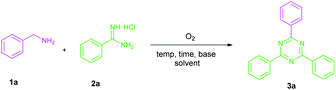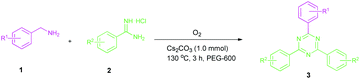Polythene glycol (PEG) as a reusable solvent system for the synthesis of 1,3,5-triazines via aerobic oxidative tandem cyclization of benzylamines and N-substituted benzylamines with amidines under transition metal-free conditions†
Abhishek R.
Tiwari
and
Bhalchandra M.
Bhanage
*
Department of Chemistry, Institute of Chemical Technology (ICT), Mumbai, India. E-mail: bm.bhanage@gmail.com; bm.bhanage@ictmumbai.edu.in; Fax: +91 2233611020; Tel: +91 2233612601
First published on 1st September 2015
Abstract
A green and highly efficient protocol for the synthesis of 1,3,5-triazines from benzylamines and N-substituted benzylamines with amidines in PEG-600 has been developed. This protocol is transition-metal free, phosphine ligand free and uses inexpensive, easily available molecular oxygen (O2) as an oxidant. A series of 1,3,5-triazines derivatives were synthesized in good to excellent yields in a shorter reaction time. The ease of the product separation and reusability of PEG-600 makes it more environmentally benign and economically affordable for gram-scale synthesis.
Green Chemistry emphasizes the development of economically feasible, synthetic procedures that avoid the use of toxic transition-metals and the utilization of environmentally benign substances and non-toxic solvents.1 Recently, transition-metal free reactions have gained prominence in organic synthesis.2 Not only does it avoid the use of toxic transition-metals, but it also reduces the cost of the developed procedure. Oxidation reactions are very important transformations in organic chemistry. Molecular oxygen (O2) is an ideal oxidant as it is inexpensive and easily available.3 In addition, having no toxic by-products make it a highly attractive reagent from the viewpoint of green sustainable chemistry.4 Most organic reactions are affected by a solvent as it plays an important role in mixing the ingredients to make the system homogeneous and allows molecular interactions to be more efficient.5 Thus, selection of a solvent is crucial. The solvent should be inexpensive, non-toxic, non-volatile and have easy recyclability. In this context, environmentally benign solvents such as water6 and ionic-liquids7 have been employed in various organic reactions. However, most organic moieties have a very low solubility in water which results in low yields of products. Further, the toxicity and environmental burden data for most of the ionic-liquids are still unknown. Over the past decade, polythene glycol (PEG) has been used in several organic reactions.8 PEG and its monomethyl ethers possess unique properties such as being thermally stable, commercially available, recyclable, immiscible with various organic solvents and non-toxic media for phase transfer catalysts.9 Further, PEG is inexpensive, completely non-halogenated and complete toxicity profiles are available for a range of polyethylene glycol (PEG) molecular weights. Many are approved for internal utilization by the US FDA.10
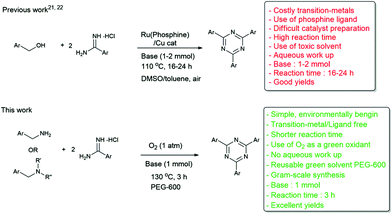
1,3,5-Triazine chemistry is of great importance due to their wide applications in biological and medicinal activities such as antimicrobial,11a antimalarial,11b antitumor agents11c antituberculosis11d and inhibition of photosynthetic electron transport (PET) and binding.11e In addition, they are used as chelating ligands for the preparation of organometallic materials,12 transition-metal catalysts,13 liquid crystals14 and fluorescent brighteners.15 Although 1,3,5-trianzines possess extensive functions, only a few methods for the synthesis of 1,3,5-triazines have been reported. Traditionally, they were synthesized from halogenated 1,3,5-triazines in the presence of transition metal-catalysts,16 and from the cyclotrimerization reaction of nitriles.17 However, the former requires transition-metal palladium (Pd), less-environmentally benign halogenated substrates, and it produces stoichiometric amounts of undesirable waste, and the latter usually needs an excess of amines as the co-catalysts. Alternatively, these compounds were also obtained by the cyclization of aromatic aldehydes with amidines.18 However, the use of aldehydes has several disadvantages such as the aldehydes could undergo a decarbonylation reaction under harsh reaction conditions19 and oxidation of active aldehyde groups, leading to the formation of unwanted by-products, hence they require inert conditions.20 Recently, two methods for the synthesis of 1,3,5-triazines from benzyl alcohol and amidines have been reported (Scheme 1). Nevertheless, these methods require a costly ruthenium-complex21 or less environmentally begin Cu(OAc)2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 22 as a catalyst. Moreover, the difficult preparation step of the Ru–phosphine complex, the use of toxic solvents such as DMSO and toluene, a longer reaction time, reflux conditions and an aqueous work up has imposed limitations on the applicability of these methods.
22 as a catalyst. Moreover, the difficult preparation step of the Ru–phosphine complex, the use of toxic solvents such as DMSO and toluene, a longer reaction time, reflux conditions and an aqueous work up has imposed limitations on the applicability of these methods.
In continuation with our ongoing work on transition-metal free approaches in organic synthesis,2h herein, we report a transition-metal free method for the synthesis of 1,3,5-triazines. Notably, this reaction proceeds via aerobic oxidative tandem cyclization of benzylamines with amidines at 130 °C for 3h.
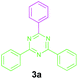
Initially, the direct reaction between benzylamine (1a) and benzamidine hydrochloride (2a) was selected as a model reaction to evaluate the feasibility of our system in an environmentally benign solvent at 100 °C for 24 h. When the reaction was carried out in H2O, ethanol and glycerol, no formation of the desired product was noted (Table 1, entries 1–3). Surprisingly, changing the solvent system to PEG-600 resulted in the formation of the desired product 3a in a 70% yield (Table 2, entry 4). This surprise result encouraged us to choose PEG-600 as the solvent. To our delight, increasing the temperature has a significant effect on the yield of 3a, providing a 95% yield at 130 °C (Table 1, entries 5–7). Increasing the reaction temperature above 130 °C didn't have a significant effect on the yield of 3a (Table 1, entry 8). The reaction time could be reduced to 3 h from 24 h (Table 1, entries 9–11). Decreasing the reaction time beyond 3 h leads to a significant decrease in the yield of the desired product 3a (Table 1, entry 12). Before heating the reaction mixture was transparent, but after heating for 3 h it turned yellow in colour. The addition of DMSO resulted in a decrease in the yield of product 3a (Table 2, entry 13). When the reaction was carried out in the absence of a base, no formation of the desired product was observed, even after running the reaction for 24 h (Table 1, entry 14). Bases such as Na2CO3 and K2CO3 did not give good results for the formation of the desired product 3a (Table 1, entry 15). Further, decreasing the amount of base from 1.0 mmol to 0.5 mmol resulted in the formation of product 3a with only a 51% yield (Table 1, entry 15). Thus, the optimized reaction conditions are: benzylamine (1a, 0.5 mmol), amidine hydrochloride (2a, 1.0 mmol), Cs2CO3 (1.0 mmol), PEG-600 (2.5 mL) for 3 h under O2.
| Entry | Solvent | Temp (°C) | Time (h) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: benzylamine (1a, 0.5 mmol), benzamidine hydrochloride (2a, 1.0 mmol), Cs2CO3 (1.0 mmol) and solvent (2.5 mL). b Isolated yield. c Na2CO3 was used instead of Cs2CO3. d K2CO3 was used instead of Cs2CO3. e Cs2CO3 (0.5 mmol). | ||||
| 1 | H2O | 100 | 24 | — |
| 2 | EtOH | 100 | 24 | — |
| 3 | Glycerol | 100 | 24 | — |
| 4 | PEG-600 | 100 | 24 | 70 |
| 5 | PEG-600 | 110 | 24 | 82 |
| 6 | PEG-600 | 120 | 24 | 90 |
| 7 | PEG-600 | 130 | 24 | 95 |
| 8 | PEG-600 | 140 | 24 | 96 |
| 9 | PEG-600 | 130 | 12 | 95 |
| 10 | PEG-600 | 130 | 6 | 95 |
| 11 | PEG-600 | 130 | 3 | 95 |
| 12 | PEG-600 | 130 | 2 | 71 |
| 13 | PEG-600![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) DMSO (1 DMSO (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
130 | 3 | 64 |
| 14 | PEG-600 | 130 | 3, 24 | —, — |
| 15 | PEG-600 | 130 | 3 | 57c, 78d, 51e |
| Entry | Amines (1) | Amidines (2) | Products (3) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: benzylamine (1, 0.5 mmol), amidine hydrochlorides (2, 1.0 mmol), Cs2CO3 (1.0 mmol), solvent (2.5 mL) for 3 h. b Isolated yield. | ||||
| 1 |
|
|
|
95 |
| 2 |
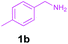
|
2a |
|
96 |
| 3 |
|
2a |
|
96 |
| 4 |
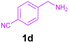
|
2a |
|
71 |
| 5 |
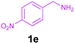
|
2a |
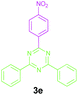
|
75 |
| 6 |
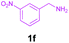
|
2a |
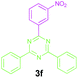
|
83 |
| 7 |
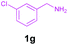
|
2a |
|
92 |
| 8 |
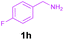
|
2a |
|
89 |
| 9 |
|
2a |
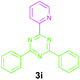
|
97 |
| 10 |
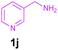
|
2a |
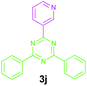
|
96 |
| 11 |
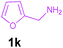
|
2a |
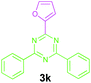
|
96 |
| 12 | 1a |
|
|
82 |
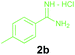
| 13 | 1c | 2b |
|
85 |
| 14 |
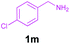
|
2b |
|
87 |
| 15 | 1c |
|
|
80 |
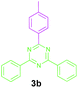
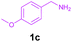
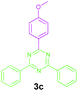
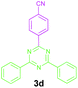
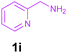
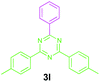
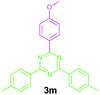
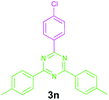
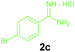
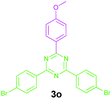
Encouraged by these results, we used various benzylamines and amidines to establish the scope and limitations of this protocol. A series of 1,3,5-triazines were synthesized in good to excellent yields under the optimized reaction conditions and representative results are listed in Table 2. Firstly, the effect of an electron donating group and an electron withdrawing group on benzylamine with benzamidine was studied (Table 2, entries 1–8). It was found that electron donating substituents such as –Me, –OMe, proceed smoothly and provided the desired products (3b–3c) in excellent isolated yields (Table 2, entries 2 and 3). Subsequently, the reaction of benzylamines bearing strong electron withdrawing groups such as –CN and –NO2 at different positions provided corresponding products (3d, 3e and 3f) in good yields (Table 2, entries 4–6). Interestingly, halogen substituents such as –Cl and –F could also be transformed in an efficient manner, providing respective products in very good yields (Table 2, entries 7 and 8). Next, heteroatom containing benzylamines such as pyridine-2-yl-2-methanamine, pyridin-3-ylmethanamine (1i and 1j) and furan-2-ylmethanamine (1k) were tested. Delightfully, all the reactions progressed efficiently affording products 3i–3k in very good yields (Table 2, entries 9–11). These obtained heteroaryl substituted 1,3,5-triazines have potential to be used as C^N or C^N^C ligands in pincer complexes.23 Next, an apparent substituent effect on amidine was also explored (Table 2, entries 12–15). Reaction of 1a, 1c and 1m with para-methylbenzamidine (2b) progressed very well affording 1,3,5-triazines (3l–3n) in excellent yields (Table 2, entries 12–14). Also, the reaction of 1c with para-bromobenzamidine resulted in the formation of the desired product (3o) with a good yield (Table 2, entry 15). Unfortunately, no formation of corresponding products could be observed when the reaction was carried out with both aliphatic amines and aliphatic amidines (results are not shown).
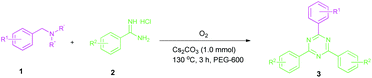
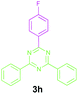
Efforts were also made to expand the scope of the method to N-mono and N,N-di substituted benzylamines and the results are summarized in Table 3. The reaction proceeded very well for N-methylbenzylamines (1aa) and N,N-dimethylbenzylamine (1aaa) providing the corresponding products in very good yields (Table 3, entries 1–6 and 8). Also, the reactions of N-ethylbenzylamine (1ab) and N-ethanolbenzylamine (1ab′) were found to be effective (Table 3, entries 7 and 10). However, the reaction of N,N-diethylbenzylamine (1abb) gave a relatively low yield of 3a compared to 1aaa (Table 3, entries 8 and 11).
| Entry | Amine (1) | Amidine (2) | Product (3) | Yieldb (%) |
|---|---|---|---|---|
| a Reaction conditions: benzylamine (1, 0.5 mmol), amidine hydrochlorides (2, 1.0 mmol), Cs2CO3 (1.0 mmol), solvent (2.5 mL) for 3 h. b Isolated yield. c Reaction was carried out for 8 h. | ||||
| 1 |
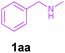
|
2a | 3a | 94 |
| 2 |
|
2a | 3c | 95 |
| 3 |
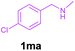
|
2a |
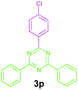
|
89 |
| 4 | 1aa | 2b | 3m | 83 |
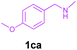
| 5 | 1ca | 2b | 3n | 84 |
| 6 | 1ma | 2b | 3o | 86 |
| 7 |
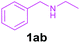
|
2a | 3a | 90 |
| 8 |

|
2a | 3a | 78, 82c |
| 9 | 1aaa | 2b | 3m | 81 |
| 10 |
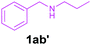
|
2a | 3a | 86 |
| 11 |
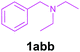
|
2a | 3a | 70c |
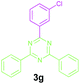
To show the synthetic utility of this protocol, gram-scale reactions were carried out by using substrates 1a (2 g, 18.87 mmol) with 2a (5.89 g, 37.74 mmol) and 1c (2.0 g, 16.53 mmol) with 2a (5.16 g, 33.06 mmol) under the optimized reaction conditions. As per our expectation, the reaction preceded well by providing 3c and 3g in 91% and 85% isolated yields, respectively.
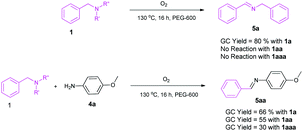
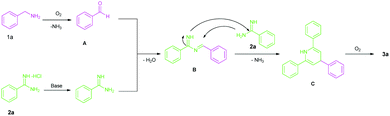
In order to understand the mechanism of these reactions, some control experiments were carried out (Scheme 2). When the reaction was carried out in the absence of amidines and Cs2CO3 formation of the imine (5a) was noted by self coupling of benzylamine. On the other hand, N-methyl and N,N-dimethylbenzylamine remain unchanged. This is in agreement with the previous report on the synthesis of imines.24 However, in the presence of para-methoxyaniline (4a) formation of the imine (5aa) was observed from 1a, 1aa and 1aaa. The yields of these imines are very low even after running the reaction for 16 h. This is possibly because 4a is much less basic than the amidines. The formation of these imines (5a and 5aa) was confirmed by GCMS. This proves that the presence of another amine influences the reaction and leads to the formation of an aldehyde via oxidative cleavage of the benzylic carbon and nitrogen bond. Based on the observations of control experiments, a plausible reaction mechanism has been illustrated (Scheme 3). The reaction proceeds with the in situ generation of an aldehyde (A). Meanwhile, the amidine salt (2) is neutralized by Cs2CO3 from its hydrochloride salt. Consequentially, reacting with A to give 1,3,5-tirazine (3a) via dehydrogenative aromatisation of C. This proposed mechanism is consistent with the previous reports.21,22 To the best of our knowledge, there is no report on the synthesis of imines from N-mono substituted benzylamine under transition-metal free conditions. This is the first time we have shown the formation of an imine from N-mono and di-substituted benzylamine under transition-metal free conditions. Further, unlike the previous reports,24,25 the self coupling reaction of benzylamine does not involve the use of any activating agents such as a catalyst or acid.
At last, we attempted to reuse the PEG-600. After completion of the reaction, PEG was recovered and subjected to another run, affording the product in almost the same yield. This process was repeated three more times, affording the product in excellent yields (Table 4). It is important to note that weight loss of ∼10% of PEG was observed for every run due to handling loss. The simple experimental and ease of product separation combined with the easy recovery and reuse of PEG is expected to contribute to the development of a green methodology for the synthesis of 1,3,5-triazines.
Conclusions
In conclusion, a highly efficient, transition-metal free synthesis of 1,3,5-triazines has been developed by employing molecular oxygen as a green oxidant in PEG-600. The use of molecular oxygen and PEG-600 as a non toxic solvent has made this protocol economical, environmentally benign and potentially viable for commercial and academic applications. Various 1,3,5-triazines were synthesized in good to excellent yields. The developed methodology is suitable for gram-scale synthesis, owing to its simple non-aqueous work up, shorter reaction time and higher yield. This protocol offers an excellent chance to avoid toxic solvents, catalysts and use of natural resources compared to previous reports. Noteworthily, for the first time we have demonstrated the synthesis of imines from N-mono and N,N-di substituted benzylamines. Moreover, after extraction with ether, PEG-600 was recovered and could be reused for up to three consecutive cycles.Acknowledgements
The author (ART) is greatly thankful to UGC (University Grant Commission) India, New Delhi for providing BSR fellowship.Notes and references
- R. A. Sheldon, I. Arends and U. Hanefeld, Green Chemistry and Catalysis, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, 2007 Search PubMed.
- (a) Y. Yuan, I. Thomé, S. H. Kim, D. Chen, A. Beyer, J. Bonnamour, E. Zuidema, S. Chang and C. Bolm, Adv. Synth. Catal., 2010, 352, 2892 CrossRef CAS; (b) R. Cano, D. J. Ramón and M. Yus, J. Org. Chem., 2011, 76, 654 CrossRef CAS PubMed; (c) Y. Fang, Y. Zheng and Z. Wang, Eur. J. Org. Chem., 2012, 1495 CrossRef CAS; (d) L. H. Zou, J. Reball, J. Mottweiler and C. Bolm, Chem. Commun., 2012, 48, 11307 RSC; (e) F. Diness and D. P. Fairlie, Angew. Chem., Int. Ed., 2012, 51, 8012 CrossRef CAS PubMed; (f) M. C. Pérez-Aguilar and C. Valdés, Angew. Chem., Int. Ed., 2012, 51, 5953 CrossRef PubMed; (g) N. Jalalian, T. B. Petersen and B. Olofsson, Chem. – Eur. J., 2012, 18, 14140 CrossRef CAS PubMed; (h) A. R. Tiwari and B. M. Bhanage, RSC Adv., 2015, 5, 57235 RSC.
- A. N. Campbell and S. S. Stahl, Acc. Chem. Res., 2012, 45, 851 CrossRef CAS PubMed.
- (a) B. M. Trost, Science, 1991, 254, 1471 CAS; (b) R. A. Sheldon, I. W. C. E. Arends, G. J. Ten Brink and A. Dijksman, Acc. Chem. Res., 2002, 35, 774 CrossRef CAS PubMed; (c) A. S. K. Hashmi and G. J. Hutchings, Angew. Chem., Int. Ed., 2006, 45, 7896 CrossRef PubMed; (d) A. Corma and H. Garcia, Chem. Soc. Rev., 2008, 37, 2096 RSC; (e) Y. Chen, D. M. Ho and C. Lee, J. Am. Chem. Soc., 2005, 127, 12184 CrossRef CAS PubMed; (f) L. Ackermann and L. T. Kaspar, J. Org. Chem., 2007, 72, 6149 CrossRef CAS PubMed; (g) For a review, see: Z. Shi, C. Zhang, C. Tanga and N. Jiao, Chem. Soc. Rev., 2012, 41, 3381 RSC.
- C. Reichardt and T. Welton, Solvents and Solvent Effects in Organic Chemistry, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim, 2011 Search PubMed.
- (a) Organic Synthesis in Water, ed. P. A. Grieco, Blackie Academic and Professional, London, 1998 Search PubMed; (b) Organic Reactions in Aqueous Media, ed. C. J. Li and T. H. Chan, John Wiley and Sons, New York, 1997 Search PubMed; (c) R. Breslow, Acc. Chem. Res., 1991, 24, 159 CrossRef CAS; (d) E. Rangel Rangel, E. M. Maya, F. Sánchez, J. G. de la Campaa and M. Iglesias, Green Chem., 2015, 17, 466 RSC; (e) P. Puthiaraja and K. Pitchumani, Green Chem., 2014, 16, 4223 RSC; (f) For a review, see: M. B. Gawande, V. D. B. Bonifácio, R. Luque, P. S. Brancoa and R. S. Varma, Chem. Soc. Rev., 2013, 42, 5522 RSC.
- (a) R. A. Sheldon, Chem. Commun., 2001, 2399 RSC; (b) C. L. Hussey, Pure Appl. Chem., 1988, 60, 1763 Search PubMed; (c) M. J. Earle and K. R. Seddon, Pure Appl. Chem., 2000, 72, 1391 Search PubMed; (d) T. Welton, Chem. Rev., 1999, 99, 2071 CrossRef CAS PubMed; (e) P. Wasserscheid and W. Keim, Angew. Chem., Int. Ed., 2000, 39, 3772 CrossRef CAS; (f) K. V. Wagh and B. M. Bhanage, Green Chem., 2015, 17, 4446 RSC; (g) K. V. Wagh and B. M. Bhanage, RSC Adv., 2014, 4, 22763 RSC.
- (a) For a review, see: J. Chen, S. K. Spear, J. G. Huddleston and R. D. Rogers, Green Chem., 2005, 7, 64 RSC; (b) R. Kumar, P. Chaudhary, S. Nimesh and R. Chandra, Green Chem., 2006, 8, 356 RSC; (c) G. P. Lu, L. Y. Zeng and C. Cai, Green Chem., 2011, 13, 998 RSC; (d) K. S. Feu, A. F. de la Torre, S. Silva, M. A. F de M. Junior, A. G. Corrêa and M. W. Paixão, Green Chem., 2014, 16, 3169 RSC.
- (a) Poly(ethylene Glycol) Chemistry, Biotechnological and Biomedical Applications, ed. J. M. Harris, Plenum Press, New York, 1992 Search PubMed; (b) Polyethylene Glycol: Chemistry and Biological Application, ed. J. M. Harris and S. Zalipsky, American Chemical Society, Washington, DC, 1997 Search PubMed.
- (a) N. Akiya and P. E. Savage, Chem. Rev., 2002, 102, 2725 CrossRef CAS PubMed; (b) U. M. Lindström, Chem. Rev., 2002, 102, 2751 Search PubMed.
- (a) M. Saleh, S. Abbott, V. Perron, C. Lauzon, C. Penney and B. Zacharie, Bioorg. Med. Chem. Lett., 2010, 20, 945 CrossRef CAS PubMed; (b) S. Melato, D. Prosperi, P. Coghi, B. Basilico and D. Monti, ChemMedChem, 2008, 3, 873 CrossRef CAS PubMed and references cited therein; (c) W. Zhu, Y. Liu, Y. Zhao, H. Wang, L. Tan, W. Fan and P. Gong, Arch. Pharm. Chem. Life Sci., 2012, 345, 812 CrossRef CAS PubMed; (d) R. V. Patel, P. Kumari, D. P. Rajani and K. H. Chikhalia, Eur. J. Med. Chem., 2011, 46, 4354 CrossRef CAS PubMed; (e) A. Ohki, N. Kuboyama, K. Koizumi, A. Tanaka, Y. Sato, H. Kohno, P. Böger and K. Wakabayashi, J. Agric. Food Chem., 1999, 47, 4398 CrossRef CAS PubMed.
- (a) S. Naik, M. Kumaravel, J. T. Mague and M. S. Balakrishna, Inorg. Chem., 2014, 53, 1370 CrossRef CAS PubMed; (b) C. Xiao, Y. Li, H. Lun, C. Cui and Y. Xu, J. Solid State Chem., 2013, 208, 127 CrossRef CAS.
- (a) M. H. Juàrez, M. Vaquero, E. Álvarez, V. Salazar and A. Suàrez, Dalton Trans., 2013, 42, 351 RSC; (b) P. K. Santra and P. Sagar, J. Mol. Catal. A: Chem., 2003, 197, 37 CrossRef CAS.
- (a) S. Kotha, D. Kashinath and S. Kumar, Tetrahedron Lett., 2008, 49, 5419 CrossRef CAS; (b) C. H. Lee and T. Yamamoto, Bull. Chem. Soc. Jpn., 2002, 75, 615 Search PubMed.
- A. García, B. Insuasty, M. Herranz, R. M. Álvarez and N. Martín, Org. Lett., 2009, 13, 5398 CrossRef PubMed.
- (a) D. Janietz and M. Bauer, Synthesis, 1993, 33 CrossRef CAS; (b) A. L. Isfahani, I. M. Baltork, V. Mirkhani, A. R. Khosropour, M. Moghadam, S. Tangestaninejad and R. Kia, Adv. Synth. Catal., 2013, 355, 957 CrossRef CAS.
- (a) A. Diaz-Ortiz, A. de la Hoz, A. Moreno, A. S. Migallon and G. Valiente, Green Chem., 2002, 4, 339 Search PubMed; (b) F. Xu, J. H. Sun, H. B. Yan and Q. Shen, Synth. Commun., 2000, 30, 1017 CrossRef CAS; (c) R. D. Spencer and B. H. Beggs, Anal. Chem., 1963, 35, 1633 CrossRef CAS.
- S. Biswas and S. Batra, Eur. J. Org. Chem., 2012, 3492 CrossRef CAS.
- (a) A. Modak, A. Deb, T. Patra, S. Rana, S. Maity and D. Maiti, Chem. Commun., 2012, 48, 4253 RSC; (b) T. Iwai, T. Fujihara and Y. Tsuji, Chem. Commun., 2008, 6215 RSC.
- G. V. R. Sharma and A. Robert, Res. Chem. Intermed., 2013, 39, 3251 CrossRef CAS.
- F. Xie, M. Chen, X. Wang, H. Jiangb and M. Zhang, Org. Biomol. Chem., 2014, 12, 2761 CAS.
- Q. You, F. Wang, C. Wu, T. Shi, D. Min, H. Chen and W. Zhang, Org. Biomol. Chem., 2015, 13, 6723 CAS.
- (a) W. Yang, H. Fu, Q. Song, M. Zhang and Y. Ding, Organometallics, 2010, 30, 77 CrossRef; (b) W. Wei, Y. Qin, M. Luo, P. Xia and M. S. Wong, Organometallics, 2008, 27, 2268 CrossRef CAS; (c) D. A. Smith, D. A. Roşca and M. Bochmann, Organometallics, 2012, 31, 5998 CrossRef CAS.
- T. B. Nguyen, L. Ermolenko and A. Al Mourabit, Green Chem., 2013, 15, 2713 RSC.
- (a) Z. Hu and F. M. Kerton, Org. Biomol. Chem., 2012, 10, 1618 RSC; (b) R. D. Patil and S. Adimurthy, Adv. Synth. Catal., 2011, 353, 1695 CrossRef CAS; (c) L. Aschwanden, T. Mallat, F. Krumeich and A. Baiker, J. Mol. Catal. A: Chem., 2009, 309, 57 CrossRef CAS; (d) B. Zhu, M. Lazar, B. G. Trewyna and R. J. Angelici, J. Catal., 2008, 260 Search PubMed; (e) H. Guo, M. Kemell, A. Al Hunaiti, S. Rautiainen, M. Leskelä and T. Repo, Catal. Commun., 2011, 12, 1260 CrossRef CAS; (f) S. Naya, K. Kimura and H. Tada, ACS Catal., 2013, 3, 10 CrossRef CAS; (g) T. Hirao, M. Higuchi, I. Ikeda and Y. Ohshiro, J. Chem. Soc., Chem. Commun., 1993, 194 RSC; (h) K. Shimizu, K. Shimura, K. Ohshima, M. Tamura and A. Satsuma, Green Chem., 2011, 13, 3096 RSC; (i) S. Furukawa, Y. Ohno, T. Shishido, K. Teramura and T. Tanaka, ACS Catal., 2011, 1, 1150 CrossRef CAS; (j) A. E. Wendlandt and S. S. Stahl, Org. Lett., 2012, 14, 2850 CrossRef CAS PubMed; (k) C. Su, M. Acik, K. Takai, J. Lu, S. Hao, Y. Zheng, P. Wu, Q. Bao, T. Enoki, Y. J. Chabal and K. P. Loh, Nat. Commun., 2012, 3, 1298 CrossRef PubMed; (l) H. Huang, J. Huang, Y. Liu, H. He, Y. Cao and K. Fan, Green Chem., 2012, 14, 930 RSC; (m) X. Lang, H. Ji, C. Chen, W. Ma and J. Zhao, Angew. Chem., Int. Ed., 2011, 50, 3934 CrossRef CAS PubMed; (n) E. Zhang, H. Tian, S. Xu, X. Yu and Q. Xu, Org. Lett., 2013, 15, 2704 CrossRef CAS PubMed; (o) F. Su, M. Antonietti, X. Wang, S. C. Mathew, L. Moehlmann and S. Blechert, Angew. Chem., Int. Ed., 2011, 50, 657 CrossRef CAS PubMed; (p) H. Yuan, W. Yoo, H. Miyamura and S. Kobayashi, J. Am. Chem. Soc., 2012, 134, 13970 CrossRef CAS PubMed; (q) M. Largeron and M. B. Fleury, Angew. Chem., Int. Ed., 2012, 51, 5409 CrossRef CAS PubMed; (r) M. Largeron and M. B. Fleury, Science, 2013, 339, 43 CrossRef PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: 1H NMR, 13C NMR, HRMS and GCMS. See DOI: 10.1039/c5gc01884f |
| This journal is © The Royal Society of Chemistry 2016 |