corrected threshold value of p = 0.05/6 = 0.0083, indicating that these two sludge types behave similarly. The confidence interval associated with the t-test included zero, also showing that there was no significant difference between the sorption behaviour of these two sludges.
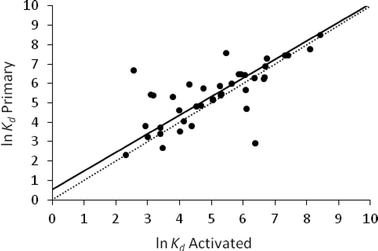
The orthogonal regression of ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd activated against ln
Kd activated against ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd primary gave a regression coefficient of 0.96, an intercept of 0.54 and a RMSE (the root mean square of the orthogonal distance from the data points to the regression line) of 0.81 (Fig. 2). These results showed that activated and primary sludge have similar sorption properties for a diverse set of APIs (n = 40). These findings are consistent with the observation that primary and activated sludge have similar physico-chemical properties; both have a relatively neutral pH, high TOC and comparable COD (Table 1).
Kd primary gave a regression coefficient of 0.96, an intercept of 0.54 and a RMSE (the root mean square of the orthogonal distance from the data points to the regression line) of 0.81 (Fig. 2). These results showed that activated and primary sludge have similar sorption properties for a diverse set of APIs (n = 40). These findings are consistent with the observation that primary and activated sludge have similar physico-chemical properties; both have a relatively neutral pH, high TOC and comparable COD (Table 1).
Within a WWTP there are several distinct stages where partitioning takes place. The first of these occurs in the primary clarifier.22 Hence primary sludge is the result of all the partitioning processes occurring prior to and including the primary clarifier (Fig. 1). As shown above, partitioning in activated sludge was similar to that in primary sludge. This means that partitioning in the WWTP would be similar in all compartments of the WWTP up to and including the activation tank.
It should be noted that, although the average reported Kd values for activated and primary sludge were found to be similar, a higher variability in Kd values was observed for some APIs with primary sludge than for activated sludge. For example, in primary sludge, Kd values ranging from 194 to 501 were reported for the anti-inflamatory diclofenac, from 20 to 314 for the anti-convulsant carbamazepine, from 46 to 460 for the cardiovascular drug atenolol, from 32 to 320 for the antibiotic sulfamethoxazole and from 251 to 427 for the antibiotic trimethoprim. This may have been due to differences in the location of the sampling point. In most cases primary sludge was collected after the first clarifier, while in a few instances material was collected by sedimentation/filtration of influent wastewater. By comparison, in activated sludge the variability in Kd values was lower, ranging from 16 to 128 for diclofenac, 17 to 135 for carbamazepine, 30 to 64 for atenolol, 77 to 282 for sulfamethoxazole and 119 to 253 for trimethoprim (Table 2). This was attributed to the activated sludge being a more homogeneous material due to the aeration process used in the activation tank. These differences aside, analysis of the available data suggests that using either activated or primary sludge would lead to similar measured values of Kd.
Activated and secondary sludge
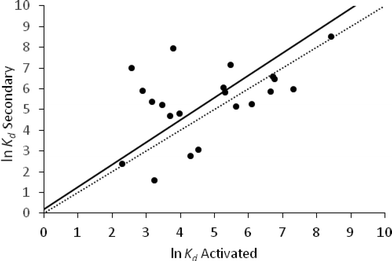
The correlation coefficient between the ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd values of activated and secondary sludge was 0.48 with a p-value = 0.027 (Table 3). This was lower than the correlation coefficient between activated and primary sludge. The paired sample t-test indicated that there was no significant difference between the ln
Kd values of activated and secondary sludge was 0.48 with a p-value = 0.027 (Table 3). This was lower than the correlation coefficient between activated and primary sludge. The paired sample t-test indicated that there was no significant difference between the ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd values for activated and secondary sludge.
Kd values for activated and secondary sludge.
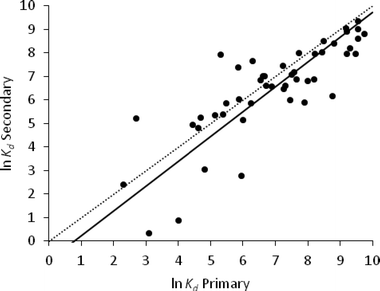
The regression analysis of these two sludge types for the available dataset (n = 21) gave a RMSE of 1.22 (Fig. 3).
In a WWTP, secondary sludge is formed mainly from activated sludge which has been allowed to settle, and their physico-chemical properties, with the exception of TSS, are broadly similar (Table 1). This would suggest that the sorption properties of these two sludges would be similar, but this was not supported by our analysis of the data reported in the literature. It is difficult to explain these statistical results. Sorption of pharmaceuticals to sewage sludge is a complex process. Unlike some other environmental media, such as soils, the partitioning mechanisms involved are not well understood. The bulk properties commonly used to characterise sewage sludge may not be adequate to describe fully these interactions and the inclusion of other properties of the matrix such as cation-exchange capacity may be required.
Activated and digested sludge
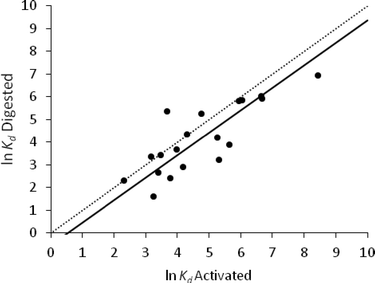
A higher correlation coefficient (0.82, p < 0.001) was found between the ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd values of activated and digested sludge (Table 3). The paired sample t-test gave p = 0.014, showing no difference between activated and digested sludge; however, the confidence interval did not contain 0 (Table 4). The orthogonal regression of these ln
Kd values of activated and digested sludge (Table 3). The paired sample t-test gave p = 0.014, showing no difference between activated and digested sludge; however, the confidence interval did not contain 0 (Table 4). The orthogonal regression of these ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd values (n = 19) gave a RMSE of 0.84 with a regression coefficient of 0.99 and intercept 0.20 (Fig. 4).
Kd values (n = 19) gave a RMSE of 0.84 with a regression coefficient of 0.99 and intercept 0.20 (Fig. 4).
These observed differences between the sorptive properties of activated and digested sludge may be influenced by their physico-chemical properties and the availability of oxygen. Generally, digested sludge is more basic than activated sludge (Table 1). The pH of the sludge affects the sorption of ionic compounds and will be more influential for APIs with pKa values in the range 6–9. There were differences in other physico-chemical properties, such as TOC, TSS and VSS, between these sludge types but their effect on sorption is more difficult to predict. Sorption may also have been affected by local variations of the bacterial population in the digester.
Primary and secondary sludge
In a WWTP a portion of primary and secondary sludge is mixed to form the sludge which is fed in to the anaerobic digester (Fig. 1). Secondary sludge is formed after the activation treatment in which microorganisms biodegrade some compounds and generate new biomass material. Therefore, the primary and secondary sludge may be expected to have different sorption characteristics for APIs. The correlation coefficient between the ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd values of primary and secondary sludge was 0.85 with a p-value <0.001 (Table 3). The paired sample t-test gave a p-value of 0.013, above the critical value, but the confidence interval was slightly to the right of 0, providing some evidence that primary and secondary sludge were similar in terms of their partitioning properties.
Kd values of primary and secondary sludge was 0.85 with a p-value <0.001 (Table 3). The paired sample t-test gave a p-value of 0.013, above the critical value, but the confidence interval was slightly to the right of 0, providing some evidence that primary and secondary sludge were similar in terms of their partitioning properties.
The orthogonal regression of the ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd values for primary and secondary sludge (n = 51) gave a RMSE of 0.81, a regression coefficient of 1.06 and intercept −0.81 (Fig. 5). This indicated that the sorption properties of APIs between primary and secondary sludge were correlated, with APIs sorbed more strongly to primary sludge than to secondary sludge (Fig. 5).
Kd values for primary and secondary sludge (n = 51) gave a RMSE of 0.81, a regression coefficient of 1.06 and intercept −0.81 (Fig. 5). This indicated that the sorption properties of APIs between primary and secondary sludge were correlated, with APIs sorbed more strongly to primary sludge than to secondary sludge (Fig. 5).
Some differences in the physico-chemical properties of primary and secondary sludge are apparent (Table 1). Most notably, TSS is markedly lower for secondary sludge compared with primary sludge. A smaller difference between TOC values suggests that the higher TSS value in primary sludge is mainly due to inorganic material.
Primary/secondary and digested sludge
Anaerobic digestion of sludge leads to changes in the physico-chemical properties of the sludge (Table 1). Therefore the partitioning behaviour for both primary and secondary sludge was expected to differ from the behaviour for anaerobically digested sludge. However, both primary and secondary sludges are used as feedstocks to form the digested sludge within a WWTP (Fig. 1), so their relationships with digested sludge were investigated.The correlation coefficients of the ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd values for primary and secondary sludge with digested sludge were 0.88 and 0.80, respectively (both with p-values <0.001) (Table 3). The paired sample t-tests show that these sludge pairs behave differently, with p-values below the threshold and confidence intervals not containing the origin (Table 4). The orthogonal regressions of the ln
Kd values for primary and secondary sludge with digested sludge were 0.88 and 0.80, respectively (both with p-values <0.001) (Table 3). The paired sample t-tests show that these sludge pairs behave differently, with p-values below the threshold and confidence intervals not containing the origin (Table 4). The orthogonal regressions of the ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd values between primary and digested sludge (n = 19), and between secondary and digested sludge (n = 16) gave RMSE values of 0.65 and 1.00, respectively. Their corresponding regression coefficients were 0.86 and 0.76.
Kd values between primary and digested sludge (n = 19), and between secondary and digested sludge (n = 16) gave RMSE values of 0.65 and 1.00, respectively. Their corresponding regression coefficients were 0.86 and 0.76.
The statistical analysis of the different sludge pairs is summarized in Table 5. This includes suggested criteria for the pooling of Kd values based on the three statistical tests.
| Correlation matrix | Paired t-test | Orthogonal regression | ||||||
|---|---|---|---|---|---|---|---|---|
| Pearson coefficient | p-value | p-value | Confidence interval | Regression coefficient | Intercept | RMSE | Pooled | |
| Pooling criteria | Close to 1 | <0.05 | >0.0083 | To include 0 | Close to 1 | Close to 0 | Close to 0 | |
| Sludge pairs | ||||||||
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd activated–ln Kd activated–ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd primary Kd primary |
0.72 | <0.001 | 0.082 | Yes | 0.96 | 0.54 | 0.81 | Yes |
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd activated–ln Kd activated–ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd secondary Kd secondary |
0.48 | 0.027 | 0.157 | Yes | 1.08 | 0.20 | 1.22 | No |
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd activated–ln Kd activated–ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd digested Kd digested |
0.82 | <0.001 | 0.014 | No | 0.99 | −0.54 | 0.64 | No |
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd primary–ln Kd primary–ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd secondary Kd secondary |
0.85 | <0.001 | 0.013 | No | 1.06 | −0.81 | 0.81 | No |
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd primary–ln Kd primary–ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd digested Kd digested |
0.88 | <0.001 | <0.001 | No | 0.86 | −0.33 | 0.65 | No |
ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd secondary–ln Kd secondary–ln![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kd digested Kd digested |
0.80 | <0.001 | 0.001 | No | 0.76 | −0.02 | 1.00 | No |
Factors affecting sorption of APIs to sludge
The sludges formed in a WWTP are complex matrices, with variable properties depending in part on design, operating temperature and hydraulic retention times within the plant. It is possible to identify the broad physico-chemical characteristics of the different sludge types. However, there is often a wide variation in properties (Table 1), making it hard to identify the specific interactions contributing to the sorption of APIs throughout the treatment process. As many APIs, in contrast to other types of pollutant (e.g. PAHs, PCBs, several pesticides), are ionisable, their partitioning behaviour, for example, may be particularly sensitive to small changes in pH. This will affect the charge on the dominant species and could vary across different compartments of a WWTP. Attempts have been made to incorporate this behaviour in predictive models.23 Due to the complexity of the media within the different compartments of a WWTP, the direct influence of differences in pH are hard to predict from physico-chemical properties with confidence and further investigation is warranted. The retention time of the sludge within the different compartments of the plant may also influence sorption, as shown by Hörsing et al.16 for secondary sludge. Highly aged materials may eventually show a reduction in their overall sorption capacity for APIs and other chemicals due to the reduction in available sites for binding. In addition, some characteristics of sewage sludge may change with improvements to treatment processes mandated by stricter environmental regulations.APIs can also undergo biodegradation within a WWTP. Different plant designs will influence the extent of these aerobic and anaerobic processes. The treatment of urban effluents can be further complicated by the presence of both APIs and their metabolites. Often, metabolites can be biodegraded (e.g. conjugation products) during various treatments to yield the parent compound, which may then undergo sorption to a sludge in later compartments of the plant.
This is in contrast to soils, where the matrices are more clearly defined, for example as clays, loams, sands and silts, which are available commercially as standard materials for testing the sorption behaviour of compounds. Activated sewage sludge is recommended for use in regulatory sorption tests (EPA, 1998). The data (Kd) from such tests may be used to model the distribution of a chemical in a WWTP and these results can be used to assesses the potential for release of APIs from the WWTP in aqueous effluent. However, it is important to understand the partitioning of chemicals with other sludge types. For example, if digested sludge is disposed of to land, there is a subsequent potential for desorption of the API from the sludge and the leaching of APIs into ground and surface waters.
APIs form a diverse set of chemicals, but tend to occupy specific regions in physico-chemical property space, for example satisfying the Lipinski rules.24 Unlike many pollutants, the majority of APIs exhibit ionic behaviour which must be considered when their environmental fate is assessed. The sorption behaviour of APIs reported in the literature covers a large range of Kd values (up to ~40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), but these are not distributed uniformly, with large regions containing few values. To strengthen the statistical analysis of the reported Kd values with all the different sewage types, further measurements are needed to obtain a more comprehensive dataset with more even coverage of the range of the observed partitioning behaviour of these compounds. In particular, there is a paucity of data for digested sludge.
000), but these are not distributed uniformly, with large regions containing few values. To strengthen the statistical analysis of the reported Kd values with all the different sewage types, further measurements are needed to obtain a more comprehensive dataset with more even coverage of the range of the observed partitioning behaviour of these compounds. In particular, there is a paucity of data for digested sludge.
An extensive body of work has been undertaken on the fate of regulated (priority) pollutants in sewage sludge and soils. This has been necessary as frequently sludge has been applied to land as a means of disposal.25 Emerging pollutants of recent concern such as APIs have received much less attention, although this situation is changing rapidly.26 As awareness of the presence of these chemicals in the environment increases, there is an urgent need to understand fully their fate. The analysis carried out in this study brings together the available sorption data for APIs and may be beneficial to those responsible for performing environmental risk assessment for these types of substances.
Conclusions
Despite the lack of available Kd values for some sludge types and the variability of Kd measurements between different studies, there is sufficient published data to undertake an initial meta-analysis. The statistical analysis showed that the sorption behaviour of the APIs in the dataset with activated and primary sludge was similar. More data points were available for the comparison of these sludge types (n = 40) and this dataset included compounds with a range of Kd values. Either matrix could be used in laboratory tests to assess sorption of APIs in a WWTP. However, it may be advantageous to use activated sludge to avoid the higher variability observed in Kd measurements with primary sludge. For mathematical modelling, where it is beneficial to have large datasets for the development of predictive models, Kd values for these sludge types may be pooled.Comparisons of the sorption behaviour of other sludge pairs did not show such strong similarities. Therefore, it is not recommended that the experimental Kd values for these matrices are combined directly. The orthogonal regressions between the different sludge pairs did not provide evidence for different sorption behaviour, with the 95% confidence intervals for the slope and intercept containing 1 and 0, respectively, in each case. However, the RMSE values for the activated–secondary and secondary–digested pairs were above one ln unit. The paired t-tests showed that the primary–digested and secondary–digested pairs could not be pooled and the correlation coefficient for the activated–secondary pair was very low, showing these sludge types behaved differently in terms of their sorption properties.
This analysis shows that there are statistical grounds for pooling the Kd values for APIs in activated and primary sludge. The evidence for pooling the other sludge types is weaker and is therefore not recommended for the purposes of preliminary ERA. In order to obtain a full understanding of the sorption behaviour of APIs within a WWTP additional experimental measurements are needed to ascertain Kd values for secondary and digested sludge.
Acknowledgements
This research was funded by the Biotechnology and Biological Sciences Research Council (BBSRC), as part of an industrial Collaboration Award in Science and Engineering (BB/I532853/1) between the University of Portsmouth and AstraZeneca's Brixham Environmental Laboratory, Devon, UK.References
- S. D. Richardson and T. A. Ternes, Anal. Chem., 2011, 83, 4614–4648 CrossRef CAS PubMed.
- A. Nikolaou, S. Meric and D. Fatta, Anal. Bioanal. Chem., 2007, 387, 1225–1234 CrossRef CAS PubMed.
- S. C. Monteiro and A. B. A. Boxall, in Reviews of Environmental Contamination and Toxicology, ed. D. M. Whitacre, Springer, New York, 2010, vol. 202, pp. 53–154 Search PubMed.
- T. A. Ternes and A. Joss, Human pharmaceuticals, hormones and fragrances: the challenge of micropollutants in urban water management, IWA Publishing, London, 2007 Search PubMed.
- F. Stuer-Lauridsen, M. Birkved, L. P. Hansen, H. C. Holten Lützhøft and B. Halling-Sørensen, Chemosphere, 2000, 40, 783–793 CrossRef CAS PubMed.
- T. A. Ternes, N. Herrmann, M. Bonerz, T. Knacker, H. Siegrist and A. Joss, Water Res., 2004, 38, 4075–4084 CrossRef CAS PubMed.
- A. Göbel, A. Thomsen, C. S. McArdell, A. Joss and W. Giger, Environ. Sci. Technol., 2005, 39, 3981–3989 CrossRef.
- A. Joss, E. Keller, A. C. Alder, A. Göbel, C. S. McArdell, T. A. Ternes and H. Siegrist, Water Res., 2005, 39, 3139–3152 CrossRef CAS PubMed.
- T. Urase and T. Kikuta, Water Res., 2005, 39, 1289–1300 CrossRef CAS PubMed.
- M. Carballa, G. Fink, F. Omil, J. M. Lema and T. A. Ternes, Water Res., 2008, 42, 287–295 CrossRef CAS PubMed.
- S. Suárez, M. Carballa, F. Omil and J. Lema, Rev. Environ. Sci. Biotechnol., 2008, 7, 125–138 CrossRef.
- L. Barron, J. Havel, M. Purcell, M. Szpak, B. Kelleher and B. Paull, Analyst, 2009, 134, 663–670 RSC.
- J. Radjenović, M. Petrović and D. Barceló, Water Res., 2009, 43, 831–841 CrossRef PubMed.
- A. Wick, G. Fink, A. Joss, H. Siegrist and T. A. Ternes, Water Res., 2009, 43, 1060–1074 CrossRef CAS PubMed.
- H.-J. Kim, D. S. Lee and J.-H. Kwon, Chemosphere, 2010, 80, 256–262 CrossRef CAS PubMed.
- M. Hörsing, A. Ledin, R. Grabic, J. Fick, M. Tysklind, J. L. C. Jansen and H. R. Andersen, Water Res., 2011, 45, 4470–4482 CrossRef PubMed.
- J. Stevens-Garmon, J. E. Drewes, S. J. Khan, J. A. McDonald and E. R. V. Dickenson, Water Res., 2011, 45, 3417–3426 CrossRef CAS PubMed.
- A. Lajeunesse, S. A. Smyth, K. Barclay, S. Sauvé and C. Gagnon, Water Res., 2012, 46, 5600–5612 CrossRef CAS PubMed.
- OECD, Test No. 106: Adsorption - Desorption Using a Batch Equilibrium Method, OECD Publishing, 2000 Search PubMed.
- OECD, Test No. 121: Estimation of the Adsorption Coefficient (Koc) on Soil and on Sewage Sludge using High Performance Liquid Chromatography (HPLC), OECD Publishing, 2001 Search PubMed.
- EPA, Fate, Transport and Transformation Test Guidelines, OPPTS 835.1110, Activated Sludge Sorption Isotherm. Prevention, Pesticides and Toxic Substances (7101), EPA 712–C–98–298, US Environmental Protection Agency, 1998 Search PubMed.
- G. Tchobanoglous, F. Burton and H. D. Stensel, Wastewater Engineering: Treatment and Reuse, Metcalf & Eddy, Inc., McGraw-Hill, New York, 4th edn., 2002 Search PubMed.
- S. Sathymoorthy and C. A. Ramsburg, Chemosphere, 2013, 92, 639–646 CrossRef PubMed.
- C. A. Lipinski, F. Lombardo, B. W. Dominy and P. J. Feeney, Adv. Drug Delivery Rev., 1997, 23, 3–25 CrossRef CAS.
- D. Schwanek, R. Carr, H. David, P. Douben, J. Hall, H. Kirchmann, L. Patria, P. Sequi, S. Smith and S. Webb, Regul. Toxicol. Pharmacol., 2004, 40, 227–251 CrossRef PubMed.
- P. Verlicchi and E. Zambello, Sci. Total Environ., 2015, 538, 750–767 CrossRef CAS PubMed.
- A. Wick, O. Marincas, Z. Moldovan and T. A. Ternes, Water Res., 2011, 45, 3638–3652 CrossRef CAS PubMed.
- M. Carballa, F. Omil, T. Ternes and J. M. Lema, Water Res., 2007, 41, 2139–2150 CrossRef CAS PubMed.
- H. R. Andersen, M. Hansen, J. Kjølholt, F. Stuer-Lauridsen, T. Ternes and B. Halling-Sørensen, Chemosphere, 2005, 61, 139–146 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ew00171d |
| ‡ Current address: AstraZeneca UK Ltd., Alderley Park, Macclesfield, SK10 8TG, UK. |
| This journal is © The Royal Society of Chemistry 2016 |