Open Access Article
Open Access ArticleSurfactant technology applied toward an active pharmaceutical ingredient: more than a simple green chemistry advance
Fabrice
Gallou
*a,
Nicholas A.
Isley†
b,
Adnan
Ganic
a,
Ulrich
Onken
a and
Michael
Parmentier
a
aChemical & Analytical Development, Novartis Pharma AG, 4056 Basel, Switzerland. E-mail: fabrice.gallou@novartis.com
bWork carried out while in Chemical & Analytical Development, Novartis Pharma AG, 4056 Basel, Switzerland
First published on 9th November 2015
Abstract
During our evaluation of the potential of surfactant technology, we rapidly experienced a straightforward and highly advantageous technology, which we applied on-scale. This resulted into significant benefits across our entire synthetic route, not just from an environmental standpoint but also from an economic and productivity perspective. To name a few: reduction of organic solvent use, reduction of water use, reduction of metric such as PMI, reduction of cost, reduction of cycle time, milder reaction conditions, improved yields, and improved process performances. Quantatively, the differences for some of these virtues approached 50% in favor of surfactant technology, all of which realised in multi-purpose facilities already within the infrastructure of standard pharmaceutical or chemical organizations. All of these benefits were achieved using a catalytic amount of a nonionic designer surfactant (e.g. TPGS-750-M) in water instead of traditional organic solvents.
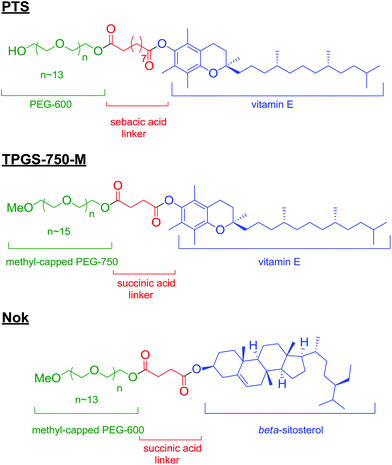
Surfactants have long been known for their remarkable physical properties as solubilizers. They have been used as a means of mixing oil in water,1 in the oil industry,2 and more recently as an excipient in food,3 and pharmaceuticals.4 Renewed interest has come with the continuous development of an expanding toolbox of synthetic applications based on newly engineered amphiphiles. Recent efforts in micellar catalysis from Lipshutz and co-workers, using powerful and versatile nonionic surfactants PTS,5 TPGS-750-M,6 or Nok7 (Scheme 1), has indeed led to the development of a variety of transformations mediated in water. These novel so-called “designer” surfactants fulfil not only all the requirements of a surfactant to be used for synthetic purposes, but also come with no safety or environmental baggage, being “benign-by-design.” Equipped with these surfactants, several laboratories devised improved protocols for a wide-range of transformations, and in particular, cross-coupling reactions.8 These new protocols not only illustrated the remarkable benefits of simple and harmless systems, but also paved the way for better practices leading to high yields, high selectivities in addition to decreasing our environmental footprint. Herein, we illustrate quantitatively the virtues of this technology that go far beyond the obvious environmental benefits.
Organic synthesis and sustainability
It is well recognized that organic solvents represent the vast majority of mass consumption and waste generated by the chemical industry (over 60% of the whole mass consumption in the pharmaceutical industry based on a recent benchmark).9 In an effort to address this issue and to reduce our environmental impact, we initiated a variety of projects aimed at the development and implementation for on-scale chemistry with an appropriate sustainable media, while decreasing our environmental footprint. Additionally, we were hoping to simultaneously address resource scarcity of other required substances such as transition-metal catalysts and complex intermediates requiring intense mass utilization. Moreover, we wanted to improve the overall efficiency of our processes with routes that were operationally simple, with more streamlined processes requiring reduced energetic investments with improved cycle time(s). Clearly, this was a nontrivial task for our department.Despite the known and obvious limited solubility of pharmaceutical compounds in water, we still seriously considered this medium as an option, but it was unrealistic to envision reactions ran under dilute conditions to allow for sufficient solubility. Our attention, therefore, rapidly turned to the use of nonionic surfactants since they were becoming more versatile compared to other polarized alternatives as a means to process our desired intermediates within our synthetic routes toward Active Pharmaceutical Ingredients (APIs). These surfactants, with both polar and nonpolar termini, undergo self-assembly in water to form nanomicelles when available in amounts above their critical micelle concentrations (CMC), typically ca. 10−4 M. Once organized, these surfactants form nanosized apolar aggregates in water that favor dissolution, through a dynamic process, of various components of a reaction system. The components are additionally in high concentration within the interior of the micelle due to the hydrophobic effect, and this concept rationalises the enhanced productivity and efficiency observed, as well as regio-, diastereo- and enantioselectivity in several reactions.10
At the time of our evaluation and implementation of the technology on large scale, the chemical toolbox was already large, encompassing various synthetic methodologies.11 The development of catalytic systems that were functional in water in the presence of surfactants, prompted us to focus on their potential as a simple, economic, and green reaction medium with many other important aspects, such as recyclability, activity, product and/substrate selectivity—the end goal being to utilize this chemistry on-scale. We wanted to quantitatively assess and push the limits of the technology to also include novel chemistry, such as aromatic nucleophilic substitution (SNAr) reactions, and amide bond formation, transformations with little-to-no precedent in water at the time of our journey, while developing technical solutions to enhance the robustness and scalability of these systems.
Results and discussion
In the following discussion, although at a very early stage of our development, we would like to illustrate the power of the technology due to the impact we predict it will have, and the precedence towards much superior practices it could create. We however had to mask the specific nature of the targeted drug substance and of the intermediates through the sequence for commercial sensitivity reasons. Our targeted sequence started with a SNAr reaction on a heteroaromatic trisubstituted system 1, ideally carried out in the presence of an unprotected hydroxyl functionality on our nucleophilic partner (Scheme 2, top). This N-arylation with compound 2 was carried out in the presence of a potentially competing O-arylation, but avoided protecting groups. It was to be followed by a Suzuki–Miyaura cross-coupling with either a boronate ester or boronic acid of 4. The resulting ester product 5 would be hydrolyzed in situ to afford the free acid 6, and subsequently submitted to the key amide bond-forming event.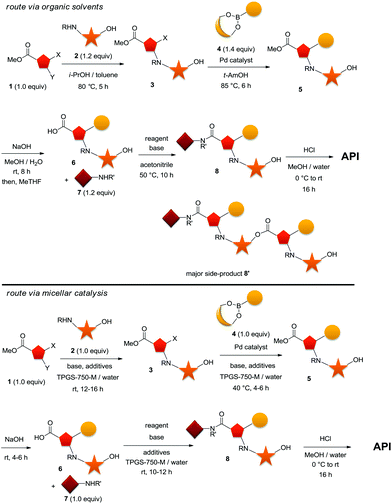 | ||
| Scheme 2 1st generation process of our API in organic solvents (top). 2nd generation process of our API using surfactant technology in water (bottom). | ||
Under standard organic solvent conditions, and after major rounds of optimization, the SNAr between compounds 1 and 2 proceeded to 3 in 87% yield with a minor amount of O-arylated material, which required further removal via crystallization. The extent of this side-reaction could be reduced by increasing the excess (1.2 to 1.4 equiv.) of nucleophile 2, but resulted in a loss of conversion. Milder conditions at reduced temperatures could also be used to minimize the extent of this side-product, but this came at an expense of a dramatically longer cycle time, making the whole process impractical on scale. The subsequent Suzuki–Miyaura cross-coupling between heteroarylhalide 3 and boronate ester 4 proceeded smoothly to 5, but required 1.4 to 1.5 equivalents of an unstable boronate ester 4. The relatively unstable boronate ester 4 was a major challenge and still remained an issue even under the optimized conditions (t-AmOH at 85 °C). Furthermore, to minimize cycle time, a rather high loading of palladium catalyst was required which triggered tedious downstream operations for its removal to acceptable levels. Hydrolysis of coupling product 5 to carboxylic acid 6 proceeded smoothly in wet methanol under basic conditions in an overall 60% yield for two steps. The subsequent amide-bond formation to 8 was, at the time, the most problematic, and continued to remain the major challenge even after significant optimization. Indeed, the presence of the free hydroxyl allowed for the formation of an ester dimer-like side product 8′. Significant optimization led to its minimization (ca. 12%), still unacceptable at such a late stage in the synthesis. In addition, an undesirable dipolar, aprotic solvent was required even after extensive experimentation while developing this first-generation process. Ultimately, a 76% yield could be obtained, although 20% excess of the expensive amine 7 was required. The final step consisted of basic hydrolysis and proceeded smoothly in a mixture of methanol/water.
Due to these problems within the development process, the entire sequence was re-evaluated using surfactant technology in water, and later would be implemented on-scale when appropriate. To our delight, we rapidly found that the whole sequence could be carried out advantageously in water with TPGS-750-M as the surfactant of choice (Scheme 2, bottom). The SNAr could proceed very smoothly and selectively at room temperature with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 stoichiometry of both the electrophile 1 and nucleophile 2 in 75% isolated yield (first crop) due to incomplete conversion (later on, this was addressed to afford a yield in the low 90% range). Surprisingly, a highly pure crude product mixture could be isolated after extraction with isopropyl acetate and precipitation with heptanes on-scale, and used as such in the subsequent Suzuki–Miyaura cross-coupling.
1 stoichiometry of both the electrophile 1 and nucleophile 2 in 75% isolated yield (first crop) due to incomplete conversion (later on, this was addressed to afford a yield in the low 90% range). Surprisingly, a highly pure crude product mixture could be isolated after extraction with isopropyl acetate and precipitation with heptanes on-scale, and used as such in the subsequent Suzuki–Miyaura cross-coupling.
For the generation of 5, the benefits of the mild conditions associated with the surfactant chemistry were especially prominent. Indeed, we observed sufficient reactivity at room temperature, which allowed for the reduction in stoichiometry of the boronate ester 4 to a much improved 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 ratio between the heteroaryl halide 3 and boronate ester 4. This led to a high quality crude product mixture owing to the mild reaction temperature (40 °C), affording an 87% isolated yield, with less than 1% protodeborylation. This allowed us to reduce the catalyst loading by a factor of two compared to the previous process, resulting in improved and streamlined downstream purification operations. The transformation now by-passed the need for a high boiling, polar solvent otherwise required for the cross-coupling, a major improvement for our processes as far as the European chemical legislation is concerned (REACH-SVHC).12 Nevertheless, it required use of ethyl acetate as an extraction solvent and TBME as an anti-solvent for the crystallization. The final key bond-forming step, i.e., the amide bond formation, displayed an even more dramatic result. Under mild, room temperature conditions, the extent of side-product decreased from ca. 12% in the optimal organic solvent conditions, despite extensive optimization, to below 1% after minimal effort in a water-based process. The 80% yield observed also required no excess of the amine, compared to the 20% excess needed in the organic solvent-mediated process. In each of the above surfactant-mediated transformations, it is important to point out that isolation of each product was not optimized, and accounted for most of the loss in the isolated yield. For example, we know that a much higher chemical yield is actually observed in the SNAr step, but at the time of our initial scale-up, we could only isolate 75% of the desired product. For the sake of discussion, we decided to keep these numbers on the conservative side to illustrate the power of the technology that, ultimately, proved to still be far superior to traditional approaches in organic solvents. The final hydrolysis step was conducted as in the earlier process under acidic conditions in a mixture of methanol and water.
1.1 ratio between the heteroaryl halide 3 and boronate ester 4. This led to a high quality crude product mixture owing to the mild reaction temperature (40 °C), affording an 87% isolated yield, with less than 1% protodeborylation. This allowed us to reduce the catalyst loading by a factor of two compared to the previous process, resulting in improved and streamlined downstream purification operations. The transformation now by-passed the need for a high boiling, polar solvent otherwise required for the cross-coupling, a major improvement for our processes as far as the European chemical legislation is concerned (REACH-SVHC).12 Nevertheless, it required use of ethyl acetate as an extraction solvent and TBME as an anti-solvent for the crystallization. The final key bond-forming step, i.e., the amide bond formation, displayed an even more dramatic result. Under mild, room temperature conditions, the extent of side-product decreased from ca. 12% in the optimal organic solvent conditions, despite extensive optimization, to below 1% after minimal effort in a water-based process. The 80% yield observed also required no excess of the amine, compared to the 20% excess needed in the organic solvent-mediated process. In each of the above surfactant-mediated transformations, it is important to point out that isolation of each product was not optimized, and accounted for most of the loss in the isolated yield. For example, we know that a much higher chemical yield is actually observed in the SNAr step, but at the time of our initial scale-up, we could only isolate 75% of the desired product. For the sake of discussion, we decided to keep these numbers on the conservative side to illustrate the power of the technology that, ultimately, proved to still be far superior to traditional approaches in organic solvents. The final hydrolysis step was conducted as in the earlier process under acidic conditions in a mixture of methanol and water.
Impact on quality
The overall quality of the final compound and of the process is of paramount importance in the pharmaceutical industry. This topic was obviously the first item of discussion insofar as implementation of the technology on-scale in our portfolio. We were cognizant of the mild conditions associated with this surfactant technology, which would lead to increased yields and selectivities while preserving high reactivity and minimizing or even avoiding decomposition pathways.8 In all steps described, very high quality of the crude intermediate products was observed. The intermediates could simply be isolated and purified by crystallization, thus ensuring the necessary control points. A final step involving a mixture of an alcohol and water led to the removal of any potential organic residue, after which the purity of the targeted drug substance turned out to be reliably 99.5% or greater, which is well above the routine purity obtained in the original process done in organic solvents.As for the potential presence of residual surfactant, it must be appreciated at the outset that it was designed as a harmless chemical. This translates, conservatively, into allowance of up to 0.15% TPGS-750-M and of its various components in the drug substance,13 a limit very easily achieved with our synthetic strategy.
Lastly, an additional benefit was the substantial depletion in residual metal required for the cross-coupling step within our API.
Metric analysis and fate of effluents
In recent years, Process Mass Intensity (PMI) has been typically recognized as a standard method of choice to measure the environmental performance of processes. The Process Mass Intensity (PMI) being defined as the quantity of raw material input (kg)/quantity of bulk API (kg). We used this metric to illustrate the benefits of this surfactant technology compared to organic solvents, and several PMIs were calculated on the solvents, water, and total organic mass, including raw materials; namely, all the intermediates 1 to 8, and the reagents.14,15 This included all steps in the synthetic path starting from the readily available heteroaryl halide 1 and amino alcohol 2. Raw materials are defined as all in-process materials that are used directly in the chemistry of synthesizing, isolating, and purifying the bulk API. The bulk API is the final form of the active ingredient that is produced in the synthesis, dried to the expected specifications, prior to any physical modification steps such as milling or formulation.The results shown in Table 1 below clearly indicate the improvement in ecological impact and overall mass efficiency for the synthesis performed under aqueous surfactant conditions. The overall mass intensity was indeed reduced by over 30%, mostly due to reduced solvent consumption, and better process performance. The overall yield has, likewise, increased by more than 5% (from 42.5% to 48%, overall), directly translating into direct economic benefits.
| Process in organic solvent | Process in water with surfactant | |
|---|---|---|
| Yield SNAr to 3 | 87% | 75% |
| Yield Suzuki–Miyaura to 5 | — | — |
| Yield hydrolysis to 6 | 70% | 87% |
| Yield amide-bond to 8 | 76% | 80% |
| Yield hydrolysis to API | 92% | |
| Overall yield | 42.5% | 48% |
| Overall PMI | 238 | 161 |
| Overall PMI change | −32% | |
| Overall PMI water change | −8% | |
| Overall PMI solvent change | −48% | |
| Overall PMI organic mass change | −52% | |
The SNAr, Suzuki–Miyaura cross-coupling, and the amide bond formation contributes to reduced costs based on lower stoichiometry of the amino alcohol 2, boronate ester 4, and amine 7, illustrated in the evolution of the PMI for substrates and reagents (a >50% reduction) (see Fig. 1). It is a rather general observation we have now experienced numerous times which provides significant economic benefits when the requirements for complex fragments can be minimized. This is mostly due to the desirable combination of sufficient reactivity and improved stability under the mild conditions used in the aqueous surfactant systems. From an environmental standpoint, the overall PMI also decreased by ca. 30% (from 238 to 161.5). The main reason, as anticipated, is the reduction in solvent usage by about 50% (from 105 to 55). It is also interesting to point out that while undesirable solvents must be used in the standard organic solvent-mediated sequence, the surfactant technology minimizes the requirement for an organic solvent to that of a mere solubilizer for extraction purposes. Our final overall process utilizes, for example, only toluene, and isopropyl acetate, instead of a variety of undesirable polar aprotic solvents (e.g. acetonitrile). The resulting organic waste can be easily discarded in standard fashion by incineration. The amount of water waste is also particularly noteworthy. The surfactants, indeed, allow us to work at high concentration, thus minimizing the absolute amount of water, maintaining these levels to those used in a standard process. In other words, there is no more water waste coming from the surfactant process compared to the original process in organic solvents, as demonstrated by our calculation (ca. 10% PMI reduction from 98 to 90). In addition, the nature of the contaminated water waste does not vary from that observed in our earlier process. We are currently designing and tailoring the nature of the reagents used in water to make the water effluent even easier to treat at the end of its lifetime and will report our first results shortly. For this kilogram-demo campaign, no recycling of the catalytic system has been applied, although recycling in the Suzuki–Miyaura cross-coupling step has already been demonstrated on a laboratory scale for this specific process.
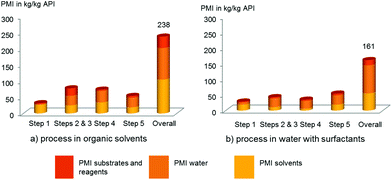 | ||
| Fig. 1 Comparison of evolution of the Process Mass Intensity (PMI): (a) the process in organic solvents vs. (b) process in water with surfactants. | ||
No specific economic benefit has been attached as yet to this process for obvious confidentiality reasons. However, a comparative analysis indicates that our overall scaled-up process in organic solvents was ca. 17% more expensive based on raw material costs only, omitting the processing costs, which would further reduce the economic balance for the process in water due to a much lower number of manipulations, and ease of operations (see Fig. 2). Disposal costs have also not been included, but our assessment indicates a profoundly positive effect, as no extra water waste is generated that is no more contaminated than in the original process in organic solvents.
The major financial gain, perhaps not surprisingly, is in the cross-coupling step due to a superior mass utilization and improved 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.1 stoichiometry, leading to a significant reduction in the excess of the (expensive) boronate ester 4. Although this situation is not always the case, it is a general trend in the pharmaceutical industry to involve complex and expensive boronic acid derivatives, which clearly highlights the economic benefits of this technology. Further opportunities to reduce costs have now been demonstrated through recent results coming from our collaboration with the Lipshutz group. Indeed, recent development around the surfactant technology now allows for the reduction in palladium catalyst from the typical 1 to 5 mol% to a few hundred ppm level, which has been demonstrated in more recent work,16 leading to a dramatic cost reduction.
1.1 stoichiometry, leading to a significant reduction in the excess of the (expensive) boronate ester 4. Although this situation is not always the case, it is a general trend in the pharmaceutical industry to involve complex and expensive boronic acid derivatives, which clearly highlights the economic benefits of this technology. Further opportunities to reduce costs have now been demonstrated through recent results coming from our collaboration with the Lipshutz group. Indeed, recent development around the surfactant technology now allows for the reduction in palladium catalyst from the typical 1 to 5 mol% to a few hundred ppm level, which has been demonstrated in more recent work,16 leading to a dramatic cost reduction.
Another important parameter that has to be taken into account when discussing the benefits of this technology is the operational aspect in the pilot plant. Each unit operation in the pilot plant is, indeed, time consuming and requires manpower, i.e. a short and straightforward process will be cheaper by definition. The outcome of the analysis demonstrates that both the SNAr and the amidation reactions are the most operationally complex steps in our standard process. The same reactions performed in water containing surfactants indicate that this operational complexity is reduced by 50%, as reflected in the cycle time. The hydrolysis reaction as such is not a complex process and is effective in both media. The main benefit comes from the previous cross-coupling step which allows for direct hydrolysis of the ester when performed in water. On the other hand, this same reaction requires tremendous effort when organic solvents are used given the solvent switch for this hydrolysis. Regarding the amidation reaction, the main reason for the drop in complexity index is the high selectivity observed during the reaction, allowing for direct filtration of 8 without the follow up basic treatment to degrade the ester by-product.
Analysis of our two processes reveals that using surfactants in water as the medium reduced productivity by nearly twofold compared to the original standard organic solvents processes. The main reasons for such streamlined processes are the complete removal of phase separations, extractions, washings or solvent switches (Table 2).
| Step | Cycle time (h) | |
|---|---|---|
| Organic solvent | TPGS-750-M/water | |
| SNAr to 3 | 104 | 61 |
| Cross-coupling to 5 | 61 | 24 |
| hydrolysis to 6 | 137 | 53 |
| Amide-bond formation to 8 | 105 | 76 |
| Final deprotection to API | 62 | 62 |
| Total | 469 (19.5 days) | 276 (11.5 days) |
It is interesting to note that this approach to synthesis can be implemented in any plant, without capital investment, which can be a major opportunity for innovation within more risk-adverse organizations. For big production facilities and especially in the context of a large portfolio of projects, this novel technology provides additional opportunities, as the footprint of some of the surrounding infrastructure can be further streamlined. For example, among several benefits from a safety standpoint, the commonly encountered tank farms sitting next to production facilities can be streamlined to a handful of much more desirable solvents, replacing undesirable and unsustainable solvents.
Conclusions
The successful multi-step kilogram scale process in water with a surfactant is to the best of our knowledge a first in the pharmaceutical industry and constitutes a real milestone. It internally triggered a paradigm shift that has since contributed to more systematic evaluation and implementation of the technology on scale, thus solving such issues such as undesirable solvents, decreasing our environmental footprint, employing simpler processes, while providing advantageous economic benefits. Although the initial view held was that it represented a mere environmental innovation, this kilogram-demo campaign demonstrated how powerful and transformative the technology could be. The more recent developments in the technology17 in highly efficient catalysis especially go far beyond the few improvements highlighted in the above manuscript and bode very well for the successful implementation of the technology and the impact it will undoubtedly have. Nonetheless, it remains in its infancy, and still requires significant efforts to better understand the new rules that govern such chemistry in water. How the various components of a given reaction and the surfactant interact, for example, are certainly under-investigated. Thus far, a mainly pragmatic path has been taken, and awaits more light to be shed onto the detailed exchange phenomena and mechanisms operating under the hydrophobic effect. Whether it involves a micellar-assisted mechanism, where reactants are dissolved in one of the phases, or an interfacial mechanism, where the reactants are located on one end of the surfactant, still needs to be answered.18 A cross-functional and multi-disciplinary effort will, therefore, be required as a next step in an effort to understand the overall process, and hence, to be able to further improve such processes long-term.Acknowledgements
We would like to particularly thank Professor Bruce Lipshutz and Dr Lawrence Hamann for the inspiration and helpful draft edits, along with current and past members of the Lipshutz group involved in paving the way in this surfactant technology, Professor Dieter Seebach for the encouragement and helpful discussions, and Dr Daniel Kaufmann for the active support.Notes and references
- H. Kunieda and M. Yamagata, Langmuir, 1993, 9, 3345 CrossRef CAS.
- A. A. Olajire, Energy, 2014, 77, 963 CrossRef CAS.
- M. J. L. Castro, C. Ojeda and A. Fernandez Cirelli, Environ. Chem. Lett., 2014, 12, 85 CrossRef CAS.
- G. La Sorella, G. Strukul and A. Scarso, Green Chem., 2015, 17, 644 RSC.
- B. H. Lipshutz and B. R. Taft, Org. Lett., 2008, 10, 1329 CrossRef CAS PubMed.
- B. H. Lipshutz, S. Ghorai, A. R. Abela, R. Moser, T. Nishikata, C. Duplais and A. Krasovskiy, J. Org. Chem., 2011, 76, 4379 CrossRef CAS PubMed.
- P. Klumphu and B. H. Lipshutz, J. Org. Chem., 2014, 79, 888 CrossRef CAS PubMed.
- B. H. Lipshutz and S. Ghorai, Tetrahedron, 2010, 66, 1057 CrossRef CAS; T. Nishikata, A. R. Abela and B. H. Lipshutz, Angew. Chem., Int. Ed., 2010, 49, 781 CrossRef PubMed; A. Krasovskiy, C. Duplais and B. H. Lipshutz, Org. Lett., 2010, 12, 4742 CrossRef PubMed; A. Abela, Z. Boskovic, T. Nishikata, C. Duplais, A. Krasovsky and B. H. Lipshutz, Top. Catal., 2010, 53, 985 CrossRef; A. Krasovskiy, I. Thome, J. Graff, V. Krasovskaya, P. Konopelski, C. Duplais and B. H. Lipshutz, Tetrahedron Lett., 2011, 52, 2203 CrossRef; B. H. Lipshutz, S. Ghorai, A. R. Abela, R. Moser, T. Nishikata, C. Duplais and A. Krasovskiy, J. Org. Chem., 2011, 76, 4379 CrossRef PubMed; B. H. Lipshutz, S. Ghorai, W. W. Y. Leong, R. Moser and B. R. Taft, J. Org. Chem., 2011, 76, 5061 CrossRef PubMed; C. Duplais, A. Krasovskiy and B. H. Lipshutz, Organometallics, 2011, 30, 6090 CrossRef PubMed; S. R. K. Minkler, B. H. Lipshutz and N. Krause, Angew. Chem., Int. Ed., 2011, 50, 7820 CrossRef PubMed; B. H. Lipshutz, S. Huang, W. W. Y. Leong, G. Zhong and N. A. Isley, J. Am. Chem. Soc., 2012, 134, 19985 CrossRef PubMed; G.-P. Lu, C. Cai and B. H. Lipshutz, Green Chem., 2013, 15, 105 RSC; N. A. Isley, F. Gallou and B. H. Lipshutz, J. Am. Chem. Soc., 2013, 135, 17707 CrossRef PubMed; C. Salome, P. Wagner, M. Bollenbach, F. Bihel, J.-J. Bourguignon and M. Schmitt, Tetrahedron, 2014, 70, 3413 CrossRef; P. Wagner, M. Bollenbach, C. Doebelin, F. Bihel, J.-J. Bourguignon, C. Salome and M. Schmitt, Green Chem., 2014, 16, 4170 RSC.
- P. Dunn, R. Henderson, I. Mergelsberg and A. Wells, ACS GCI Pharmaceutical Roundtable - Collaboration to Deliver a Solvent Selection Guide for the Pharmaceutical Industry Moving towards Greener Solvents for Pharmaceutical Manufacturing - An Industry Perspective, 2009, http://acs.confex.com/acs/green09/recordingredirect.cgi/id/510, (accessed Sept 21, 2015); R. A. Sheldon, I. W. C. E. Arends and U. Hanefeld, Green Chemistry and Catalysis, Wiley-VCH, Weinheim, Germany, 2007 Search PubMed.
- A. Krasovskiy, C. Duplais and B. H. Lipshutz, Org. Lett., 2010, 12, 4742 CrossRef CAS PubMed; B. H. Lipshutz, M. Hageman, J. C. Fennewald, R. Linstadt, E. Slack and K. Voigtritter, Chem. Commun., 2014, 50, 11378 RSC; R. T. H. Linstadt, C. A. Peterson, D. J. Lippincott, C. I. Jette and B. H. Lipshutz, Angew. Chem., Int. Ed., 2014, 53, 4159 CrossRef PubMed; E. D. Slack, C. M. Gabriel and B. H. Lipshutz, Angew. Chem., Int. Ed., 2014, 53, 14051 CrossRef PubMed; S. Handa, D. J. Lippincott, D. H. Aue and B. H. Lipshutz, Angew. Chem., Int. Ed., 2014, 53, 10658 CrossRef PubMed; S. Huang, K. Voigtritter, J. B. Unger and B. H. Lipshutz, Synlett, 2010, 2041 Search PubMed.
- B. H. Lipshutz and S. Ghorai, Aldrichimica Acta, 2008, 41, 59 CAS; B. H. Lipshutz and S. Ghorai, Aldrichimica Acta, 2012, 45, 3 Search PubMed.
- European Chemical Agency (ECHA), http://echa.europa.eu/web/guest/addressing-chemicals-of-concern/authorisation/substances-of-very-high-concern-identification, (accessed Sept 21, 2015).
- L. Navarro, A review of health and safety for Eastman Vitamin E TPGS, Product safety & Health, Eastman Chemical Company, Kingsport, Tennessee 37662-5054, 2005 Search PubMed.
- C. Jimenez-Gonzalez, C. S. Ponder, Q. B. Broxterman and J. B. Manley, Org. Proc. Res. Dev., 2011, 15, 912 CrossRef CAS . For a recent overview of various metrics, see: F. Roschangar, R. A. Sheldon and C. H. Senanayake, Green Chem., 2015, 17, 752 RSC.
- B. H. Lipshutz, N. A. Isley, J. C. Fennewald and E. D. Slack, Angew. Chem., Int. Ed., 2013, 52, 10911 CrossRef CAS.
- For “ppm-Level Pd-catalyzed cross-couplings in water at room temperature,” see: S. Handa, Y. Wang, F. Gallou and B. H. Lipshutz, Science, 2015, 349, 1087 CrossRef CAS PubMed; B. H. Lipshutz, presented in part at the 249th ACS National Meeting & Exposition, Denver, CO, USA, 2015; S. Handa, M. P. Anderson, F. Gallou, J. Reilly and B. H. Lipshutz, manuscript under review.
- N. A. Isley, M. S. Hageman and B. H. Lipshutz, Green Chem., 2015, 17, 893 RSC; J. C. Fennewald, E. B. Landstrom and B. H. Lipshutz, Tetrahedron Lett., 2015, 56, 3608 CrossRef CAS PubMed; C. M. Gabriel, M. Keener, F. Gallou and B. H. Lipshutz, Org. Lett., 2015, 17, 3968 CrossRef PubMed; S. Handa, E. D. Slack and B. H. Lipshutz, Angew. Chem., Int. Ed., 2015, 54, 11994–11998 CrossRef PubMed; A. Bhattacharjya, P. Klumphu and B. H. Lipshutz, Nat. Commun., 2015, 6, 7401 CrossRef PubMed; A. Bhattacharjya, P. Klumphu and B. H. Lipshutz, Org. Lett., 2015, 17, 1122 CrossRef PubMed; N. A. Isley, R. T. H. Linstadt, S. M. Kelly, F. Gallou and B. H. Lipshutz, Org. Lett., 2015, 17, 4734 CrossRef PubMed.
- A. Marracino, F. Amadei, G. Apollonio, G. d'Inzeo, M. Liberti, A. di Crescenzo, A. Fontana, R. Zappacosta, R. And and M. Aschi, J. Phys. Chem. B, 2011, 115, 8102 CrossRef CAS PubMed; M. Pera-Titus, L. Leclercq, J.-M. Clacens, F. De Campo and V. Nardello-Rataj, Angew. Chem., Int. Ed., 2015, 54, 2006 CrossRef PubMed.
Footnote |
| † Current address: Department of Chemistry and The Skaggs Institute for Chemical Biology, The Scripps Research Institute, 10550 North Torrey Pines Road, La Jolla, California 92037, USA |
| This journal is © The Royal Society of Chemistry 2016 |