Suitability of Chemcatcher® passive sampling in monitoring organotin compounds at a wastewater treatment plant
H.
Ahkola
*a,
J.
Juntunen
a,
K.
Krogerus
a,
T.
Huttula
a,
S.
Herve
a and
A.
Witick
b
aFinnish Environment Institute (SYKE), Survontie 9A (Technopolis), 40500 Jyväskylä, Finland. E-mail: heidi.ahkola@ymparisto.fi
bNab Labs Oy, Survontie 9 (YAD), 40500 Jyväskylä, Finland
First published on 17th June 2016
Abstract
Municipal wastewater contains a number of harmful chemicals whose concentrations can fluctuate dramatically. Therefore studying concentrations of harmful chemicals by conventional grab water sampling can give a misleading picture of the true chemical contents. In grab samples the contents can often remain below the quantification limit which does not necessary mean the compound is not present in the aquatic environment but rather implies unsuccessful timing of sampling or limited sample volume. Alternative techniques are needed to improve the reliability of monitoring harmful chemicals and thus in this study the presence of organotin compounds (OTCs) was monitored at a wastewater treatment plant (WWTP) with Chemcatcher® passive sampling. Extreme conditions complicate the passive sampling, particularly in influent stream. However, the OTC concentrations determined with passive samplers were similar with those measured from grab samples. In conclusion, fewer OTCs were found with grab sampling than with passive sampling, particularly in effluent. The passive samplers seemed to be more suitable than grab samples for monitoring OTCs in wastewaters. As long-term sampling techniques give a more representative picture of the true chemical contents, passive sampling should be considered as an emerging tool for environmental authorities to implement the monitoring of harmful chemicals at WWTP.
Water impactMunicipal wastewater contains a number of harmful chemicals whose concentrations can fluctuate dramatically. Therefore studying concentrations of harmful chemicals by conventional grab water sampling can give a misleading picture of the true chemical contents. Since long-term sampling techniques give a more representative picture of the true chemical contents, environmental authorities should consider passive sampling as an emerging tool for the monitoring of harmful chemicals at waste water treatment plant. |
Introduction
Our water quality is threatened by harmful substances, originating from anthropogenic sources. The chemicals from households and industry end up in wastewater treatment plants (WWTPs) and parts of these chemicals go through the wastewater treatment process and end up in receiving waters. Treatment, collection and discharge of urban waste waters are controlled by European Commission Directive 91/271/EEC1 but the regulation does not currently include monitoring of organic substances in effluents or waste water sludge.During the sewage treatment process part of the chemicals are retained in wastewater sludge. Despite further treatment (e.g. composting) sludge can still contain a number of harmful substances such as organotin compounds (OTCs)2 which can leach back to the environment when the treated sludge is used in landscaping or as fertilizers. In Finland tributyltin (TBT), dibutyltin (DBT) and monobutyltin (MBT) end up in aquatic environments via WWTP effluents.3 TBT, DBT and MBT were also frequently found in landfill runoff waters while triphenyltin (TPhT) and dioctyltin (DOT) were detected only in a few samples.3 However, diphenyltin (DPhT) and monophenyltin (MPhT) were not detected at all. Pinel-Raffaitin et al.4,5 observed inorganic tin to be one of the main contaminants which leach from the landfills and its concentration in leachates and biogases clearly exceeded the ones detected in aquatic and atmospheric ecosystems in Finland.6 The reuse of wastewater sludge is estimated to bring 1.1–2.6 kilograms of TBT into the environment and with effluents 0.01–0.4 kilograms of TBT ends up in surface waters.7
TBT is regulated due to its persistent and ubiquitous characteristics.8 It is one of the priority substances listed in European Union Water Framework Directive (WFD)9 since it presents a significant risk to aquatic environments. The EU restricted the use of OTCs in ship paints in 2003,10 and the use of tri-substituted OTCs for other purposes was banned in 2010.11 In 2010 the restriction was extended to imported products by setting a limit on their acceptable content per item (0.1% by weight of tin).11 In 2012 the restriction was extended to include DBT.12 The concentrations of OTCs in the environment are low but their toxicological effects appear in concentrations which are close to the limit of quantification (LOQ) since the environmental quality standard concentration (AA-EQS) for TBT is 0.2 ng L−1.13 This causes challenges to analytical methods since in most laboratories the LOQ for TBT can be higher by order of magnitude than its AA-EQS.
The OTCs are anthropogenic substances which are not formed in nature.8,14 There is a wide usage of DBT which covers e.g. glues, paints, plastic items and other household and personal care products from baking paper to earplugs.3,10 MBT has mainly been used in preparing plastic products. In Finland TBT has been applied as a wood protecting agent, slimicide and fungicide in the pulp and paper industry, disinfecting fishnets in fishfarms and as biocide in agriculture.3 During recent years the main source of TBT in Finland has been imported wood products.15 Monobutyltin (MBT), dibutyltin (DBT) and octyltins are still used outside the EU as PVC stabilizers in packaging materials from which they leach to foodstuff and beverages.16–19 Despite the restrictions these substances are still found and released into aquatic environments for example via WWTP.
According to Mehtonen et al.3 there was no connection between population equivalent and the concentration of OTCs detected in wastewater. However, Huhtala et al.6 observed that population equivalent contributed to MBT and DBT concentration but not TBT content. They suggested that the loading of TBT is constant and originates mainly from consumer products imported from outside the EU. The contribution of industrial wastewater and stormwater on the amount of DBT and MBT at WWTP was recognized as well. The high variation of DBT concentrations between different WWTPs can derive from its extensive industrial use. The source of fluctuating MBT loading in wastewaters is still unclear as its industrial use is minor and data on its application in consumer products is inadequate. One possibility would be that during the treatment process DBT is transformed to MBT.17 Due to low TBT contents at Finnish WWTPs transformation of TBT to DBT is not considered as a relevant DBT source. The degradation of TBT in water is ambiguously reported, since it strongly depends on the prevailing aquatic environment.8,20 The half-life of TBT in seawater depends on several things such as pH, temperature, turbidity and light.21,22 Seligman et al.23 observed a half-life of 6 or 7 days for TBT in marine waters in light and dark, respectively. However, the degradation of DBT was considered to be slower varying from 9 to 19 days in clean surface water.24 If suspended solids are present in water, TBT can attach to particles, which further hinder its degradation and increases the half-life.
Chemcatcher® passive samplers have been deployed for monitoring harmful substances in natural waters.25–29 If the concentration of an analyte is below LOQ in grab sample, it can be concentrated to a measurable level by passive sampling. Chemcatcher® has been proposed as the preferred sampling method over grab sampling as the latter technique e.g. requires pretreatment to avoid the clogging of solid phase extraction cartridge.30–33 Only a few Chemcatcher® passive sampler studies have been reported in wastewaters.33 There are some studies where polar organic integrative sampler (POCIS) has been deployed in wastewater effluent34,35 or hospital sewage waters.36 In general for POCIS as well as Chemcatcher® passive sampler the sampling rates are higher in laboratory conditions than in wastewater.33,36–38 When a Chemcatcher sampler is deployed without a diffusion membrane it detects far lower concentrations of harmful substances than e.g. POCIS sampler. Therefore uncovered Chemcatchers can be deployed at the sampling site for a shorter time than POCIS samplers.
The aim of this study was to assess the suitability of Chemcatcher® passive sampling as a semi-quantitative tool for determining OTCs in wastewater. The passive sampling technique was compared with grab sampling when studying the presence of OTCs at WWTP. Finally, the suitability of Chemcatcher® passive sampling technique on wastewater screening was estimated.
Materials and methods
Waste water treatment plant
The waste waters of the studied WWTP are mainly originated from sewage waters of 150![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 inhabitants in Jyväskylä region in central Finland. It also includes 7% of industrial waste water discharges from food-, chemical-, metal- and forest industries, power production, laundries and waste management.39,40 In 2012–2013 the average annual waste water discharge was 40
000 inhabitants in Jyväskylä region in central Finland. It also includes 7% of industrial waste water discharges from food-, chemical-, metal- and forest industries, power production, laundries and waste management.39,40 In 2012–2013 the average annual waste water discharge was 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 m3 d−1 (463 L s−1). The highest waste water volume is introduced to sewage system late in the morning and early in the evening. At the studied WWTP the purification process is based on simultaneous biological and chemical precipitation.40–42 The primary treatment includes addition of ferric salt, which coagulates phosphorus, screening, grit removal and sedimentation. In secondary treatment biologically degradable organic matter is removed with conventional activated sludge treatment, which is followed by addition of ferric salt and clarification by sedimentation. At the WWTP the retention time of solids was 2 days and residence time 16 hours. The effluents are discharged to Lake Päijänne which is a raw water source for drinking water production to capital region.
000 m3 d−1 (463 L s−1). The highest waste water volume is introduced to sewage system late in the morning and early in the evening. At the studied WWTP the purification process is based on simultaneous biological and chemical precipitation.40–42 The primary treatment includes addition of ferric salt, which coagulates phosphorus, screening, grit removal and sedimentation. In secondary treatment biologically degradable organic matter is removed with conventional activated sludge treatment, which is followed by addition of ferric salt and clarification by sedimentation. At the WWTP the retention time of solids was 2 days and residence time 16 hours. The effluents are discharged to Lake Päijänne which is a raw water source for drinking water production to capital region.
Passive sampling trials
The passive samplers were deployed at WWTP for three days. Three replicate samplers were kept both in raw influent stream where the water flow was high and in effluent tank with calm, continuous water flow. Thus, the hydrodynamic conditions were not similar due to the construction of WWTP. Four separate experiments were conducted during 10th–13th July 2012 (trial 1), 16th–19th July 2013 (trial 2), 27th–30th August 2013 (trial 3) and 3rd-6th September 2013 (trial 4) and during the trials grab samples (V = 300 mL) were taken daily. The removal percent of OTCs during the waste water treatment process was calculated based on both passive- and grab sampling results.Calculating the time weighted average (TWA) concentration
During the linear regime the passive sampler acts as an infinite sink for OTCs and the following applies:25,43–45| MS(t) = CWRSt | (1) |
Grab and composite sampling
The OTC concentrations measured in pooled 24 h composite samples were compared with the ones found in instant grab samples which were taken daily from raw influent and effluent waters (V = 300 mL). Three replicate analyses were performed for samples taken with both techniques. OTC concentrations were also measured weekly in raw influent and effluent waters between May and September in years 2012 and 2013. These samples were taken as 24 h composite samples. In addition the effect of filtration on the OTC concentration was studied since the OTCs were assumed to retain in particulate matter. The OTCs were analysed from filtered and unfiltered waste water samples during a three weeks' time period. Part of the effluent and influent water sample was filtered through GF/A glass fiber filter and analysed similarly as the unfiltered part. The amount of suspended solids was determined from both effluent and influent samples but due to relatively low amount of particles the OTCs were measured only in water phase.Materials and chemicals
The used solvents were HPLC-grade and the reagents were certified reference materials. The suspended solids were measured with GF/A glass fiber filters (pore size 1.6 μm) manufactured by Whatman (Whatman International Ltd, Kent, UK). The C-18 Empore disks (47 mm diameter) were ordered from 3M via Agilent Technologies Finland Ltd. The polycarbonate Chemcatcher® sampler housing was purchased from MP-Plast Inc. (Muurame, Finland). The Empore disk was conditioned with ultra high quality (UHQ) water (internal resistance ≥18 MΩ cm−1 at 25 °C).Sample preparation and analysis
The sample treatment and analysis were performed according to Ahkola et al.45 All waste water samples were collected into 1 L acid washed amber glass bottles and the samples were kept at 4 °C before analysing them at the following day of sampling. The volume of analysed grab water sample was 300 mL. In brief, internal standard (tri-n-propyltin), acetate buffer (1 M, pH 5.4) and sodium tetraethylborate NaB(C2H5)4 were added to water sample following by 5 mL hexane. The sample was kept in magnetic stirrer for 10 minutes. Hexane part was separated, evaporated to a smaller volume under nitrogen stream and transferred to a sample vial. Laboratory blank was prepared in each sample batch. The extracts were measured using GC-ICP-MS (Agilent 6890N gas chromatograph coupled with Agilent 7500ce ICP-MS). The GC column was HP 5 (30 m × 0.32 mm × 0.25 μm) and the analysis of OTCs was performed with following conditions: splitless injection mode (volume 1 μL), oven program 60 °C (1 min) to 300 °C (5 min) at 30 °C min−1 and carrier gas (He) flow 2 mL min−1. ICP-MS operated with carrier gas flow of 1.05 mL min−1, power of 900 W and dwell time of 0.1 s. Transfer line between GC and ICP-MS was kept at 300 °C. Studied OTCs were MBT, DBT, TBT, MPhT, DPhT, TPhT, MOT, DOT and trioctyltin (TOT). The LOQs were 0.2 ng L−1 for TBT and 0.5 ng L−1 for other OTCs when determining them in waste water grab samples.Before deployment the receiving phase of Chemcatcher® passive sampler, C-18 Empore disk, was conditioned by immersing it in methanol for 20 min. After that the disk was placed into filtration apparatus and 10 mL methanol was passed through the disk followed by 20 mL UHQ-water. No diffusion membrane was used in this study. The sampler housing parts were soaked in methanol overnight. The moist disk was placed into the assembled sampler housing and stored in a zip-lock bag at 4 °C until deployment. After the deployment the sampler was disassembled and the disk was extracted in ultrasonic bath with acetic acid/methanol (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) mixture and using tri-n-propyltin as an internal standard. The disk was removed and 4 mL acetate buffer (1 M, pH 5.4), 200 μL sodium tetraethylborate (NaB(C2H5)4) and 1.5 mL hexane were added to the extract and shaken for 5 minutes. The hexane part was separated and evaporated to a smaller volume and the OTCs were analysed using GC-ICP-MS. OTCs were also determined in sludge samples to estimate whether they are retained in sludge during the waste water treatment process. Nine sludge samples before digestion were taken at WWTP in three different sampling events. About 5 g of each sludge sample was extracted according to the procedure of Chemcatcher® passive sampler. The results are expressed per dry weight of solid matter. The LOQ of the passive sampling procedure was 0.05 ng per disk for TBT and 0.1 ng per disk for other OTCs. For sludge samples the LOQs were 0.2 ng g−1 dry weight for TBT and 0.5 ng g−1 for other OTCs.
1) mixture and using tri-n-propyltin as an internal standard. The disk was removed and 4 mL acetate buffer (1 M, pH 5.4), 200 μL sodium tetraethylborate (NaB(C2H5)4) and 1.5 mL hexane were added to the extract and shaken for 5 minutes. The hexane part was separated and evaporated to a smaller volume and the OTCs were analysed using GC-ICP-MS. OTCs were also determined in sludge samples to estimate whether they are retained in sludge during the waste water treatment process. Nine sludge samples before digestion were taken at WWTP in three different sampling events. About 5 g of each sludge sample was extracted according to the procedure of Chemcatcher® passive sampler. The results are expressed per dry weight of solid matter. The LOQ of the passive sampling procedure was 0.05 ng per disk for TBT and 0.1 ng per disk for other OTCs. For sludge samples the LOQs were 0.2 ng g−1 dry weight for TBT and 0.5 ng g−1 for other OTCs.
All analyses were conducted at laboratory certified by FINAS (Finnish Accreditation Service) as T142 (EN ISO/IEC 17025). Water chemistry characteristics were analysed by following standard procedures and their removal efficiency at WWTP was calculated as well. The determined characteristics were biological oxygen demand (BOD7), chemical oxygen demand (CODCr), suspended solids, total nitrogen (Ntot) and total phosphorus (Ptot).
Results and discussion
Passive sampling trials at WWTP
The sampling rates, RS, were determined at constant laboratory conditions using UHQ-water which included no solid particles. This minimizes the accumulation of OTCs into solid matter as well as the effect of other water characteristics on the sampling. The RS for each OTC is published in Ahkola et al.45 and presented for butyltins in Table 1. The conditions during laboratory calibration were certainly different from those prevailing at WWTP. For instance the flow rate in effluent was about half of the one prevailing in calibration experiment and in general, low flow rates decrease the sampling rates.45,46 In waste water the solid particles can absorb the OTCs away from the soluble phase so they are not available for passive samplers which collect only the dissolved part of the chemical.25 Based on the trials at WWTP RS in waste water was estimated by dividing the accumulated amount (MS) with deployment time (3 days) and average concentration in water during the sampling (Cw) (eqn (1)). In influent the RS were lower than in other experiments conceivably due to extreme sampling conditions (Table 1). The passive samplers were deployed in raw inflowing waste water which included polluted water, debris, trash and high water flow (463 L s−1) which disturbed the passive sampling procedure. In effluent the samplers were kept in a sink having a quiescent water flow. The sampling rates for MBT and DBT were about half of the laboratory calibrated ones which can be due to diminished water flow. For TBT the RS was quite the same in both influent and effluent waters. The presence of suspended solids in waste water could reduce the RS of TBT as well. In general, RS depends on the characteristics of the target compound.44 When studying PAH-compounds Greenwood et al.44 noticed that small, quite hydrophobic compounds had the highest RS whereas large and very hydrophobic compounds had the lowest RS. Changing the temperature from 6 to 18 °C increased the uptake rate 5.2 times.The OTC concentrations measured in influent waste water samples in 2012 (trial 1) were considerably higher than in 2013 (trials 2–4) (Table 2). During the trial 1 the average waste water volume was larger (41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 255 m3 d−1) than in other trials (trial 2 = 34
255 m3 d−1) than in other trials (trial 2 = 34![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 210 m3 d−1, trial 3 = 35
210 m3 d−1, trial 3 = 35![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 823 m3 d−1 and trial 4 = 36
823 m3 d−1 and trial 4 = 36![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 215 m3 d−1). The TWA concentrations of OTCs in water were calculated from the passive sampling results and compared with concentrations measured in grab samples (Table 2). MBT, DBT and TBT were found in all samplers deployed both in influent and effluent waters. Also MOT and DOT were observed in all trials except for trial 1, but then only MBT, DBT and TBT were measured from passive samplers. Despite TBT was found in all samplers, its concentration remained below the LOQ in all effluent grab water samples taken during trials 3 and 4 and in trial 2 it was detected once. In trial 2 DOT-concentration in influent was higher when determined with passive samplers than measured in grab samples. In trial 2 DOT was detected in grab samples only twice so the high DOT concentration pulse was probably missed due to unsuccessful timing of grab sampling. TPhT was detected only in one sampler exposed in influent (trial 4). MPhT was also found once but then it was detected in all replicate samplers (trial 3). The concentrations of phenyltins were very low as TPhT was measured only in one influent and effluent grab sample during trial 1. But again, phenyltins were not measured from the samplers at trial 1. The OTC concentrations in blank samples were below LOQ. In general the concentrations determined with passive samplers were in agreement with the concentrations measured in grab water samples. However, there were few exceptions which imply fluctuating OTC concentrations. However, passive samplers found OTCs which were not detected by conventional grab sampling. This suggests that passive sampling can be considered as a more suitable technique than grab sampling for monitoring OTCs in waste waters.
215 m3 d−1). The TWA concentrations of OTCs in water were calculated from the passive sampling results and compared with concentrations measured in grab samples (Table 2). MBT, DBT and TBT were found in all samplers deployed both in influent and effluent waters. Also MOT and DOT were observed in all trials except for trial 1, but then only MBT, DBT and TBT were measured from passive samplers. Despite TBT was found in all samplers, its concentration remained below the LOQ in all effluent grab water samples taken during trials 3 and 4 and in trial 2 it was detected once. In trial 2 DOT-concentration in influent was higher when determined with passive samplers than measured in grab samples. In trial 2 DOT was detected in grab samples only twice so the high DOT concentration pulse was probably missed due to unsuccessful timing of grab sampling. TPhT was detected only in one sampler exposed in influent (trial 4). MPhT was also found once but then it was detected in all replicate samplers (trial 3). The concentrations of phenyltins were very low as TPhT was measured only in one influent and effluent grab sample during trial 1. But again, phenyltins were not measured from the samplers at trial 1. The OTC concentrations in blank samples were below LOQ. In general the concentrations determined with passive samplers were in agreement with the concentrations measured in grab water samples. However, there were few exceptions which imply fluctuating OTC concentrations. However, passive samplers found OTCs which were not detected by conventional grab sampling. This suggests that passive sampling can be considered as a more suitable technique than grab sampling for monitoring OTCs in waste waters.
| OTC | Influent | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trial 1 | Trial 2 | Trial 3 | Trial 4 | |||||||||
| C S | C w | n | C S | C w | n | C S | C w | n | C S | C w | n | |
| MBT | 5.5 | 39–100 | 2 | 8.8 | 0.6–40 | 4 | 4.2 | 15–104 | 4 | 18 | 63–73 | 4 |
| DBT | 25 | 38–148 | 2 | 7.3 | 3.0–31 | 4 | 5.2 | 38–71 | 4 | 8 | 26–35 | 4 |
| TBT | 0.8 | <LOQ–2 | 1 | 0.07 | 0.2–3.6 | 2 | 0.04 | <LOQ–2.0 | 1 | 0.04 | 0.5–1.6 | 4 |
| TeBT | NM | <LOQ | 0 | <LOQ | <LOQ | 0 | <LOQ | <LOQ | 0 | <LOQ | <LOQ–1.2 | 1 |
| MPhT | NM | <LOQ | 0 | <LOQ | <LOQ | 0 | 0.18 | <LOQ | 0 | <LOQ | <LOQ | 0 |
| DPhT | NM | <LOQ | 0 | <LOQ | <LOQ | 0 | <LOQ | <LOQ | 0 | <LOQ | <LOQ | 0 |
| TPhT | NM | <LOQ–9 | 1 | <LOQ | <LOQ | 0 | <LOQ | <LOQ | 0 | 0.05 | <LOQ | 0 |
| MOT | NM | 10–29 | 2 | 2.8 | 2.1–6.3 | 3 | 2.6 | 17–33 | 4 | 3.2 | 9.6–14 | 4 |
| DOT | NM | 7–37 | 2 | 38 | 2.2–3.5 | 2 | 17 | 0.9–45 | 4 | 9.9 | 6.3–11 | 4 |
| TOT | NM | <LOQ–11 | 1 | <LOQ | <LOQ | <LOQ | <LOQ | 0 | <LOQ | <LOQ | 0 | |
| OTC | Effluent | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trial 1 | Trial 2 | Trial 3 | Trial 4 | |||||||||
| C S | C w | n | C S | C w | n | C S | C w | n | C S | C w | n | |
| MBT | 1.8 | 1.8–14 | 3 | 1.9 | 1–39 | 4 | 1.5 | 0.7–6.0 | 3 | 2.3 | 1.7–2.4 | 4 |
| DBT | 5.5 | 2.2–8.7 | 3 | 2.7 | 5–51 | 4 | 1.5 | 2.8–5.7 | 3 | 1.5 | 2.0–2.6 | 4 |
| TBT | 0.4 | 0.4–3.7 | 3 | 0.03 | <LOQ–0.2 | 1 | 0.04 | <LOQ | 0 | 0.02 | <LOQ | 0 |
| TeBT | NM | <LOQ | 0 | <LOQ | <LOQ | 0 | <LOQ | <LOQ | 0 | <LOQ | <LOQ | 0 |
| MPhT | NM | <LOQ | 0 | <LOQ | <LOQ | 0 | <LOQ | <LOQ | 0 | <LOQ | <LOQ | 0 |
| DPhT | NM | <LOQ | 0 | <LOQ | <LOQ | 0 | <LOQ | <LOQ | 0 | <LOQ | <LOQ | 0 |
| TPhT | NM | <LOQ–16 | 1 | <LOQ | <LOQ | 0 | <LOQ | <LOQ | 0 | <LOQ | <LOQ | 0 |
| MOT | NM | 0.5–2.5 | 3 | 0.6 | 0.7–4.8 | 3 | 0.9 | <LOQ–0.6 | 1 | 0.6 | <LOQ | 0 |
| DOT | NM | <LOQ–1.4 | 1 | 4.9 | 1.5–3.1 | 2 | 3.6 | <LOQ | 0 | 2.5 | <LOQ | 0 |
| TOT | NM | 1–35 | 2 | <LOQ | <LOQ | 0 | <LOQ | <LOQ | 0 | <LOQ | <LOQ | 0 |
Previous OTC-studies with passive samplers included diffusion membrane on which hindered the sampling rate.47–49 Only few studies involved Chemcatcher® passive sampling deployment in waste waters, however they did not concern OTCs Tan et al.33 studied endocrine disrupting compounds (EDSs) in waste water using deployment time of four days. They used uncovered styrene-divinylbenzene Empore disk with sulphonic acid functionality (SDB-RPS) as a receiving phase and determined the sampling rates for EDCs to be 1.12–3.23 L d−1, which were several times higher than the ones we determined for OTCs. However, as EDSs and OTCst have different physical and chemical properties (solubility, log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Kowetc.) their comparison is complicated. Calibration of POCIS passive sampler has been conducted in spiked waste waters to study pharmaceuticals and 15–40% lower Rs were observed in waste water than in tap water.36 The linear uptake region of the chemicals was observed to be shorter in waste water than in surface water being less than 7 days in previously mentioned. That derives from competitive sorption of analytes between the sampler receiving phase and surrounding organic matter. Tan et al.33 deployed Chemcatcher® samplers with SDB-RPS disk in waste water for four days and observed no clogging of the receiving phase. Shaw et al.50 found in their laboratory calibration that the linear region of Chemcatcher® with uncovered SDB-RPS receiving phase was 10 days. However, they deployed the samplers for 1, 5, 10 and 30 days so the uptake could have be remained at linear stage longer than just for 10 days. Vermeirssen et al.51 studied polar compounds in river and tap waters with identical Chemcatcher® configuration and only one compound from 22 approached the equilibrium after 25 days. Bailly et al.36 stated that the Rs should be determined in similar conditions as the field deployment in conducted, e.g. the content of organic matter. They observed that due to biofouling growth the deployment time in waste waters should not exceed 5 days. In our previous studies45 the linear uptake of OTCs determined in laboratory conditions using UHQ-water continued until the trial was stopped (9 days). In this study the samplers were estimated to remain at the linear region during the deployment time of three days. At retrieval only minimal biofouling was observed.
Kowetc.) their comparison is complicated. Calibration of POCIS passive sampler has been conducted in spiked waste waters to study pharmaceuticals and 15–40% lower Rs were observed in waste water than in tap water.36 The linear uptake region of the chemicals was observed to be shorter in waste water than in surface water being less than 7 days in previously mentioned. That derives from competitive sorption of analytes between the sampler receiving phase and surrounding organic matter. Tan et al.33 deployed Chemcatcher® samplers with SDB-RPS disk in waste water for four days and observed no clogging of the receiving phase. Shaw et al.50 found in their laboratory calibration that the linear region of Chemcatcher® with uncovered SDB-RPS receiving phase was 10 days. However, they deployed the samplers for 1, 5, 10 and 30 days so the uptake could have be remained at linear stage longer than just for 10 days. Vermeirssen et al.51 studied polar compounds in river and tap waters with identical Chemcatcher® configuration and only one compound from 22 approached the equilibrium after 25 days. Bailly et al.36 stated that the Rs should be determined in similar conditions as the field deployment in conducted, e.g. the content of organic matter. They observed that due to biofouling growth the deployment time in waste waters should not exceed 5 days. In our previous studies45 the linear uptake of OTCs determined in laboratory conditions using UHQ-water continued until the trial was stopped (9 days). In this study the samplers were estimated to remain at the linear region during the deployment time of three days. At retrieval only minimal biofouling was observed.
Previous studies showed that OTC concentrations measured from grab samples are higher than the TWA concentrations determined with passive samplers since grab samples also include a particle bound fraction.25,47,48 Aguilar-Martínez et al.49 observed the order of magnitude difference when studying OTCs with these two techniques. Tan et al.33 monitored endocrine disrupting compounds at WWTP and they noticed TWA concentrations calculated from passive sampling data to be lower by factor of 2 to >10 than in grab sampling. This applied also in our studies and it was especially observed in influent (Table 2). The grab water sample is taken at a certain moment and if the timing fails it can miss the contaminant peak. In addition passive samplers measure the average concentration of the compound during the whole sampling period. Difference of these two techniques is understandable, as they simply describe dissimilar issues.
Concentrations in effluent corresponded to the passive sampling results better than the ones in influent which can be explained with rather similar sampling conditions when compared to extreme circumstances prevailing in influent waters. With grab sampling only the concentrations above the LOQ can be observed which neglects the trace concentrations. On the other hand the high content of analyte in single grab sample can overestimate the true chemical content. However, due to low water flow in effluent the sampling rates of MBT and DBT were about half of the ones than in calibration trial (Table 1).
The TWA concentrations were compared with those measured from filtered and unfiltered grab samples. The results are presented as an average of four grab samples taken daily during trial 4 (Table 3). Filtering the samples did not reduce the OTC content dramatically which can be due to incomplete derivatization caused by suspended solids.52–54 It also appeared that the concentrations in filtered and in unfiltered grab samples were generally higher than the ones detected in passive samplers. However, passive samplers concentrated OTCs for three days which allowed the enrichment of trace contents to measurable level. This was discovered with TBT, MOT and DOT in effluent (Table 3).
| Influent | Effluent | |||||
|---|---|---|---|---|---|---|
| OTC | C sampler | C water | C water filtered | C sampler | C water | C water filtered |
| MBT | 18 | 68 | 76 | 2.3 | 2.0 | 1.8 |
| DBT | 7.8 | 32 | 27 | 1.5 | 2.3 | 2.1 |
| TBT | 0.04 | 1.0 | 0.5 | 0.02 | ND | ND |
| TeBT | ND | 1.2 | ND | ND | ND | ND |
| MPhT | ND | ND | ND | ND | ND | ND |
| DPhT | ND | ND | ND | ND | ND | ND |
| TPhT | ND | ND | ND | ND | ND | ND |
| MOT | 3.2 | 11 | 14 | 0.6 | ND | ND |
| DOT | 9.9 | 8.6 | 6.9 | 2.5 | ND | ND |
| TOT | ND | ND | ND | ND | ND | ND |
Comparison between grab water sampling and composite sampling
OTC concentrations measured from composite samples (x) were compared with the ones measured in grab samples (y). The equations were yinfluent = 1.834x and yeffluent=1.046x. The correlation coefficients were rinfluent = 0.810 and reffluent = 0.455 of which the first one is significant on the 0.1% risk level (n = 19). The reffluent is insignificant since the concentrations of OTCs in effluent were mainly below the LOQ and only six times OTCs could be analysed with both sampling techniques. The results imply that in influent grab samples the concentrations of OTCs were almost twice as high as in composite samples, which suggests that their concentration varies considerably during the composite sampling period of 24 hours. In Finland there is no standard procedure for collecting sample at WWTP to monitor harmful substances. In this study we tested both grab and composite samples since latter is used as a sampling technique when monitoring nutrient, suspended solids and oxygen contents in waste water.Monitoring of OTCs in waste water grab samples
Weekly monitoring revealed that the concentration of OTCs varied during the study period. MBT and DBT were the predominant OTCs and their concentration in incoming waste water varied between 18–350 ng L−1 and 18–300 ng L−1, respectively (Table 4). In effluent their contents dropped to 2.4–51 ng L−1 and 1–18 ng L−1. Also TBT (0.2–25 ng L−1), MOT (7.3–64 ng L−1) and DOT (3.2–150 ng L−1) were measured in nearly all influent samples. The rest of the OTCs were either mainly below the LOQ or at a level of few ng L−1 in both inflowing and outflowing waste water. The concentration ranges were mainly higher in year 2012 than in 2013 with two exceptions which were MPhT in influent and MOT in effluent samples (Table 4). TPhT was detected in effluent and in influent only once. The total number of waste water samples was 13 in 2013 whereas ten effluent and 11 influent samples were measured in 2012.| OTC | Influent 2012 | Influent 2013 | Effluent 2012 | Effluent 2013 | ||||
|---|---|---|---|---|---|---|---|---|
| (ng L−1) | n | (ng L−1) | n | (ng L−1) | n | (ng L−1) | n | |
| MBT | 18–350 | 11 | 50–150 | 13 | 3.0–51 | 10 | 2.4–6.0 | 10 |
| DBT | 29–300 | 11 | 18–100 | 13 | 1.0–18 | 10 | 1.3–6.9 | 11 |
| TBT | 0.9–25 | 8 | 0.2–1.4 | 12 | 0.2–2.0 | 6 | 0.4–1.4 | 2 |
| TeBT | ND–1.2 | 1 | ND–0.8 | 1 | ND | 0 | ND–1.0 | 1 |
| MPhT | ND–1.6 | 1 | 1.0–6.4 | 3 | ND–4.5 | 1 | ND | 0 |
| DPhT | ND | 0 | ND | 0 | ND | 0 | ND | 0 |
| TPhT | ND–4.1 | 1 | 0.5–0.9 | 3 | ND–4.5 | 1 | ND | 0 |
| MOT | 9.8–64 | 10 | 7.3–33 | 13 | 0.5–1.9 | 7 | ND–2.5 | 1 |
| DOT | 7.9–150 | 10 | 3.2–37 | 13 | 0.7–3.3 | 2 | ND–1.2 | 1 |
| TOT | 1.6–18 | 6 | ND–0.6 | 1 | 0.5–22 | 7 | ND | 0 |
In Finland TBT levels at WWTPs have been monitored earlier. Toivikko55 studied TBT concentrations in effluent samples of nine WWTPs and none of them exceeded the LOQ (1 ng L−1). Mehtonen et al.3 investigated four WWTPs and found TBT in concentrations of 2–9 ng L−1 in influent samples and <1–23 ng L−1 in effluents. DBT contents in inflowing waste water were 53–155 ng L−1 and in treated waste water 3–43 ng L−1 while for MBT the concentrations were 119–184 ng L−1 and 5–198 ng L−1, respectively. Both contents were at the same level as the ones measured in this study. Vieno7 monitored TBT from several WWTP influents (n = 36) and effluents (n = 60) and observed the concentrations to be rather low <0.2–3 ng L−1 and <0.2–1.9 ng L−1, respectively. The AA-EQS (0.2 ng L−1) was exceeded at five WWTPs. Our results follow the same trend in TBT concentration but the DBT content was somewhat higher than measured by Mehtonen et al.3 However, in our study we detected a wide range of MBT concentrations from very low contents to the high ones. In the study of Mehtonen et al.3 LOQ was 0.03–1.7 ng L−1 for water samples depending on the OTC. Toivikko et al.55 had the LOQ of 1 ng L−1 for OTCs in water which was higher than in our study.
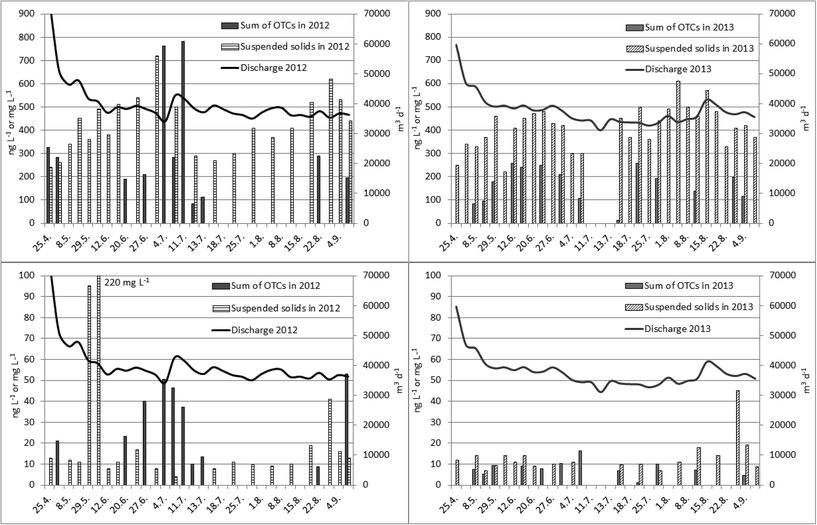
The OTC concentration followed neither the volume of waste water nor the amount of suspended solids in waste water (Fig. 1). The extraction of OTCs from solid particles may require more efficient pre-treatment with different eluents or procedures like pressurized liquid extraction56,57 or accelerated solvent extraction58 and therefore we assumed that only the dissolved part of the OTCs was measured.
Effect of filtration to the OTC concentrations
OTC concentrations in filtered (y) and unfiltered (x) waste water samples were compared as well. The influent contained mainly MBT, DBT, TBT, MOT and DOT (for OTCs found y = 0.836x, r = 0.896, significant at 0.1% risk level, n = 66). In effluents the dominating OTCs were MBT and DBT whereas TBT and MOT were detected only in a few samples (for OTCs found y = 0.883x, r = 0.676, which is significant at 0.1% risk level, n = 23). Filtering of the water samples did not reduce the OTC concentrations as much as was expected. This suggests that OTCs have been bound to fine particles which are small enough to pass through the filter (pore size 1.6 μm). One possibility would be that only the dissolved part of OTCs was measured in both cases since the extraction of OTCs from particles may require more efficient technique. The dissolved organic matter in water sample leads also to incomplete derivatization and underestimation of the concentration of OTCs.52–54 In this study the concentration of suspended solids was mainly below 20 mg L−1 except for 29th May 2012 and 5th June 2012 when the amounts were 95 mg L−1 and 220 mg L−1, respectively. At that time untreated waste water was released to Lake Päijänne, due to malfunction at WWTP. The slopes of the regression equations suggest that slightly lower concentrations of OTCs were found in filtered samples than in unfiltered ones. In general if OTCs were found in an unfiltered sample they were detected in a filtered sample as well.Removal of OTCs and water quality characteristics at WWTP
The removal percent of OTCs were calculated based on passive sampling and grab sampling results (Table 5). If the content remained <LOQ the sample was not included to the calculations. Higher removal was observed with grab sampling for all detected OTCs (MBT, DBT, MOT and DOT) except for TBT which also had the lowest removal percent. TOT concentration fluctuated having higher concentrations in effluent than in influent so the removal could not be determined. The removal percent could not be calculated for phenyltins nor TeBT since those OTCs were detected only few times at concentrations close to LOQ. The average elimination of TBT at Finnish WWTPs is 74% (Vieno7). There can be several explanations for low removal one being that in effluent samples the concentrations were near the LOQ. Passive sampling detects lower concentrations than grab sampling (TBT, MOT and DOT) and if the contents even are found in effluent the removal cannot be considered complete. The removal of suspended solids, CODCr, BOD7 and Ptot at WWTP was good being 93–98% (Table 5). First three of those characteristics describe the removal of particles and organic matter. When the coarse particles are removed during waste water treatment process the OTCs bound to them should be removed as well. This suggests that the particle bound fraction of OTCs is retained to solid matter and is removed during the process. The Ntot is not regulated by environmental authorities which explains its low removal.| OTC | Removal% | Grab sampling | Passive sampling | Waste water sludge | ||||
|---|---|---|---|---|---|---|---|---|
| Grab | Passive | n influent | n effluent | n influent | n effluent | Range (μg kg−1) | n | |
| MBT | 90 | 80 | 14 | 14 | 12 | 12 | 2.7–47 | 9 |
| DBT | 85 | 75 | 14 | 14 | 12 | 12 | 3.5–237 | 9 |
| TBT | 44 | 51 | 8 | 4 | 9 | 8 | 0.3–4 | 7 |
| TeBT | ND | ND | 1 | 0 | 0 | 0 | 7.7–12 | 3 |
| MPhT | ND | ND | 0 | 0 | 3 | 0 | ND | 0 |
| DPhT | ND | ND | 0 | 0 | 0 | 0 | ND | 0 |
| TPhT | ND | ND | 1 | 1 | 1 | 0 | ND | 0 |
| MOT | 91 | 75 | 13 | 7 | 9 | 9 | 0.2–15 | 9 |
| DOT | 87 | 83 | 12 | 3 | 8 | 7 | 1.1–15 | 7 |
| TOT | ND | ND | 1 | 2 | 0 | 0 | 4.4–8 | 2 |
| Suspended solids | 97 ± 2 | 31 | 32 | |||||
| CODCr | 93 ± 3 | 31 | 31 | |||||
| BOD7 | 98 ± 2 | 29 | 30 | |||||
| Ntot | 24 ± 8 | 31 | 32 | |||||
| Ptot | 97 ± 2 | 31 | 32 | |||||
OTCs in waste water sludge
In sludge samples MBT and DBT contents were the highest (2.7–47 μg kg−1 and 3.5–237 μg kg−1, respectively) of all the measured OTCs. According to their high removal percent during the treatment process (91% and 94%, respectively) it was expected that they were retained in waste water sludge (Table 5). TBT was observed in one sludge tank at concentration levels between 0.3–4 μg kg−1. MOT and DOT had high removal percents and were found in nearly all sludge samples. However, no phenyltins were found in sludge samples which implies they simply are not used anymore (Table 5). Mehtonen et al.3 detected much higher contents of MBT, DBT and TBT, 458–941 μg kg −1, 345–616 μg kg−1 and 9–18 μg kg−1, respectively, in sludge samples from nine WWTPs. They were close to the contents determined by Huhtala et al.6 which were 740 μg kg−1, 600 μg kg−1 and 9.1 μg kg−1 of MBT, DBT and TBT, respectively. Mehtonen et al.3 detected also TPhT in sludge at range <0.7–1.6 μg kg−1 whereas in survey conducted by Huhtala et al.6 the concentrations remained below the LOQ. Huhtala et al.6 did not include TOT in their analysis and Mehtonen et al.3 excluded MOT and TOT. They had somewhat higher LOQs since Mehtonen et al.3 had 0.4–25 ng g−1 dry weight for sludge and 0.03–1.7 ng L−1 for water samples. The LOQs of Huhtala et al.6 were 1 ng L−1 for water samples and 1–5 ng g−1 dry weight for sludge samples. The effect of sludge composting or digesting on the OTC content was not studied.Conclusions
During the wastewater treatment process the removal percent of OTCs was higher when based on grab sampling results than passive sampling results. There may be several explanations for this, one being that in effluent grab samples the concentrations were near or below the LOQ. Passive samplers found lower concentrations than was possible to detect with grab sampling. If the method is sensitive enough to detect the OTCs from effluent the elimination cannot be considered complete. The high removal of suspended solids, CODCr and BOD7 during the treatment process suggests that only the soluble fraction or the one bound to fine particles of OTCs is released from the WWTP.In influent the determined in situ RS values were reasonable but lower than in the calibration trial possibly due to extreme conditions. In effluent the estimated RS values for MBT and DBT were approximately half of the ones determined in laboratory calibration, which may be due to diminished water flow. For TBT the estimated RS was similar in both wastewaters. The presence of suspended solids in wastewaters could also reduce the sampling rate of OTCs. However, filtering of the wastewater samples did not reduce the OTC concentrations as much as expected which implies that OTCs are bound to small or colloidal particles that are able to pass the filter. The high concentration of suspended solids also hindered the derivatization of OTCs when the sample was prepared for the analysis. Overall, if OTCs attached to suspended solids are neither accumulated to passive samplers nor derivatized we can estimate that the contents measured with these two techniques are comparable.
In general the OTC-concentrations calculated based on passive sampling data were in line with those measured from grab samples. In grab samples the contents remain often below the LOQ, which does not necessary mean the compound is not present in the aquatic environment but rather implies unsuccessful timing. In conclusion, fewer OTCs were found with grab sampling than with passive sampling, particularly in effluent. The passive samplers seemed to be more suitable than grab samples for monitoring OTCs in wastewaters. Since long-term sampling techniques give a more representative picture of the true chemical contents, environmental authorities should consider passive sampling as an emerging tool for the monitoring of harmful chemicals in WWTP.
Acknowledgements
We would like to thank M.Sc. Petri Nieminen and M.Sc. Tino Hovinen for conducting the passive sampling trials. B.Sc. Saara Haapala, M.Sc. Tiina Virtanen and Ms. Rauni Kauppinen are greatly appreciated for their contribution on the sample treatment. This study was supported by the European Regional Development Fund, Maj and Tor Nessling foundation and Maa-ja vesitekniikan tuki ry.References
- EC, Directive 91/271/EEC, 1991, vol. 135, pp. 40–52.
- K. Peeters, T. Zuliani, J. Scancar and R. Milacic, Water Res., 2014, 53, 297–309 CrossRef CAS PubMed.
- J. Mehtonen, J. Mannio, K. Kalevi, S. Huhtala, J. Nuutinen, N. Perkola, P. Sainio, J. Pihlajamäki, V. Kasurinen, J. Koponen, R. Paukku and P. Rantakokko, Reports of the Finnish Environment Institute, 2012, vol. 29, p. 74 Search PubMed.
- P. Pinel-Raffaitin, M. Ponthieu, I. Le Hecho, D. Amouroux, L. Mazeas, O. F. X. Donard and M. Potin-Gautier, J. Environ. Monit., 2006, 8, 1069–1077 RSC.
- P. Pinel-Raffaitin, D. Amouroux, I. LeHecho, P. Rodriguez-Gonzalez and M. Potin-Gautier, Water Res., 2008, 42, 987–996 CrossRef CAS PubMed.
- S. Huhtala, P. Munne, T. Nakari, J. Nuutinen, N. Perkola, P. Sainio, E. Schultz and L. Schultz, Partner Report Finland, 2011, p. 111 Search PubMed.
- N. Vieno, Vesilaitosyhdistyksen monistesarja 34, p. 279 Search PubMed.
- R. F. Cole, G. A. Mills, R. Parker, T. Bolam, A. Birchenough, S. Kröger and G. R. Fones, Trends Environ. Anal. Chem., 2015, 8, 1–11 CrossRef CAS.
- EC, Directive 2000/60/EC, 2000, vol. L327, pp. 1–72.
- S. Dobson, P. Howe and P. Floyd, World Health Organization, 2006http://www.inchem.org/documents/cicads/cicads/cicad73.pdf Search PubMed.
- EC, Decision 2009/425/EC, 2009, vol. L138, pp. 11–13.
- EU, Regulation 276/2010, 2010, vol. L86, pp. 7–12.
- EC, Directive 2008/105/EC, 2008, vol. L348, pp. 84–97.
- J. Richter, I. Fettig, R. Philipp and N. Jakubowski, Environ. Sci. Pollut. Res., 2015, 22, 9589–9594 CrossRef CAS PubMed.
- A. Karvonen, T. Taina, J. Gustafsson, J. Mannio, J. Mehtonen, T. Nystén, M. Ruoppa, P. Sainio, K. Siimes, K. Silvo, S. Tuominen, M. Verta, K. M. Vuori and L. Äystö, Reports of the Ministry of the Environment, 2012, vol. 15, p. 77 Search PubMed.
- S. Takahashi, H. Mukai, S. Tanabe, K. Sakayama, T. Miyazaki and H. Masuno, Environ. Pollut., 1999, 106, 213–218 CrossRef CAS PubMed.
- M. Hoch, Appl. Geochem., 2001, 16, 719–743 CrossRef CAS.
- I. Mersiowsky, R. Brandsch and J. Ejlertsson, J. Environ. Qual., 2001, 30, 1604–1611 CrossRef CAS PubMed.
- R. Airaksinen, P. Rantakokko, A. W. Turunen, T. Vartiainen, P. J. Vuorinen, A. Lappalainen, A. Vihervuori, J. Mannio and A. Hallikainen, Environ. Res., 2010, 110, 544–547 CrossRef CAS PubMed.
- N. Watanabe, S. Sakai and H. Takatsuki, Water Sci. Technol., 1992, 25, 117–124 CAS.
- C. Stewart and S. J. Demora, Environ. Technol., 1990, 11, 565–570 CrossRef CAS.
- C. Alzieu, Ocean Coast Manage, 1998, 40, 23–36 CrossRef.
- P. F. Seligman, A. O. Valkirs, P. M. Stang and R. F. Lee, Mar. Pollut. Bull., 1988, 19, 531–534 CrossRef CAS.
- P. F. Seligman, A. O. Valkirs and R. F. Lee, Environ. Sci. Technol., 1986, 20, 1229–1235 CrossRef CAS.
- J. K. Kingston, R. Greenwood, G. A. Mills, G. M. Morrison and L. B. Persson, J. Environ. Monit., 2000, 2, 487–495 RSC.
- B. Vrana, G. A. Mills, E. Dominiak and R. Greenwood, Environ. Pollut., 2006, 142, 333–343 CrossRef CAS PubMed.
- E. L. M. Vermeirssen, N. Bramaz, J. Hollender, H. Singer and B. I. Escher, Water Res., 2009, 43, 903–914 CrossRef CAS PubMed.
- H. Ahkola, S. Herve and J. Knuutinen, Environ. Sci. Pollut. Res., 2013, 20, 1207–1218 CrossRef CAS PubMed.
- J. Petersen, D. Profrock, A. Paschke, J. A. C. Broekaert and A. Prange, Environ. Sci. Pollut. Res., 2015, 22, 16051–16059 CrossRef CAS PubMed.
- H. J. M. Verhaar, F. J. M. Busser and J. L. M. Hermens, Environ. Sci. Technol., 1995, 29, 726–734 CrossRef CAS PubMed.
- J. Namiesnik, B. Zabiegala, A. Kot-Wasik, M. Partyka and A. Wasik, Anal. Bioanal. Chem., 2005, 381, 279–301 CrossRef CAS PubMed.
- B. S. Stephens, A. Kapernick, G. Eaglesham and J. Mueller, Environ. Sci. Technol., 2005, 39, 8891–8897 CrossRef CAS PubMed.
- B. L. L. Tan, D. W. Hawker, J. F. Muller, F. D. L. Leusch, L. A. Tremblay and H. F. Chapman, Environ. Int., 2007, 33, 654–669 CrossRef CAS PubMed.
- S. L. Macleod, E. L. Mcclure and C. S. Wong, Environ. Toxicol. Chem., 2007, 26, 2517–2529 CrossRef CAS PubMed.
- S. L. Bartelt-Hunt, D. D. Snow, T. Damon, J. Shockley and K. Hoagland, Environ. Pollut., 2010, 158, 2790–2791 CrossRef CAS.
- E. Bailly, Y. Levi and S. Karolak, Environ. Pollut., 2013, 174, 100–105 CrossRef CAS PubMed.
- C. Harman, M. Reid and K. V. Thomas, Environ. Sci. Technol., 2011, 45, 5676–5682 CrossRef CAS PubMed.
- V. Yargeau, B. Taylor, H. X. Li, A. Rodayan and C. D. Metcalfe, Sci. Total Environ., 2014, 487, 722–730 CrossRef CAS PubMed.
- J. Mannio, J. Mehtonen, S. Londesborough, M. Grönroos, A. Paloheimo, P. Köngäs, K. Kalevi, K. Erkomaa, S. Huhtala, H. Kiviranta, K. Mäntykoski, J. Nuutinen, R. Paukku, H. Piha, P. Rantakokko, P. Sainio and L. Welling, The Finnish Environment, 2011, vol. 3, p. 97 Search PubMed.
- P. Tuominen, Managing director of Jyväskylän Seudun Puhdistamo Oy, 9th December 2014 private conversation Search PubMed.
- Regional State Administrative Agency for Eastern Finland, Environmental permit decision, 64/03/1, 2003, 28.10.2003.
- Supreme Administrative Court, Environmental Permit KHO 3615, 2005, 29.12.2005.
- B. Vrana, G. Mills, R. Greenwood, J. Knutsson, K. Svenssone and G. Morrison, J. Environ. Monit., 2005, 7, 612–620 RSC.
- R. Greenwood, G. A. Mills and B. Vrana, Comprehensive Analytical Chemistry Series, ed. D. Barcelo, Elsevier, Amsterdam, 2007, vol. 48, p. 453 Search PubMed.
- H. Ahkola, J. Juntunen, M. Laitinen, K. Krogerus, T. Huttula, S. Herve and A. Witick, Environ. Sci.: Processes Impacts, 2015, 17, 813–824 Search PubMed.
- K. Booij, H. M. Sleiderink and F. Smedes, Environ. Toxicol. Chem., 1998, 17, 1236–1245 CrossRef CAS.
- R. Aguilar-Martínez, R. Greenwood, G. A. Mills, B. Vrana, M. A. Palacios-Corvillo and M. M. Gomez-Gomez, Int. J. Environ. Anal. Chem., 2008, 88, 75–90 CrossRef.
- R. Aguilar-Martínez, M. A. Palacios-Corvillo, R. Greenwood, G. A. Mills, B. Vrana and M. M. Gomez-Gomez, Anal. Chim. Acta, 2008, 618, 157–167 CrossRef PubMed.
- R. Aguilar-Martínez, M. M. Gomez-Gomez and M. A. Palacios-Corvillo, Int. J. Environ. Anal. Chem., 2011, 91, 1100–1116 CrossRef.
- M. Shaw, G. Eaglesham and J. F. Mueller, Chemosphere, 2009, 75, 1–7 CrossRef CAS PubMed.
- E. L. M. Vermeirssen, C. Dietschweiler, B. I. Escher, J. van der Voet and J. Hollender, Anal. Bioanal. Chem., 2013, 405, 5225–5236 CrossRef CAS PubMed.
- O. F. X. Donard, P. Quevauviller and A. Bruchet, Water Res., 1993, 27, 1085–1089 CrossRef CAS.
- R. Vassallo and A. J. Vella, Appl. Organomet. Chem., 2002, 16, 21–26 CrossRef CAS.
- D. Said-Pullicino and A. J. Vella, Appl. Organomet. Chem., 2005, 19, 719–726 CrossRef CAS.
- S. Toivikko, Finnish Water and Waste Water Works Association, 2011 Search PubMed.
- A. Wasik, B. Radke, J. Bolalek and J. Namiesnik, Chemosphere, 2007, 68, 1–9 CrossRef CAS PubMed.
- B. Radke, A. Wasik, L. L. Jewell, U. Paczek and J. Namiesnik, Soil Sediment Contam., 2013, 22, 614–630 CrossRef CAS.
- C. G. Arnold, M. Berg, S. R. Muller, U. Dommann and R. P. Schwarzenbach, Anal. Chem., 1998, 70, 3094–3101 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |