Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Reversing aggregation: direct synthesis of nanocatalysts from bulk metal. Cellulose nanocrystals as active support to access efficient hydrogenation silver nanocatalysts†
Madhu
Kaushik
,
Alain You
Li
,
Reuben
Hudson
,
Mitra
Masnadi
,
Chao-Jun
Li
and
Audrey
Moores
*
Centre for Green Chemistry and Catalysis, Department of Chemistry, McGill University, 801 Sherbrooke St West, Montreal, QC H3A 0B8, Canada. E-mail: audrey.moores@mcgill.ca; Fax: +1 (514) 398 3797; Tel: +1 (514) 398 4654
First published on 3rd September 2015
Abstract
A highly atom-economical synthetic method to access nanocatalysts from bulk metal is described. A water suspension of cellulose nanocrystals was exposed to an Ag wire, under air and light exposure. In 2 weeks, Ag nanoparticles of size 1.3 nm ± 0.3 nm were deposited onto the biopolymer. These species were active for the hydrogenation of aldehydes, 4-nitrophenol, alkenes and alkynes.
Many different synthetic methods have been developed to access metal nanomaterials with well controlled size, shape, aspect ratio and thus properties. However, because metal nanoparticles (NPs) are kinetically stabilized materials, their synthesis often relies on intensive use of solvents, reagents, reducing and capping agents. The concept of atom economy, developed by Trost1 and adopted within the 12 principles of green chemistry for organic synthesis,2 remains to be systematically applied to nanosynthesis.3 In recent years, efforts have been made to develop more sustainable synthetic methods to access these high-value materials,3 including solvent-free methods,4 biomass-based approaches3d,5 and greener reducers development.6 An interesting avenue relies on by-passing the need to reduce metal salts and use a reduced metal form as starting material. While metal (0) organometallic species have been developed, they often require some synthetic effort and careful handling.7 Bulk metal grinding is also a well-known approach, suffering from poor NP size and shape control.4b A distinct and potentially milder approach relies on a result recently delineated by Hutchison and his group.8 Bulk Cu and Ag materials were shown to generate nanoparticles in their surface vicinity, as the results of exposure to moisture, light and air. In this interesting work, no external oxidizing, reducing agents or energy were needed to turn bulk into nanomaterials.
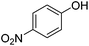
In this article, we present the novel synthetic method to access metal nanoparticles based on this principle, thus bypassing two industrial steps necessary to access such nanomaterials: (1) the need to form metal halide salts produced industrially via reacting pure metals with halohydric acids and (2) the reduction of the resulting halogenide salts, or other derivatives originating from these salts. For this study, we used cellulose nanocrystals (CNCs) as a water suspendable, and mildly reducing support. Ag NPs were produced in days at room temperature, in the presence of light, in water, from a simple Ag wire and without using any other additive than CNCs. Characterization by transmission electron microscopy (TEM) and X-ray photoelectron spectroscopy (XPS), including Auger analysis, was performed to establish that the resulting material was a hybrid composed of CNCs onto which small Ag NPs were deposited (AgNPs@CNC). In this work, we used the resulting Ag NPs in the catalytic hydrogenation of aldehydes to afford primary alcohols in water, at 100 °C and 40 bars H2.9 We (Li) had recently demonstrated the homogeneous version of this reaction using Ag salts with ligands.10 AgNPs@CNC were also effective in the hydrogenation of 4-nitrophenol, alkenes and alkynes. The aqueous suspension of AgNPs@CNC served as a biphasically separable catalytic system, recyclable up to 4 times, with negligible leaching.
CNCs are a class of functional nanomaterial readily obtained from cellulose via sulfuric acid hydrolysis.11 CNCs have well-defined size and morphology, high specific surface area, high aspect ratio, high crystalline order, chirality, high mechanical strength, and controllable surface chemistry. In addition, CNCs are inexpensive, renewable, biodegradable, non-toxic, and accessible industrially in large scale, from a local plant in Quebec.12 These properties have made CNCs useful in a range of applications, including the production of iridescent and birefringent films, reinforcing fillers in plastics and polymers, chiral materials, hydrogels, aerogels and supercapacitors.11b,12b,13 They have also been shown to act as active and water suspendable support to stabilize NPs of Pd,14 Pt,15 Au,16 Ag,16a,17 Cu16a and Se.18 Specifically, AgNPs@CNCs were previously synthesized from Ag salts, such as AgNO3, using either NaBH4,16a,17a–c or CNCs themselves as a reducing agent.17d,17e Ag NPs constitute interesting materials, used for antibacterial applications,17a,d,e,19 4-nitrophenol reductions,19a,20 oxidation,21 and alcohols cross-coupling22 reactions.
In an initial set of experiments, silver wires were immersed in CNC suspensions with concentrations of either 0.5%, 1% or 1.5% (w/w) in pure water. Some vials were exposed to light, while the others were kept in the dark (by wrapping with aluminium foil). All samples were air-exposed. After 45 days, the Ag content in the samples was measured using inductively coupled plasma-mass spectroscopy (ICP-MS) (Table 1).
| Entry | CNC concentration (in w/w) | Light exposure | Ag content (in μg L−1) |
|---|---|---|---|
| 1 | 0.5 | Yes | 163.1 |
| 2 | 1.0 | Yes | 166.3 |
| 3 | 1.5 | Yes | 144.4 |
| 4 | No CNC | Yes | 5.7 |
| 5 | 0.5 | No | 4.1 |
| 6 | 1.0 | No | 4.5 |
| 7 | 1.5 | No | 4.4 |
| 8 | No CNC | No | 5.1 |
The results were bimodal. High, and essentially similar, concentrations of Ag were observed when CNCs were present in the suspension and when the suspensions were exposed to light (Table 1, entries 1–3). Any other circumstance led to the presence of a baseline quantity of Ag (Table 1, entries 4–8). Light proved essential to silver oxidation in accordance with the work of Hutchison.8 But CNCs were also essential, proving their key role in the silver wire depletion.
The kinetics of this process was further explored with a 0.5% CNC suspension exposed to the Ag wire at room temperature (Fig. S1†). Its Ag content was monitored by ICP-MS upon sampling on days 3, 7, 10, 15 and 20. Two sets of experiment were carried out side by side, one in the presence, one in absence of light. Fig. 1 shows a steady increase of the Ag content in the CNC suspension for the first 10 days with light, after which it reached a plateau around 160 μg L−1. This value is comparable to results obtained at 45 days, indicating the achievement of an equilibrium state. Again, absence of CNC or light led to no significant increase in Ag concentration (Fig. 1). The role of air was probed by immersing a Ag wire in a degassed CNC suspension, in the presence of light. No significant amount of Ag was observed in this case either.
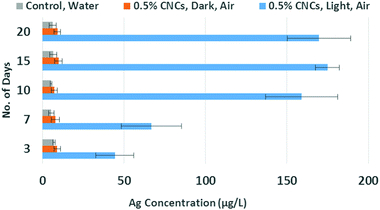 | ||
| Fig. 1 Ag content over 20 days by ICP-MS measurements. The values observed are an average of five data points for each type of experiment. | ||
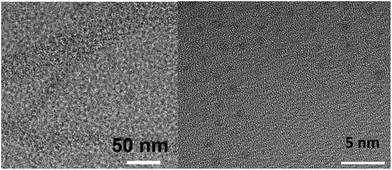
The ICP-MS measurements were performed after proper Ag digestion and were thus a means to quantify Ag in suspensions in all its possible forms, i.e. Ag salts and suspended Ag colloids. In order to gain more insight into the role of the CNC in the observed phenomenon as well as the nature of the ICP-MS measured Ag, a complete characterization of the resulting suspended material was undergone. A sample of 0.5% CNC suspension, stirred at room temperature for 20 days while exposed to Ag wire, light and air was dried onto a Cu TEM grid. On TEM images, CNCs were observed as well-dispersed, low contrast whiskers and the Ag NPs appeared as darker spots, mostly onto the CNCs, with an average size of 1.3 nm ± 0.3 nm (Fig. 2, S2–4†). The presence of Ag was confirmed by EDS (Fig. S4†). A careful selection of focusing conditions allowed to overcome the usual difficulties associated with CNCs/metal hybrid composites imaging.23 Reduction of Ag under TEM conditions was excluded based on time analysis, Ag NPs size and specific location onto CNC and XPS results (vide infra).24 These TEM results were in agreement to the previously reported CNC images,12b,23,25 showing that the CNCs did not aggregate in suspension and retained both their high surface area and rod-like form. Next, XPS experiments were performed to gain insight into the Ag oxidation state. Precise quantification of Ag(I) and Ag(0) species is difficult in the photoelectron region, because both species have binding energies which are close. In the Auger region, Ag(I) and Ag(0) each produce an individual peak, at 355.7 eV and 357.21 eV respectively. They were both observed in AgNPS@CNCs samples (Fig. S5a†). XPS being a surface technique, sputtering was performed on the samples for 15 seconds and led to Ag(I) peak disappearance, leaving the Ag(0) peak alone (Fig. S5b†). Standards for this method were obtained using Ag metal (Fig. S6†).
This study thus proved that: (1) Ag(0) NPs (with partially oxidized surface) were produced at the surface of CNCs upon immersing a Ag wire in a CNC suspension; (2) both CNCs and light were necessary for this reaction to proceed. A proposed mechanism is depicted in Fig. 3. According to the study of Hutchison and coll., light, moisture and air are able to oxidize the Ag wire into Ag(I) salts.8 In absence of CNCs, the reaction stops at this stage and the Ag concentration in suspension remains marginal. In fact, in absence of potential support, AgNPs tend to dissolve in pure water.26 When CNCs are in suspension, the Ag salts are consumed towards the growth of Ag NPs at the CNC surface, causing more Ag depleting from the wire and a sharp Ag concentration increase measured by ICP. CNCs are nanowhiskers composed of cellulose strings packed in a crystalline fashion. Cellulose itself is a polymer of cellobiose, a dimer of glucose. Therefore, the surface of CNCs is lined with hydroxyl groups, which have been shown before to enable the reduction of Ag(I) back into Ag(0).27 Cellulose was also shown to allow this reduction under microwave conditions.28 In the CNC synthesis process, by sulfuric acid hydrolysis, some hydroxyl groups are turned into sulfate esters (sulfur content = 3%). These sulfate groups have been proven to favour stabilisation and nucleation of Ag NPs in a recent research.17b A recent review on the evaluation of Ag NPs synthesis according to sustainable principles may be used to assess this novel method.3d Over the 8 evaluation criteria used, this method ranks very high on 7 of them (reducing agent, capping agent, solvent, local resources used, reaction temperature, equipment and size range).
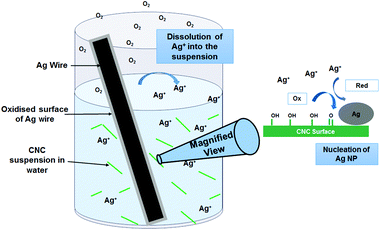 | ||
| Fig. 3 Pictorial representation of the mechanism for the generation of Ag NPs from Ag wire in aqueous CNC suspensions. | ||
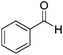
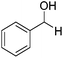
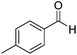
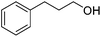
Beyond its key role in the Ag NPs synthesis, the CNC suspension also acted as a stabilizing medium for the nanocatalysts, which were stable as non-aggregated, small NPs, and a readily usable and separable catalytic system. The AgNPs@CNCs suspension obtained was tested as catalysts for the hydrogenation of unsaturated compounds, including aldehydes, alkenes, alkynes and nitro, in water (Table 2). To start with, benzaldehyde was chosen as the model substrate for optimisation of temperature and pressure conditions (Table S1†). At 100 °C and 40 bars H2, benzaldehyde was successfully hydrogenated to benzyl alcohol with a yield of 96% (Table 2, entry 1). The homogeneous counterpart of this reaction was recently demonstrated by us (Li) under comparable conditions and AgNPs@CNCs offer an appealing means of heterogenizing this process in an atom-economical fashion.10
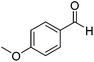
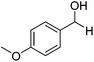
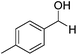
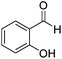
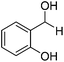
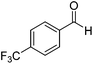
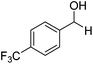
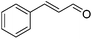
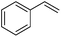
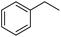
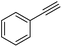
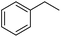
Further screening was performed on substituted aromatic aldehydes (Table 1, entries 2–5). Methyl and methoxy substitution in para position, as well as hydroxyl in ortho, afforded good yields. Trifluoromethyl however gave poor results, possibly due to solubility issues. Vinyl aldehydes also worked well, with complete reduction of both C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O and C
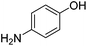
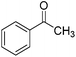
O and C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bonds (entry 6). Complete reduction of C–C double and triple bonds was also demonstrated on styrene and phenylacetylene (entries 7 and 8). We were also pleased to see that a nitroarenes (entry 9) could be turned into an aniline derivative, in a process that is more atom economical than the classic NaBH4, silver-catalysed reduction. To the best of our knowledge, this is the first example of this reaction being performed with Ag NPs and H2 as a reducer. Ketones however proved unreactive under these conditions (entry 10). The catalyst could also be easily recycled by addition of an organic solvent followed by phase separation, and the aqueous AgNPs@CNCs suspension was reused in a subsequent reaction. The yields were quantitative in the first four cycles, after which the activity reduced drastically (Fig. S7†). This sharp decrease was coincidental with the transformation of the water suspension into a gel, presumably because of water loss upon multiple workups. This gel is likely to lead to a drop in catalytic activity because of diffusion limitation. The leaching of Ag was quantified from the product of benzaldehyde hydrogenation. It was determined that from the 5 mL of a typical catalytic test, 95 ng L−1 of Ag had leached in the product. Compared to the original 168 μg L−1 concentration of Ag in the same solution, this corresponds to a marginal depletion of 0.056%. As the synthetic method described therein could be considered lengthier than comparable methods, it was checked that AgNO3 salts produced the same outcome as Ag wire exposure. A 175 μg L−1 aqueous solution of AgNO3 was stirred at room temperature in presence of CNCs (0.5 wt%) for 20 days. The resulting system performed very well for the hydrogenation of benzaldehyde (97% yield). As a control, it was checked that either a CNC suspension or a AgNO3 solution alone afforded no conversion for the same reaction (Fig. S8†).
C bonds (entry 6). Complete reduction of C–C double and triple bonds was also demonstrated on styrene and phenylacetylene (entries 7 and 8). We were also pleased to see that a nitroarenes (entry 9) could be turned into an aniline derivative, in a process that is more atom economical than the classic NaBH4, silver-catalysed reduction. To the best of our knowledge, this is the first example of this reaction being performed with Ag NPs and H2 as a reducer. Ketones however proved unreactive under these conditions (entry 10). The catalyst could also be easily recycled by addition of an organic solvent followed by phase separation, and the aqueous AgNPs@CNCs suspension was reused in a subsequent reaction. The yields were quantitative in the first four cycles, after which the activity reduced drastically (Fig. S7†). This sharp decrease was coincidental with the transformation of the water suspension into a gel, presumably because of water loss upon multiple workups. This gel is likely to lead to a drop in catalytic activity because of diffusion limitation. The leaching of Ag was quantified from the product of benzaldehyde hydrogenation. It was determined that from the 5 mL of a typical catalytic test, 95 ng L−1 of Ag had leached in the product. Compared to the original 168 μg L−1 concentration of Ag in the same solution, this corresponds to a marginal depletion of 0.056%. As the synthetic method described therein could be considered lengthier than comparable methods, it was checked that AgNO3 salts produced the same outcome as Ag wire exposure. A 175 μg L−1 aqueous solution of AgNO3 was stirred at room temperature in presence of CNCs (0.5 wt%) for 20 days. The resulting system performed very well for the hydrogenation of benzaldehyde (97% yield). As a control, it was checked that either a CNC suspension or a AgNO3 solution alone afforded no conversion for the same reaction (Fig. S8†).
Conclusions
In this article, we described for the first time the direct Ag NPs synthesis from bulk Ag metal in a one-pot setting. This facile, inexpensive synthetic method proceeded in water at room temperature. CNCs played a key role as high surface support and in situ reducer to drive the depletion of Ag from a metal wire and afford 1.3 nm ± 0.3 nm Ag NPs. The resulting hybrid was fully characterized by TEM and XPS and tested for their catalytic activity. Their ability to hydrogenate aldehydes, 4-nitrophenol, alkenes and alkynes was shown and they afford a cheap and sustainable alternative for their homogeneous counterparts.Acknowledgements
We thank the Natural Science and Engineering Research Council of Canada (NSERC) Discovery Grant program, the Canada Foundation for Innovation (CFI), the Canada Research Chairs (CRC), the Centre for Green Chemistry and Catalysis (CGCC), NSERC-Collaborative Research and Training Experience (CREATE) in Green Chemistry and McGill University for their financial support. We thank FPInnovations for providing the CNC starting material and Kevin Wilkinson and Madjid Hadioui from Université de Montreal for ICP-MS analysis. We are grateful to Hojatollah Vali and Kelly Sears for useful conversations on TEM analysis and Rajender Varma for interesting insight into silver nanoparticle synthesis. Elisabeth François-Moores is thanked for contributing to the graphical abstract material.Notes and references
- (a) B. M. Trost, Acc. Chem. Res., 2002, 35, 695–705 CrossRef CAS PubMed; (b) B. M. Trost, Angew. Chem., Int. Ed. Engl., 1995, 34, 259–281 CrossRef CAS.
- (a) P. Anastas and N. Eghbali, Chem. Soc. Rev., 2010, 39, 301–312 RSC; (b) P. T. Anastas and J. C. Warner, Green Chemistry: Theory and Practice, Oxford University Press, New York, 1998 Search PubMed.
- (a) J. A. Dahl, B. L. S. Maddux and J. E. Hutchison, Chem. Rev., 2007, 107, 2228–2269 CrossRef CAS PubMed; (b) C. J. Murphy, J. Mater. Chem., 2008, 18, 2173–2176 RSC; (c) M. J. Eckelman, J. B. Zimmerman and P. T. Anastas, J. Indian Ecol., 2008, 12, 316–328 CrossRef CAS; (d) M. Cinelli, S. R. Coles, M. N. Nadagouda, J. Błaszczyński, R. Słowiński, R. S. Varma and K. Kirwan, Green Chem., 2015, 17, 2825–2839 RSC.
- (a) M. J. Rak, N. K. Saadé, T. Friščić and A. Moores, Green Chem., 2014, 16, 86–89 Search PubMed; (b) M. J. Rak, T. Friščić and A. H. Moores, Faraday Discuss., 2014, 170, 155–167 RSC.
- J. Virkutyte and R. S. Varma, Chem. Sci., 2011, 2, 837–846 RSC.
- (a) V. Kelsen, B. Wendt, S. Werkmeister, K. Junge, M. Beller and B. Chaudret, Chem. Commun., 2013, 49, 3416–3418 RSC; (b) K. Pelzer, O. Vidoni, K. Philippot, B. Chaudret and V. Colliere, Adv. Funct. Mater., 2003, 13, 118–126 CrossRef CAS; (c) C. Pan, K. Pelzer, K. Philippot, B. Chaudret, F. Dassenoy, P. Lecante and M.-J. Casanove, J. Am. Chem. Soc., 2001, 123, 7584–7593 CrossRef CAS PubMed; (d) M. Kaushik, H. M. Friedman, M. Bateman and A. Moores, RSC Adv., 2015, 53207–53210 RSC.
- (a) K. Philippot and B. Chaudret, C. R. Chim., 2003, 6, 1019–1034 CrossRef CAS; (b) B. Chaudret, C. R. Phys., 2005, 6, 117–131 CrossRef CAS; (c) S. A. Stratton, K. L. Luska and A. Moores, Catal. Today, 2012, 183, 96–100 CrossRef CAS; (d) R. Linhardt, Q. M. Kainz, R. N. Grass, W. J. Stark and O. Reiser, RSC Adv., 2014, 4, 8541–8549 RSC.
- R. D. Glover, J. M. Miller and J. E. Hutchison, ACS Nano, 2011, 5, 8950–8957 CrossRef CAS PubMed.
- D. E. De Vos, P. G. N. Mertens, F. Cuypers, P. Vandezande, X. Ye, F. Verpoort and I. F. J. Vankelecom, Appl. Catal., A, 2007, 325, 130 CrossRef.
- Z. Jia, F. Zhou, M. Liu, X. Li, A. S. C. Chan and C.-J. Li, Angew. Chem., Int. Ed., 2013, 52, 11871–11874 Search PubMed.
- (a) D. Klemm, F. Kramer, S. Moritz, T. Lindström, M. Ankerfors, D. Gray and A. Dorris, Angew. Chem., Int. Ed., 2011, 50, 5438–5466 CrossRef CAS PubMed; (b) S. J. Eichhorn, A. Dufresne, M. Aranguren, N. E. Marcovich, J. R. Capadona, S. J. Rowan, C. Weder, W. Thielemans, M. Roman, S. Renneckar, W. Gindl, S. Veigel, J. Keckes, H. Yano, K. Abe, M. Nogi, A. N. Nakagaito, A. Mangalam, J. Simonsen, A. S. Benight, A. Bismarck, L. A. Berglund and T. Peijs, J. Mater. Sci., 2010, 45, 1–33 CrossRef CAS.
- (a) J. A. C. Gömez, U. W. Erler and D. O. Klemm, Macromol. Chem. Phys., 1996, 197, 953–964 CrossRef; (b) Y. Habibi, L. A. Lucia and O. J. Rojas, Chem. Rev., 2010, 110, 3479–3500 CrossRef CAS PubMed.
- (a) X. Cao, Y. Habibi, W. L. E. Magalhães, O. J. Rojas and L. A. Lucia, Curr. Sci., 2011, 100, 1172–1176 CAS; (b) S. J. Eichhorn, Soft Matter, 2011, 7, 303–315 RSC; (c) H. Koga, E. Tokunaga, M. Hidaka, Y. Umemura, T. Saito, A. Isogai and T. Kitaoka, Chem. Commun., 2010, 46, 8567–8569 RSC; (d) H. Koga, A. Azetsu, E. Tokunaga, T. Saito, A. Isogai and T. Kitaoka, J. Mater. Chem., 2012, 22, 5538–5542 RSC; (e) X. Yang and E. D. Cranston, Chem. Mater., 2014, 26, 6016–6025 CrossRef CAS; (f) J. Yang, C.-R. Han, J.-F. Duan, M.-G. Ma, X.-M. Zhang, F. Xu, R.-C. Sun and X.-M. Xie, J. Mater. Chem., 2012, 22, 22467–22480 Search PubMed; (g) N. L. Garcia de Rodriguez, W. Thielemans and A. Dufresne, Cellulose, 2006, 13, 261–270 Search PubMed; (h) S. Y. Liew, D. A. Walsh and W. Thielemans, RSC Adv., 2013, 3, 9158–9162 RSC; (i) S. Y. Liew, S. Shariki, A. Vuorema, D. A. Walsh, F. Marken and W. Thielemans, Cellulose nanowhiskers in electrochemical applications, in ACS Symposium Series, American Chemical Society, 2012, vol. 1107, pp. 75–106 Search PubMed; (j) K. E. Shopsowitz, H. Qi, W. Y. Hamad and M. J. MacLachlan, Nature, 2010, 468, 422–425 CrossRef CAS PubMed.
- (a) C. M. Cirtiu, A. F. Dunlop-Brière and A. Moores, Green Chem., 2011, 13, 288–291 RSC; (b) X. Wu, C. Lu, W. Zhang, G. Yuan, R. Xiong and X. Zhang, J. Mater. Chem. A, 2013, 1, 8645–8652 RSC; (c) M. Rezayat, R. K. Blundell, J. E. Camp, D. A. Walsh and W. Thielemans, ACS Sustainable Chem. Eng., 2014, 2, 1241–1250 CrossRef CAS; (d) M. Kaushik, K. Basu, C. Benoit, C. M. Cirtiu, H. Vali and A. Moores, J. Am. Chem. Soc., 2015, 137, 6124–6127 CrossRef CAS PubMed.
- (a) K. Benaissi, L. Johnson, D. A. Walsh and W. Thielemans, Green Chem., 2010, 12, 220–222 RSC; (b) F. Coccia, L. Tonucci, D. Bosco, M. Bressan and N. d'Alessandro, Green Chem., 2012, 14, 1073–1078 RSC.
- (a) S. Padalkar, J. R. Capadona, S. J. Rowan, C. Weder, Y.-H. Won, L. A. Stanciu and R. J. Moon, Langmuir, 2010, 26, 8497–8502 CrossRef CAS PubMed; (b) E. Lam, S. Hrapovic, E. Majid, J. H. Chong and J. H. T. Luong, Nanoscale, 2012, 4, 997–1002 RSC; (c) J.-L. Huang, D. G. Gray and C.-J. Li, Beilstein J. Org. Chem., 2013, 9, 1388–1396 CrossRef PubMed.
- (a) Z. Shi, J. Tang, L. Chen, C. Yan, S. Tanvir, W. A. Anderson, R. M. Berry and K. C. Tam, J. Mater. Chem. B, 2015, 3, 603–611 RSC; (b) A. R. Lokanathan, K. M. A. Uddin, O. J. Rojas and J. Laine, Biomacromolecules, 2014, 15, 373–379 CrossRef CAS PubMed; (c) H. Liu, D. Wang, Z. Song and S. Shang, Cellulose, 2011, 18, 67–74 CrossRef CAS; (d) N. Drogat, R. Granet, V. Sol, A. Memmi, N. Saad, C. Klein Koerkamp, P. Bressollier and P. Krausz, J. Nanopart. Res., 2011, 13, 1557–1562 CrossRef CAS; (e) R. Xiong, C. Lu, W. Zhang, Z. Zhou and X. Zhang, Carbohydr. Polym., 2013, 95, 214–219 CrossRef CAS PubMed.
- Y. Shin, J. M. Blackwood, I.-T. Bae, B. W. Arey and G. J. Exarhos, Mater. Lett., 2007, 61, 4297–4300 CrossRef CAS.
- (a) W. Xu, W. Jin, L. Lin, C. Zhang, Z. Li, Y. Li, R. Song and B. Li, Carbohydr. Polym., 2014, 101, 961–967 CrossRef CAS PubMed; (b) F. Cui, J. Liu, Z. Zhao and H. Feng, J. Mater. Chem., 2012, 22, 13891 RSC.
- (a) S. K. Das, M. M. R. Khan, A. K. Guha and N. Naskar, Green Chem., 2013, 15, 2548 RSC; (b) H. Liu, H. Liu, S. Yang and C. Nie, Mater. Lett., 2013, 100, 296 CrossRef; (c) K. Jiang, H.-X. Zhang, Y.-Y. Yang, R. Mothes, H. Lang and W.-B. Cai, Chem. Commun., 2011, 47, 11924–11926 RSC.
- (a) P. Singh, G. Lamanna, C. Menard-Moyon, F. M. Toma, E. Magnano, F. Bondino, M. Prato, S. Verma and A. Bianco, Angew. Chem., Int. Ed., 2011, 50, 9893–9897 CrossRef CAS PubMed; (b) A. Biabani-Ravandi, M. Rezaei and Z. Fattah, Chem. Eng. J., 2013, 219, 124–130 CrossRef CAS.
- A. Satsuma and K.-i. Shimizu, J. Jpn. Pet. Inst., 2011, 54, 347–360 CrossRef.
- (a) M. Kaushik, W. C. Chen, T. G. M. van de Ven and A. Moores, Nord. Pulp Pap. Res. J., 2014, 29, 77–84 CAS; (b) M. Kaushik, C. Fraschini, G. Chauve, J.-L. Putaux and A. Moores, in The Transmission Electron Microscopy, ed. K. Maaz, InTech, 2015, DOI:10.5772/60985.
- M. N. Nadagouda and R. S. Varma, Aust. J. Chem., 2009, 62, 260–264 CrossRef CAS.
- D. Klemm, B. Heublein, H. P. Fink and A. Bohn, Angew. Chem., Int. Ed., 2005, 44, 3358–3393 CrossRef CAS PubMed.
- C. Stephan, K. J. Wilkinson and M. Hadioui, Nanosci. Nanometrol., 2015, 1, 20–23 Search PubMed.
- P. Raveendran, J. Fu and S. L. Wallen, J. Am. Chem. Soc., 2003, 125, 13940–13941 CrossRef CAS PubMed.
- M. N. Nadagouda and R. S. Varma, Biomacromolecules, 2007, 8, 2762–2767 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5gc01281c |
| This journal is © The Royal Society of Chemistry 2016 |