Real-time modulated nanoparticle separation with an ultra-large dynamic range†‡§
Kerwin Kwek
Zeming
a,
Nitish V.
Thakor
ab,
Yong
Zhang
*cd and
Chia-Hung
Chen
*ac
aSingapore Institute for Neurotechnology (SINAPSE), 28 Medical Dr. #05-COR, 117456 Singapore
bDepartment of Biomedical Engineering, Johns Hopkins University School of Medicine, Traylor 701/720 Rutland Ave, Baltimore, MD 21205, USA
cDepartment of Biomedical Engineering, National University of Singapore, 9 Engineering Drive 1, Block EA #03-12, 117576 Singapore. E-mail: biezy@nus.edu.sg; biecch@nus.edu.sg; Tel: +65 6516 4871 Tel: +65 98514596
dCellular and Molecular Bioengineering Lab, National University of Singapore, Block E3A, #07-06, 7 Engineering Drive 1, 117574 Singapore
First published on 17th November 2015
Abstract
Nanoparticles exhibit size-dependent properties which make size-selective purification of proteins, DNA or synthetic nanoparticles essential for bio-analytics, clinical medicine, nano-plasmonics and nano-material sciences. Current purification methods of centrifugation, column chromatography and continuous-flow techniques suffer from particle aggregation, multi-stage process, complex setups and necessary nanofabrication. These increase process costs and time, reduce efficiency and limit dynamic range. Here, we achieve an unprecedented real-time nanoparticle separation (51–1500 nm) using a large-pore (2 μm) deterministic lateral displacement (DLD) device. No external force fields or nanofabrication are required. Instead, we investigated innate long-range electrostatic influences on nanoparticles within a fluid medium at different NaCl ionic concentrations. In this study we account for the electrostatic forces beyond Debye length and showed that they cannot be assumed as negligible especially for precise nanoparticle separation methods such as DLD. Our findings have enabled us to develop a model to simultaneously quantify and modulate the electrostatic force interactions between nanoparticle and micropore. By simply controlling buffer solutions, we achieve dynamic nanoparticle size separation on a single device with a rapid response time (<20 s) and an enlarged dynamic range (>1200%), outperforming standard benchtop centrifuge systems. This novel method and model combines device simplicity, isolation precision and dynamic flexibility, opening opportunities for high-throughput applications in nano-separation for industrial and biological applications.
Introduction
Nanoparticles exhibit size-dependent properties based on their morphology, structure, chemical composition and synthesis process.1–3 The size-selective purification of nanoscopic objects is essential for a broad spectrum of applications in bio-analytics, clinical medicine, bead-based assays, high-resolution optical imaging, nanoparticle catalytic activity and nanocomposite materials in which the nanoparticle size determines the overall properties such as antibacterial functions.4–8 Conventional methods for nanoparticle separation and purification, such as gel electrophoresis, column chromatography and centrifugation, have high separation resolutions and well-established protocols;9 however, they require multistage extraction processes without real-time control over particle separation, which inevitably limits the dynamic range of nanoparticle separation that can be achieved. For instance, the affinity binding chromatography method of purifying proteins or drugs requires multiple processing steps for column preparation, sample binding, washing, eluting and purification for a period of 12 to 48 hours. Centrifugal systems are the most common methods of purifying nanoparticles because they offer highly reproducible separation based on particle size and density.10,11 However, without continuous flow separation, sample loss occurs due to nanoparticle aggregation and the difficulty of re-suspending a centrifuged sample.12Continuous flow separation is a new class of separation that can be achieved using microfluidic devices. It is low in cost and offers reduced sample/reagent requirements, scalable throughput, device automation and real-time control of the separation ranges.13,14 A wide spectrum of methods ranging from active acoustic,15,16 electrophoretic,17 and centrifugal18 force fields to passive pore-based filtration19 and nano-structured traps20 have been developed for nanoparticle separation in fluidic devices. Among these methods, the pore-based deterministic lateral displacement (DLD) method is highly promising due to its versatility for passive or integrated active sorting modes, scalability and potential nanometer-resolution separation.16,21–23 Attempts have been made to use DLD for nano-separation, but the results showed poor separation of 190 nm particle separations.24 To date, Huang et al. have succeeded in electrokinetically separating large bacterial DNA using DLD devices characterized by nanometer-scale polystyrene particles ranging from 1000 nm to 600 nm;21 Chen et al. separated a 1000 nm blob of genomic DNA using a 1.7 μm DLD pore in a high concentration of polyethylene glycol (10% w/v).25 Despite these advances, effective continuous flow nano-separation is highly dependent on active force fields or nano-fabricated constructs, resulting in a trade-off between dynamic range and separation resolution. Active force fields allow for real-time particle separation manipulation, although achieving high-resolution separation remains challenging and additional equipment is required. By contrast, nano-fabricated constructs enable remarkable control over separation resolution, albeit with limited dynamic range control, complex nano-lithography requirements and low throughput.
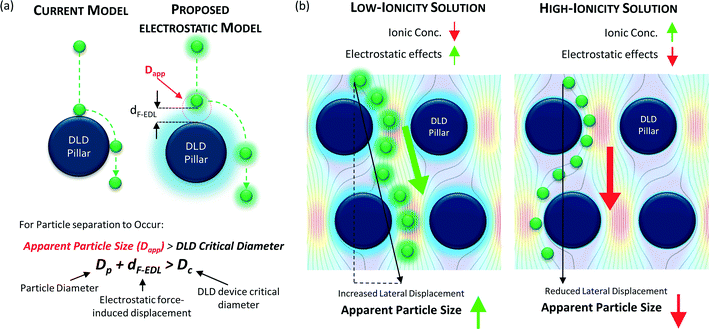
In this study, we explore a novel nano-separation method based on real-time nanoparticle size modulation in micro-fabricated DLD devices without applying any external force field. We managed to separate a large range of particle sizes from 51 nm to 1500 nm in a single DLD device. We modulate the nanoparticle size by simply changing the buffer reservoir solution with different ionic concentrations. Differing salt ionic concentrations create different thicknesses of the electric double layer (EDL) which modulates the electrostatic force interactions between the nanoparticle and the walls of the DLD device (Fig. 1).26 The nanoparticle displacement induced by the electrostatic and hydrodynamic forces alters the apparent size of the particle for nano-separation in DLD devices. Accordingly, the sizes of the separated nanoparticles can therefore be regulated by adjusting the ionic concentration of the buffer.
Notably, modifying the buffer’s ionic concentration can actively modulate nanostructure and nanoparticle interactions.27–31 Fu et al.30 and Regtmeier et al.31 have explored similar ionic methods of modulating overlapping Debye layers to control nanofluidic pore sizes for selective particle separation. The Debye length is the characteristic parameter that is used to approximate the EDL thickness within these pores and nanostructures. Fu et al. successfully separated DNA and proteins using a pore size of 300 nm, whereas Regtmeier et al. separated 15 nm particles using a 515 nm gap. However, in both works, an external electric field source was necessary to electrokinetically drive the separation of the particles. These systems do not permit the adjustment of the buffer’s ionic concentration in real time and require the fabrication of nanoscale gaps, thereby restricting device throughput and dynamic range. Moreover, Debye length approximations of particle and pore interactions understate the true EDL effects. Thus, a novel model to account for the long-range electrostatic effects is explored here in addition to the real-time nanoparticle size modulation for precision separation control.
By developing the model, the novelty of this communication is two-fold: 1) we precisely predict and control the long-range EDL electrostatic effects on a particle in fluid medium to control the particle apparent size. 2) This enables us to develop a method for continuous flow real-time nano-separation modulation without the use of external force fields in micro-fabricated DLD systems. By changing the apparent particle size through the injection of ionic buffer solutions, the dynamic range was significantly amplified (>1200%), increasing the pore-to-particle size ratio (~40 times). Dynamic nano-separation was demonstrated to discriminate a spectrum of particles with varied diameters (51 nm to 1500 nm) within a rapid response time of less than 20 seconds. To our knowledge, there are no other methods that offer a sufficiently precise separation and dynamic flexibility to separate particles with sizes ranging from 51 nm to the micron scale under continuous flow conditions.24 The modulated separation of 51 nm polystyrene particles outperformed a conventional benchtop centrifugal system. The size modulation of the nanoparticles was achieved by simply changing the ionic concentration of the buffer reservoir solution. With future refinements and scaling up of the device, high-throughput nano-separation for industrial applications can be approached, and precise diagnoses can be achieved based on the isolation of circulating biomarkers such as viruses, exosomes, proteins and DNA.
Materials and methods
The DLD device
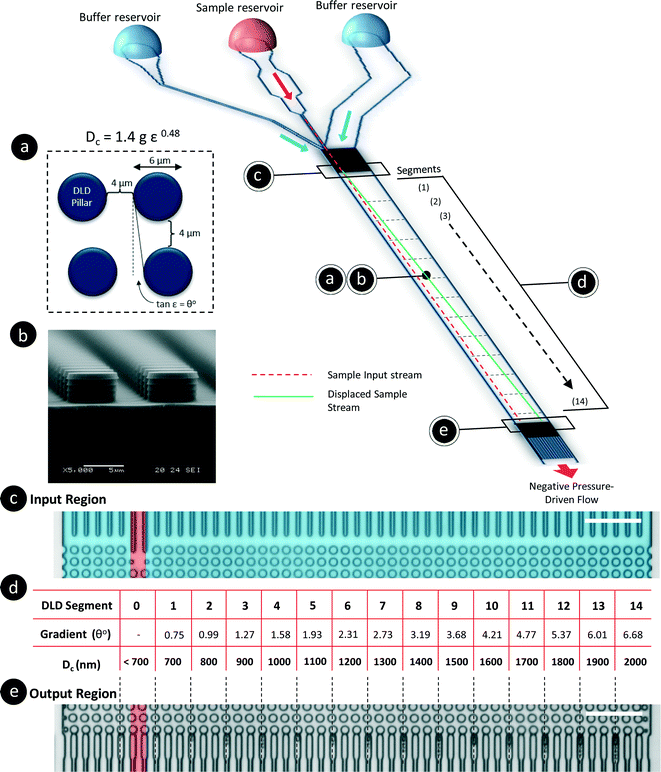
The DLD method for particle separation in microfluidic devices has been extensively studied for the past decade since it was first reported by Huang et al.21 DLD is a continuous flow separation technique based on a pillar array gradient that determines the displacement of a particle in the lateral direction. The critical cut-off size for the particles is determined by the gradient and the gap between adjacent pillars in the array. Particles larger than the critical cut-off diameter (Dc) will be displaced from the sample streamline, whereas smaller particles will seemingly flow unhindered through the pillar array by traveling within the fluid streamlines. The empirical formula derived by Davis et al.32 is found in eqn (1).In this paper, all DLD devices discussed are multi-segmented devices with 14 critical diameter segments, as shown in Fig. 2(d), with each segment in a continuous sequence. Each DLD segment is responsible for the lateral displacement of particles of a specific size, and these particles will exit at their respective outputs (Fig. 2(e)). The particle separation behavior is determined by the design of the DLD devices. Using a pore size of 4 μm and varying pillar array gradients such that ε = 1/N, we developed a DLD system with critical diameters ranging from 700 nm to 2000 nm, as determined based on eqn (1).
This device has a very thin, single-stream input spanning 4 μm, which is sandwiched between two buffer streams (Fig. 2(c)). As particles flow through the device, they will be displaced until the DLD critical diameter is larger than the particle size. The sample particles will exit the device at various lateral positions based on their hydrodynamic sizes. As such, particles of less than 700 nm in size will not be laterally displaced from the sample stream, whereas particles larger than 2000 nm will remain in the stream until they reach the exit location corresponding to Dc = 2000 nm. We initially calibrated the device with beads using the same method as in our previous works and found that the behavior of the DLD device was consistent with the established empirical model (see Fig. S1§). Additionally, to prevent contamination through the residual adsorption of NaCl ions into the PDMS, we completed all flow tests with various bead sizes at lower ionic concentrations before increasing the ionic concentration of the buffer. The ionic concentration was increased from 10 μM to 500 μM, and the values were selected based on the characteristic Debye length of the solution (see Fig. S2§). To change the buffer, DI water was used to flush the reservoir three times before new buffer was added.
The fabrication of the device uses standard photolithography techniques with deep reactive ion etching to fabricate the DLD channels. The fabrication diagrams and steps can be found in Notes S3.† The system is driven by a negative pressure at the outlet, whereas the sample and buffer inlets are open-air reservoirs to facilitate rapid changes of the ionic buffer inputs (see Fig. S4§).
A second DLD device was fabricated to test the limits and dynamic range of nanoparticle separation. The specifications for the second DLD device were all identical except for a reduction in the gap size to 2 μm. This reduction in gap size resulted in a separation range of 350 nm to 1000 nm. These specifications can be derived from eqn (1).
Samples and buffer solutions
The particles used in this study included NIST standard polystyrene beads (Bangs Laboratories, USA) ranging from 500 nm to 1000 nm in size and fluorescence-coded polystyrene beads of 51 nm, 191 nm, 520 nm and 780 nm in size with excitation/emission wavelengths of 480/520 nm (Bangs Laboratories). The beads were diluted in ultrapure 18 ohm filtered DI water (Millipore) by a factor of 10 and mixed in an ultrasonic bath for 5 min to reach a final concentration of 0.1% w/v.The primary ionic salt used was sodium chloride, NaCl (Sigma, Singapore), dissolved in ultrapure DI water in concentrations ranging from 10 μM to 500 μM. The high salt buffer solution used was 1× phosphate-buffered saline (PBS) solution (Sigma-Aldrich, Singapore), with a ~135 mM NaCl concentration. Non-ionic surfactant was prepared using Pluronic F-127 at 1 mM (Sigma).
Experimental setup and data analysis
Pre-treatment of the devices was performed by applying a surface coating of (tridecafluoro-1,1,2,2-tetrahydrooctyl)-1-trichlorosilane (Sigma) via chemical vapor deposition and a pre-flow treatment with 1% (w/v) Pluronic F-127 solution to prevent the attachment of bio-particles to the device surface. After 30 min of Pluronic treatment, the device was washed by loading fresh ultrapure DI water into the reservoirs and flushing for 30 min. To drive the flow process, a 100 μl gas-tight Hamilton glass syringe was attached to a 150 μm diameter tube, which, in turn, was inserted into the device outlet. A syringe pump (Chemyx) was attached to the syringe, and the syringe piston was retracted at a flow rate of 0.05 μl min−1. At this flow rate, the beads were traveling at a velocity of approximately 250 μm s−1 in the microfluidic device with a channel depth of 4 μm.To capture the separated beads at the output of the DLD device, a high-speed Phantom camera (M310) was attached to a bright-field upright microscope at a magnification of 400×. Images of the NIST beads at the output positions were captured from each video, and the beads were counted manually from these images. A sample video is provided in the ESI.† The lowest possible bead size that could be visually distinguished at 400× magnification and a 1280 × 800 pixel resolution was 500 nm. To capture fluorescence images, a CCD camera was used to capture the 520 nm wavelength emission of the green fluorescent nano-sized beads. The images were processed using ImageJ to convert the fluorescence intensities into grayscale images to measure the intensities and locations of the fluorescence streaks.
Results and discussion
Apparent particle size in the dynamic DLD system
The electrostatic interactions between the particles and the device will impact the hydrodynamic particle size and drastically influence the separation of sub-micron particles and nanoparticles. Although the Debye length is commonly used to estimate the electrostatic influence on surfaces, we hypothesize that electrostatic forces beyond the Debye length are sufficient to induce nanoparticle displacements that can be detected by size-sensitive DLD systems. A model was established to predict the long-range repulsive electrostatic effects acting on DLD structures and particles (Fig. 1). Attractive electrostatic forces are not considered because the attraction of particles to surfaces would imply the possibility of physical or electrostatic attachment, thus preventing separation.Conventionally, DLD systems do not account for electrostatic effects (Fig. 1a). Particle separation is theoretically governed by the critical size at which separation occurs only if the particle size is greater than that critical size, namely, Dc (eqn (1)).
| Dc = 1.4gε0.48 | (1) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ = ε), where θ is the angle of the pillar array gradient.
θ = ε), where θ is the angle of the pillar array gradient.
Our proposed approach introduces electrostatic interactions into the modeling of particle separation; in this case, the effective critical size for particle separation that applies in the DLD device due to electrostatic interactions is called the apparent particle size (Dapp). Dapp must be greater than the Dc of the DLD system for separation to occur such that:
| Dp + dF-EDL > Dc | (2) |
We can determine the apparent particle size (Dapp) by summing particle diameter (Dp) and the displacement caused by electrostatic interactions between the particles and the surface of the DLD device (dF-EDL). By modulating Dapp, one can achieve different separation outcomes for particles with the same Dp, as shown in Fig. 1b for an enhanced separation size and in Fig. 1c for a reduced separation size.
Because Dp is fixed, we can modulate Dapp only by changing the electrostatic force-induced displacement dF-EDL. Decreasing the ionic concentration of the buffer medium will increase the electrostatic force interactions between the particles and the device surface, which will, in turn, increase Dapp. This increase in apparent particle size aids in the effective separation of smaller particles in pore-based microfluidic systems. The converse is true for an increase in the ionic concentration of the fluid medium (Fig. 1c), which causes a reduction in electrostatic effects that reduces Dapp in the same microfluidic setup. Thus, the dynamic range of the device can be significantly modulated by tuning the electrostatic interaction variable dF-EDL.
Separating nanoparticles under different ionic concentrations
To determine and understand the extent of electrostatic effects on modulating size-sensitive particle separation behavior from the microscale to the nanoscale, we first investigated the sub-micron regime before proceeding to the nano regime. We designed a DLD system with a large pore size of 4 μm and a pillar diameter of 6 μm (Fig. 2a). The device was fabricated using standard photolithography and etched to a depth of 4 μm (Fig. 2b). The particle sample and buffers are stored in open reservoirs and driven into the device by negative pressure. This design permits rapid changes of the buffer solutions in the reservoirs while ensuring that a thin width of the sample stream is sandwiched between two buffer streams (Fig. 2c). The DLD device is a multi-segmented device with 14 critical diameter segments, with each segment in a continuous sequence (Fig. 2d). The gradient in each DLD segment increases such that each successive segment is responsible for the lateral displacement of particles with increasing Dc, from 700 nm to 2000 nm (Fig. 2e). The details of the experimental protocols can be found in the Materials and methods section.Beads of sizes ranging from 600 nm to 1000 nm were tested in the DLD device in varying NaCl ionic buffer concentrations that varied from 10 μM to 500 μM. Ultrapure DI water was used to test the effects of a non-ionic solution, and a 1× PBS solution at a pH of 7.4 was used to investigate the effects of a high salt solution. The separation results were plotted to show the variation in the apparent particle size with different ionic solutions (Fig. 3). Theoretically, when electrostatic considerations are ignored, the particles should exhibit a Dapp close to Dp and should exit the DLD device from a segment corresponding to a size close to the Dc of the device (Fig. S2§). However, the apparent particle sizes in the DLD device significantly responded to changes in the ionic concentration of the buffer streams. Ultrapure DI water resulted in the most significant shift in the separation spectrum of the particles, with Dp = 1000 nm particles exhibiting a Dapp of 1847 nm. Meanwhile, none of the particles that exhibited a Dapp of less than 700 nm were separated from the input stream in the PBS buffer. Increasing the ionic concentration reduced the Dapp of the particles, as hypothesized in Fig. 1. This phenomenon is unique and remarkable, because a simple manipulation of the ionic buffer can drastically shift the Dapp of a 1000 nm particle from a value of 1847 nm to less than 700 nm. The lower boundary on Dapp is unknown, as it is beyond the limits of the DLD particle separation range. The total variation in Dapp that can be achieved by changing from a non-ionic to a highly ionic solution is greater than 114% for a 1000 nm particle. This suggests that fine modulation of particle size separation behavior can be achieved by using optimized ionic concentrations. To our knowledge, this large variation in the apparent particle size during pore-based particle separation has never previously been observed, and thus, the results reported here represent the first quantitative measurements of this phenomenon using a DLD system.
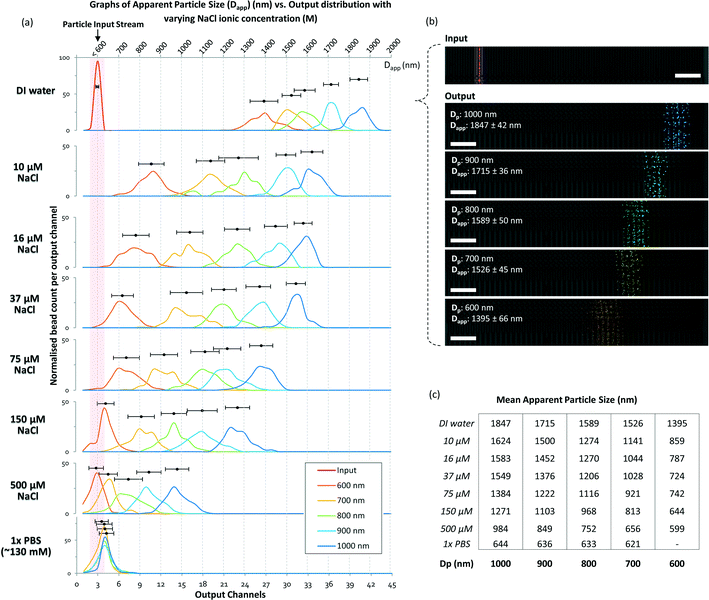 | ||
| Fig. 3 DLD separation spectra of sub-micron particles in media with different ionic concentrations. The particle input stream is shown in (a) as a red line in the DI water spectrum and highlighted in the red region for the rest of the spectra. The separation spectra are marked in various colors: dark blue for 1000 nm, sky blue for 900 nm, green for 800 nm, yellow for 700 nm, and orange for 600 nm. The output distributions are normalized individually. The top axis denotes Dapp, which ranges from 700 nm to 2000 nm, and the bottom axis shows the output channels. (b) The particle separation in the raw video footage with pseudo-colors added to highlight the particles. The extracted information is overlaid on the image of the corresponding output channel. The raw footage can be seen in Movie S1.§ The scale bars represent 50 μm. In (c), the mean displacement of the particles in different ionic NaCl concentrations is tabulated. | ||
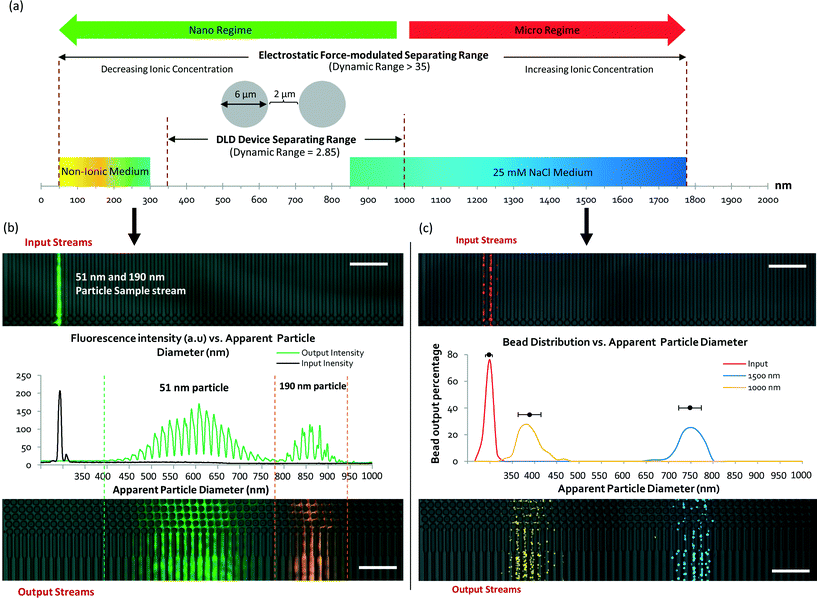
At a 500 μM concentration of NaCl, the ionic shielding sufficiently reduced the interaction such that Dapp was comparable to the particle size predicted for DLD using eqn (1). Using the knowledge obtained by characterizing the modulation of the separation spectrum by electrostatic forces, we tested the limits of the device and found that it was able to effectively separate a sample mixture of nanoparticles of 190 nm, 520 nm and 780 nm in size (see Fig. S3§). In theory, conventional DLD systems can separate nanoparticles. However, previous attempts to separate nanoparticles of less than 500 nm in size in DLD systems have not been successful for pore sizes larger than 1 μm.24 Here, we demonstrated distinct separation using a 4 μm DLD gap size and direct separation from a particle mixture.
Electrostatic force model vs. Debye length model
The DLD separation method is known for its high resolution and precision for particle separation. The inclusion of electrostatic forces in DLD, as suggested in the proposed model (Fig. 1), enables dynamic modulation of the size sensitivity for the separation of micro- and nanoparticles. The mean Dapp is plotted against Dp in Fig. 4(a), wherein the dotted line represents the theoretical model in which electrostatic forces are neglected (dF-EDL = 0). Data points above the dotted line suggest the presence of a positive repulsive electrostatic force due to dF-EDL > 0, resulting in Dapp > Dp. When dF-EDL < 0, the force between the particles and the device surface becomes attractive and the apparent particle size becomes smaller than the actual particle diameter. This is probably caused by the onset of attractive van der Waals forces dominating the repulsive electrostatic forces at low Debye length scales of ~1 nm.33 Particles suspended in ionic salt buffer media with NaCl concentrations lower than 500 μM appear to exhibit an increase in apparent particle size, whereas particles in ionic solutions with NaCl concentrations greater than 500 μM will exhibit a reduced apparent particle size in the DLD device. Because the lower limit on Dc for the empirical model of the DLD system is 700 nm, we are unable to accurately predict a reduction in the apparent particle size to less than 700 nm. Certainly, a salt concentration of greater than 200 mM will induce an average reduction in apparent particle size in the DLD system of more than 263 nm.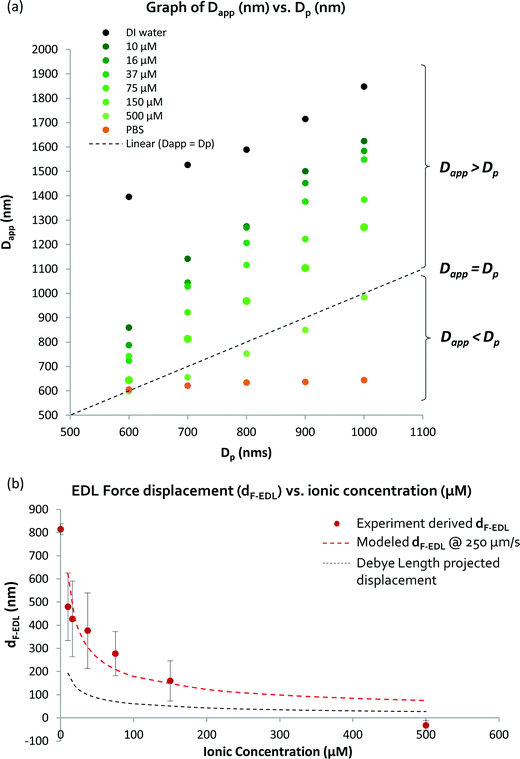 | ||
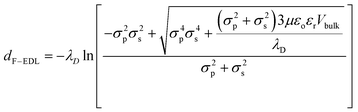
| Fig. 4 Measurement and derivation of the apparent particle size (Dapp): (a) shows how the tabulated average Dapp varies with respect to the particle diameter. The dotted line shows where the Dapp is the same as the actual particle diameter (Dp). Dapp is the sum of Dp and dF-EDL. The electrostatic force-induced displacement (dF-EDL) for ionic concentrations between 0 and 500 μM is represented by red points (b). The red-dotted lines show the predicted enhanced displacement (dF-EDL) from eqn (1). | ||
In this pioneering study of the quantitative effects of electrostatic forces on DLD nanoparticle separation, we limit the electrostatic effects included in the model to the repulsive electrostatic forces acting in solutions with ionic concentrations below 500 μM NaCl. Attractive electrostatic forces would cause the nanoparticles to attach to the walls, forming aggregated particles, and would reduce the apparent particle size to a sufficient extent that particle separation would become impossible. A more detailed derivation of dF-EDL and a further explanation can be found in the ESI§ S4 and S5. The electrostatic interaction between the nanoparticles and the DLD pillars is described as follows:
 | (2) |
Here, the electrostatic force displacement dF-EDL is dependent on the Debye length (λD), the electrical permittivities of the medium (ε) and the free space (εo), the surface charge densities of the particles (σp) and the device surface (σs), the viscosity of the fluid (μ) and the velocity of the surrounding fluid (Vbulk). Surprisingly, dF-EDL is predicted to be independent of particle size and dependent only on the charge density, ionic strength and fluid velocity (eqn (2)). Because the physical size of each particle is fixed and the electrostatic component of the force, dF-EDL, is variable, we plot the average dF-EDL against the ionic concentration from 0 to 500 μM (Fig. 4b). The average experimental dF-EDL values were calculated by subtracting the particle diameter from the apparent particle size at various concentrations. The modeled dF-EDL results (red dotted line) at a fluid flow velocity of 250 μm s−1 are largely in agreement with the experimental values. The conventional Debye length approximation for nanoparticle separation (black dotted line) deviates from the proposed model by more than 100% (Fig. 4b). Thus, our model predicts that long-range electrostatic forces acting on nanoparticles cannot be assumed to be negligible in ionic media with concentrations lower than 500 μM. A slight change in the ionic medium will have a significant effect on the apparent size of the nanoparticles.
Expanded dynamic range for nano- to microparticle separation
To build on the results presented above by investigating the electrostatic influence and the limits on nanoparticle size manipulation in large-pore DLD devices, we designed and fabricated a new DLD device with a smaller gap size of 2.0 μm (see Fig. 5a and Fig. S6§). This device offers a separation spectrum with Dc values ranging from 350 nm to 1000 nm in steps of 50 nm. The electrostatic force-dependent DLD separation was tested using fluorescent polystyrene beads of 51 nm and 190 nm in size. Using a sheath flow of ultrapure DI water, we succeeded in distinctly separating these particles from the original flow stream (Fig. 5b). To our knowledge, the ability to separate 51 nm particles using a 2.0 μm pore-based filter is unprecedented. The pore-to-particle size ratio is approximately 40, greater than that of Regtmeier et al., who achieved a pore-to-particle size ratio of 33.The 51 nm and 190 nm particles exhibited Dapp values of 650 nm and 910 nm, respectively. The mean Dapp difference was 260 nm, compared with a Dp size difference of 139 nm – an 87% greater size difference between the particles. The separation spectrum presented in Fig. 5a shows a shift toward the left side of the scale bar, indicating a dynamic increase in Dapp that enabled particles of smaller Dp to be separated in the same DLD device. In a conventional DLD model (eqn (1)), separating 51 nm particles would require an impractical 2-meter-long device with a channel width of 0.5 mm.
The separation spread and distribution of the 51 nm particles were relatively wide, with a standard deviation of approximately 125 nm, greater than the size of the particles themselves. This was due to the diffusion of the particles. At a flow rate of 250 μm s−1, the diffusion length 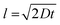 of the flow stream is approximately 100 μm. Therefore, the DLD nanoparticle separation resolution is limited only by diffusion. Moreover, the fabrication of the device pillars using DRIE introduced scalloping effects (Fig. 2b), which may have introduced microscale variations in fluid flow, resulting in a reduced DLD separation efficiency.
of the flow stream is approximately 100 μm. Therefore, the DLD nanoparticle separation resolution is limited only by diffusion. Moreover, the fabrication of the device pillars using DRIE introduced scalloping effects (Fig. 2b), which may have introduced microscale variations in fluid flow, resulting in a reduced DLD separation efficiency.
To test the other extreme of the separation range in the same device, a high-ionicity medium consisting of a 25 mM NaCl solution was used for the separation of 1000 nm and 1500 nm particles. Fig. 5c shows the particle separation distribution with added pseudo-color. The separation of the particles is distinct, with Dapp values of 441 nm and 801 nm for the 1000 nm and 1500 nm particles, respectively, and an average of dF-EDL = −630 nm, indicating a decrease in the apparent particle size. Hence, using the same DLD device, a 1000 nm particle can appear “smaller” than a 51 nm particle in a different ionic medium.
The electrostatic modulation of nanoparticle sizes enables unprecedented continuous nanoparticle separation and increases the effective dynamic range of pore-based DLD systems for separation from 2.85 to 35. Moreover, it also allows for the separation of particles with different sizes using the same device while outperforming conventional benchtop centrifuge methods (Fig. S7§). We compared the separation of 51 nm particles in a conventional benchtop centrifuge at 22![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000g and were unable to fully separate these particles from the original fluid sample.
000g and were unable to fully separate these particles from the original fluid sample.
Response time for real-time nanoparticle separation manipulation
To our knowledge, a passive flow (non-external force-field-based) method for the real-time manipulation of nanoparticle separation has never before been achieved. Most real-time separation involves centrifugal, electric, acoustic or magnetic fields. These systems require additional equipment or experimental setups to enable fine control of the particle separation behavior. Although Arosio et al. used a microfluidic centrifuge system for the separation of 50 nm to 200 nm particles, the setup was not a continuous flow system and the separation spectra considerably overlapped.18 Using a combination of EDL effects, electrokinetics and nano-fabrication, Regtmeier et al. successfully separately nanoparticles of 15 nm and 36 nm in size.31 Here, we demonstrated that by simply changing the buffer solutions in the reservoirs of our system, it is possible to control the separation of nanoparticles with a response time of 20 seconds or less (Fig. 6 and Movie S2§).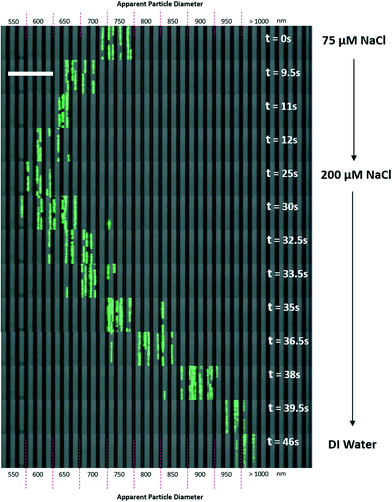 | ||
| Fig. 6 Response time test for real-time particle separation manipulation. 500 nm beads were used as test beads for response time in the separation test. Screen capture from video footages were extracted and sequenced from 0 s to 46 s (see Movie S1§). Salt solutions in buffer reservoirs were changed in real-time and the separation of beads was observed under a microscope using a video camera. The white bar depicts a 40 μm distance. | ||
To test the real-time separation response of ionic buffer separation, 500 nm fluorescent beads were tested in three different buffer solutions, as shown in Fig. 6. To capture video fluorescence images, a 5D Mark III DSLR camera was used at ISO 25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 600 at 1/30 fps. A bright-field image of the channels was overlaid on the fluorescence video. Thus, the effects of real-time changes to the ionic buffers were captured (see Movie S2§).
600 at 1/30 fps. A bright-field image of the channels was overlaid on the fluorescence video. Thus, the effects of real-time changes to the ionic buffers were captured (see Movie S2§).
A 75 μM NaCl solution was used as the initial buffer, and the fluorescent particle stream settled within an apparent diameter range of 700 nm to 800 nm. Then, a 200 μM NaCl solution was flushed into the buffer reservoirs at t = 0 s. When the 200 μM NaCl solution was introduced, it took approximately 12 seconds for the full effect of the ionic solution to shift the separation spectrum of the particles to an apparent diameter range of 600 nm to 650 nm. However, when DI water was flushed into the reservoirs, the particle stream shifted in the opposite direction to apparent diameters of more than 1000 nm. This shift required approximately 20 seconds as the DI water flushed the NaCl solution out of the device.
This response time is dependent on the flow velocity of the fluids in the channel, which governs the time required to flush the previous buffer out of the system. Reducing the response time would be possible with much higher flow velocities, which would also increase the throughput of separation. However, as seen from eqn (2) and Fig. S5,§ the apparent particle size would simultaneously decrease due to higher hydrodynamic drag forces opposing the electrostatic force. Despite this reduction in Dapp, our model still exhibits a greater electrostatic influence compared with the Debye length approximation at microfluidic velocities as high as 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 μm s−1.
000 μm s−1.
Because ions can diffuse into surrounding channels consisting of PDMS and silicon, we did observe that prolonged use of high-ionicity solutions in PDMS-covered channels resulted in a less effective response because the ions were able to diffuse and accumulate in the PDMS channels. Hence, even if ultrapure DI water is used, the effects may be reduced as a result of residual NaCl ions in the PDMS diffusing back into the fluid medium.
Conclusion
By virtue of its sensitivity to particle size, DLD microfluidics is a promising separation method with potential applications including blood purification,34,35 bacteria separation,36,37 cancer cell concentration,38etc.23 The high-throughput scalability of its fluid flow rates toward ml min−1 enables its application in a regime similar to that of traditional centrifugal methods. Although DLD using large-pore structures (~1.4 μm) has demonstrated promise for the distinct, passive separation of sub-micron particles (600–1000 nm),21 separating particles of less than 500 nm in diameter has proven to be challenging because of additional relevant variables such as diffusion and electrostatic forces.24,39 To address these effects, in this study, we investigated a long-range EDL electrostatic model for DLD. By considering the electrostatic forces arising due to the ionic strength of the buffer medium, our model uniquely accounts for the electrostatic forces between the surfaces of the DLD pillars and the particles, resulting in modifications to the apparent particle size and allowing for the simple and precise prediction of the induced displacement. Based on this model, the particle-wall interaction forces can be manipulated by tuning the ionic strength of the surrounding fluid, thereby enabling real-time manipulation of the particle size and separation without using magnetic, acoustic or electric fields. Accordingly, the separation of 51 nm particles using a micro-fabricated device with large pores of 2 μm in size was achieved. Moreover, we demonstrated continuous flow nano-separation with dynamic control of the separation spectrum, ranging from 51 nm to 1500 nm, in a DLD device. The dynamic range for particle separation was thus increased by ~1200% in comparison with that of conventional DLD separation. The results of our approach represent the first demonstration of real-time, label-free nano-separation in a continuous flow microfluidic system, and the simplicity of this method provides an opportunity for rapid, distinct, high-throughput separation of synthetic nanoparticles or biologically relevant samples such as viruses, exosomes, proteins and DNA.39Acknowledgements
We would like to acknowledge the funding support from National University of Singapore (NUS), Singapore Institute for Neurotechnology (SINAPSE), and Ministry of Education Academic Research Fund (AcRF: R-397-000-183-112, R-397-000-153-112). The experiments were mostly performed in Cellular and Molecular Bioengineering Lab in NUS. We also would like to thank Dr. Shashi Ranjan for the useful discussions on the study of solute ionic effect in DLD separation. Postgraduate PhD student Wang Ming Kevin assisted in the confirmation of the formulation and model of electrostatic DLD.References
- Z. Nie, A. Petukhova and E. Kumacheva, Properties and emerging applications of self-assembled structures made from inorganic nanoparticles, Nat. Nanotechnol., 2010, 5, 15–25 CrossRef CAS PubMed.
- I. A. Rahman, P. Vejayakumaran, C. S. Sipaut, J. Ismail and C. K. Chee, Size-dependent physicochemical and optical properties of silica nanoparticles, Mater. Chem. Phys., 2009, 114, 328–332 CrossRef CAS.
- Q. Yu, L. Qi, R. K. Mishra, X. Zeng and A. M. Minor, Size-dependent mechanical properties of Mg nanoparticles used for hydrogen storage, Appl. Phys. Lett., 2015, 106, 261903 Search PubMed.
- S. Zhang, J. Li, G. Lykotrafitis, G. Bao and S. Suresh, Size-Dependent Endocytosis of Nanoparticles, Adv. Mater., 2009, 21, 419–424 CrossRef CAS PubMed.
- M. De, P. S. Ghosh and V. M. Rotello, Applications of Nanoparticles in Biology, Adv. Mater., 2008, 20, 4225–4241 CrossRef CAS.
- O. Salata, Applications of nanoparticles in biology and medicine, J. Nanobiotechnol., 2004, 2, 3 CrossRef PubMed.
- E. C. Wang and A. Z. Wang, Nanoparticles and their applications in cell and molecular biology, Integr. Biol., 2014, 6, 9–26 RSC.
- W. J. Stark, P. R. Stoessel, W. Wohlleben and A. Hafner, Industrial applications of nanoparticles, Chem. Soc. Rev., 2015, 44, 5793–5805 RSC.
- B. Kowalczyk, I. Lagzi and B. A. Grzybowski, Nanoseparations: Strategies for size and/or shape-selective purification of nanoparticles, Curr. Opin. Colloid Interface Sci., 2011, 16, 135–148 CrossRef CAS.
- T. M. Laue and W. F. Stafford III, Modern applications of analytical ultracentrifugation, Annu. Rev. Biophys. Biomol. Struct., 1999, 28, 75–100 CrossRef CAS PubMed.
- J. A. Fagan, M. L. Becker, J. Chun and E. K. Hobbie, Length Fractionation of Carbon Nanotubes Using Centrifugation, Adv. Mater., 2008, 20, 1609–1613 CrossRef CAS.
- C. Vauthier, B. Cabane and D. Labarre, How to concentrate nanoparticles and avoid aggregation?, Eur. J. Pharm. Biopharm., 2008, 69, 466–475 CrossRef CAS PubMed.
- A. Lenshof and T. Laurell, Continuous separation of cells and particles in microfluidic systems, Chem. Soc. Rev., 2010, 39, 1203–1217 RSC.
- N. Pamme, Continuous flow separations in microfluidic devices, Lab Chip, 2007, 7, 1644–1659 RSC.
- B. Hammarstrom, T. Laurell and J. Nilsson, Seed particle-enabled acoustic trapping of bacteria and nanoparticles in continuous flow systems, Lab Chip, 2012, 12, 4296–4304 RSC.
- D. J. Collins, T. Alan and A. Neild, Particle separation using virtual deterministic lateral displacement (vDLD), Lab Chip, 2014, 14, 1595–1603 RSC.
- M. Hanauer, S. Pierrat, I. Zins, A. Lotz and C. Sonnichsen, Separation of nanoparticles by gel electrophoresis according to size and shape, Nano Lett., 2007, 7, 2881–2885 CrossRef CAS PubMed.
- P. Arosio, T. Müller, L. Mahadevan and T. P. J. Knowles, Density-Gradient-Free Microfluidic Centrifugation for Analytical and Preparative Separation of Nanoparticles, Nano Lett., 2014, 14, 2365–2371 CrossRef CAS PubMed.
- A. S. Prabhu, et al., Chemically modified solid state nanopores for high throughput nanoparticle separation, J. Phys.: Condens. Matter, 2010, 22, 454107 CrossRef PubMed.
- S. M. Stavis, J. Geist and M. Gaitan, Separation and metrology of nanoparticles by nanofluidic size exclusion, Lab Chip, 2010, 10, 2618–2621 RSC.
- L. R. Huang, E. C. Cox, R. H. Austin and J. C. Sturm, Continuous particle separation through deterministic lateral displacement, Science, 2004, 304, 987–990 CrossRef CAS PubMed.
- J. P. Beech, P. Jonsson and J. O. Tegenfeldt, Tipping the balance of deterministic lateral displacement devices using dielectrophoresis, Lab Chip, 2009, 9, 2698–2706 RSC.
- J. McGrath, M. Jimenez and H. Bridle, Deterministic lateral displacement for particle separation: a review, Lab Chip, 2014, 14, 4139–4158 RSC.
- S. M. Santana, M. A. Antonyak, R. A. Cerione and B. J. Kirby, Microfluidic isolation of cancer-cell-derived microvesicles from hetergeneous extracellular shed vesicle populations, Biomed. Microdevices, 2014, 16, 869–877 CrossRef CAS PubMed.
- Y. Chen, et al., Concentrating Genomic Length DNA in a Microfabricated Array, Phys. Rev. Lett., 2015, 114, 198303 CrossRef PubMed.
- J. J. López-García, J. Horno and C. Grosse, Poisson–Boltzmann Description of the Electrical Double Layer Including Ion Size Effects, Langmuir, 2011, 27, 13970–13974 CrossRef PubMed.
- D. G. Haywood, A. Saha-Shah, L. A. Baker and S. C. Jacobson, Fundamental Studies of Nanofluidics: Nanopores, Nanochannels, and Nanopipets, Anal. Chem., 2015, 87, 172–187 CrossRef CAS PubMed.
- Y. Zeng, et al., Effect of particle size and Debye length on order parameters of colloidal silica suspensions under confinement, Soft Matter, 2011, 7, 10899–10909 RSC.
- M. Krishnan, N. Mojarad, P. Kukura and V. Sandoghdar, Geometry-induced electrostatic trapping of nanometric objects in a fluid, Nature, 2010, 467, 692–695 CrossRef CAS PubMed.
- J. Fu, R. B. Schoch, A. L. Stevens, S. R. Tannenbaum and J. Han, A patterned anisotropic nanofluidic sieving structure for continuous-flow separation of DNA and proteins, Nat. Nanotechnol., 2007, 2, 121–128 CrossRef CAS PubMed.
- J. Regtmeier, J. Kasewieter, M. Everwand and D. Anselmetti, Continuous-flow separation of nanoparticles by electrostatic sieving at a micro-nanofluidic interface, J. Sep. Sci., 2011, 34, 1180–1183 CrossRef CAS PubMed.
- J. A. Davis, Microfluidic Separation of Blood Components through Deterministic Lateral Displacement, Doctor of Philosophy thesis, Princeton University, 2008 Search PubMed.
- H.-J. Butt, Measuring electrostatic, van der Waals, and hydration forces in electrolyte solutions with an atomic force microscope, Biophys. J., 1991, 60, 1438–1444 CrossRef CAS PubMed.
- J. A. Davis, et al., Deterministic hydrodynamics: taking blood apart, Proc. Natl. Acad. Sci. U. S. A., 2006, 103, 14779–14784 CrossRef CAS PubMed.
- K. K. Zeming, S. Ranjan and Y. Zhang, Rotational separation of non-spherical bioparticles using I-shaped pillar arrays in a microfluidic device, Nat. Commun., 2013, 4, 1625 CrossRef PubMed.
- S. Ranjan, K. K. Zeming, R. Jureen, D. Fisher and Y. Zhang, DLD pillar shape design for efficient separation of spherical and non-spherical bioparticles, Lab Chip, 2014, 14, 4250–4262 RSC.
- K. J. Morton, et al., Crossing microfluidic streamlines to lyse, label and wash cells, Lab Chip, 2008, 8, 1448–1453 RSC.
- K. Loutherback, et al., Deterministic separation of cancer cells from blood at 10 mL/min, AIP Adv., 2012, 2(4), 042107 CrossRef PubMed.
- J. C. Sturm, E. C. Cox, B. Comella and R. H. Austin, Ratchets in hydrodynamic flow: more than waterwheels, Interface Focus, 2014, 4, 20140054 CrossRef PubMed.
Footnotes |
| † K. K. Z., Y. Z., C. H. C. conceived the idea and designed the experiments. K. K. Z. performed the experiments and wrote the paper. K. K. Z., N. V. T., Y. Z., C. H. C. analyzed the results and revised the paper. |
| ‡ There are no competing financial interests for the duration of the experiment. |
| § Electronic supplementary information (ESI) available. See DOI: 10.1039/c5lc01051a |
| This journal is © The Royal Society of Chemistry 2016 |