Magnetically tunable colloidal micromirrors†
James
Goebl
a,
Yiding
Liu
ab,
Sandy
Wong
a,
Serkan
Zorba
c and
Yadong
Yin
*ab
aDepartment of Chemistry, University of California, Riverside, California 92521, USA. E-mail: yadong.yin@ucr.edu
bMaterials Science and Engineering Program, University of California, Riverside, California 92521, USA
cDepartment of Physics and Astronomy, Whittier College, Whittier, California 90608, USA
First published on 22nd September 2015
Abstract
Herein we demonstrate a method for decorating highly reflective 2D gold microplates with magnetic nanoparticles to produce an optical colloid that can be actuated using an applied magnetic field. These magnetic micromirrors can be rapidly rotated and exhibit a strong contrast in reflectance between the “on” and “off” states.
Conceptual insightsIn this work, we show that by conjugating superparamagnetic nanoparticles to the surface of highly reflective and anisotropic metallic microplates, we can create a composite material possessing both sets of properties. The result is a system of micromirrors which can be rapidly aligned and rotated by applying a magnetic field to function as optical valves, with an excellent contrast between the “on” and “off” states. Because the superparamagnetic particles are attached directly to the microplate surface, only a small amount of magnetic material is needed in the system and the performance of the optical portion of the composite is uncompromised. This work represents a substantial improvement over previous demonstrations of magnetically-actuated metallic microplate systems, which relied on suspension of the microplates in a concentrated ferrofluid that absorbed the majority of incident light. This concept holds promise for many applications, including micron scale optical systems and smart windows. |
Over the past decade, research on the synthesis of nanomaterials has grown to produce innovative materials with a broad array of functional properties, including superparamagnetism,1 plasmonic properties,2 and fluorescence,3 as well as an assortment of anisotropic structures, such as rods,4 cubes,5 octahedra,6 and plates.7 By taking advantage of this expanding library of nanomaterials, subsequent research efforts have focused on producing nanostructures with a combination of properties by incorporating two or more materials with different properties into a single composite. In this vein, there is much recent work involving the pairing of magnetic particles with anisotropic nano- and micromaterials via chemical bonding through surface moieties; this imbues the resultant hybrid materials with both anisotropic and magnetic properties, effectively creating a magnetically anisotropic nanostructure, which also possesses the properties of the original base materials. One example of this concept is magnetically actuated Au nanorods,8 which by virtue of the anisotropic nature of the Au rods and the magnetic properties of the adjacent magnetic nanoparticles represent a tuneable plasmonic system, which holds promise for novel optical devices.
Incorporation of a magnetic response into two-dimensional (2D) microstructures is of particular interest as it allows effective control of their orientation for better utilization of their highly anisotropic properties. Studart and coworkers explored the orientational control of alumina/iron oxide microplates via a magnetic field to enhance the toughness of ceramic–polymer composite films.9 Magnetic responsiveness has also been enabled by suspending reflective metal microplates in a concentrated ferrofluid to create anisotropic optical valves for magnetically tuneable mirror applications.10,11 While the orientation of the microplates could be adjusted by changing the direction of the applied magnetic field to alter the transparency and reflectivity of the system, the performance of such systems suffered from the fact that the ferrofluid component is highly opaque at the concentration necessary to control the plate orientation, and as a result the system permits only limited light transmittance, which is less than optimal for many optical applications.
In this work, we present a more effective magnetically tuneable optical microplate system, in which high aspect ratio (∼100) Au microplates are rendered magnetically functional via conjugation to amine terminated superparamagnetic γ-Fe2O3@SiO2 nanoparticles. Since only a thin layer of magnetic material is necessary to render the plates magnetic, they retain their high reflectivity and their surrounding solution remains transparent, while their optical properties can be readily and rapidly tuned by applying a magnetic field oriented in varying directions. We expect this new material to be useful for novel microscale optical systems. By following the same design principle but replacing Au microplates with inexpensive alternatives such as mica or many other reflective metal oxides with similar morphologies, we also expect that this system might be a good candidate as an active component for smart window applications.
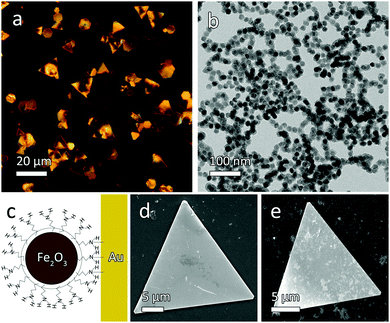
The Au microplates were first prepared using a modified version of a previously published synthesis (Fig. 1a),12 by the reduction of HAuCl4 with salicylic acid at 80 °C in the presence of high molecular weight poly(diallyldimethylammonium) chloride (PDDA), which serves as a growth directing agent. Superparamagnetic maghemite (γ-Fe2O3) nanocrystals were synthesized using a high-temperature oil-phase synthesis,13 subsequently rendered water-soluble through treatment by tetramethylammonium hydroxide (TMAH),14 then coated with a thin layer of silica via a modified Stöber process, and finally amine functionalized by depositing APTES on the particle surface.15 The resultant material is shown in Fig. 1b. The TMAH treatment is believed to slightly etch the iron oxide surface and replace the original ligands of oleic acid with hydrophilic TMAH molecules, allowing the particles to be transferred into water while protecting the particles from aggregation by adding positive surface charges. In order to impart magnetic functionality to the microplates, a small amount of amine-functionalized magnetic nanoparticles was mixed with the Au plates. After mixing for a few minutes, the plates became noticeably magnetic due to the strong coordination of the amine with the Au surface (Fig. 1c). After removing the excess magnetic nanoparticles, the Au plate surface was found to be covered with a conformal coating of γ-Fe2O3. As a result, the composite microplates became magnetically responsive, and the conjugated structure remained stable during subsequent magnetic manipulation and testing. The microplates would remain suspended in water for several minutes, but could readily be redispersed by gentle shaking. It should be noted that the magnetic nanoparticles needed to be introduced into the microplate dispersion gradually until the microplates became sufficiently responsive to the external field (requiring 100–200 μL of magnetic nanoparticle solution), as addition of a large excess of magnetic nanoparticles (more than ∼500 μL) would result in significant aggregation.
Following conjugation to the magnetic nanoparticles, the Au microplates could then be magnetically manipulated, leading to changes in the optical properties of the bulk system that could easily be observed by the naked eye, as depicted in Fig. 2. In the absence of a magnetic stimulus, the plate solution exhibited a shiny, golden color reminiscent of bulk Au. Upon the application of a magnetic field oriented parallel to the viewing direction, the plates rapidly aligned with the applied field and the solution became transparent due to the minimized cross section of the microplates, with a faint brown tint due to the native color of γ-Fe2O3. As the applied magnetic field was rotated such that the angle between the magnetic field and the viewing direction (θ) increased, the plate solution transitioned from being clear and transparent to opaque and reflective. This optical change was due to the plates in the solution becoming aligned perpendicular to the viewing angle (θ = 90°), which maximized the projected cross section of the plates to reduce transmitted light, as well as direct more reflected light back to the observer.
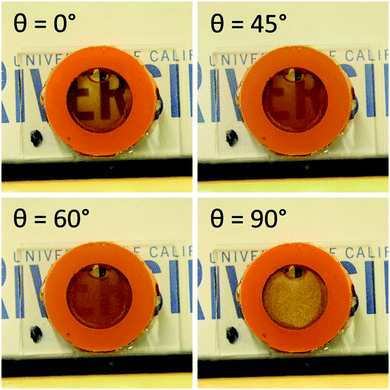 | ||
| Fig. 2 Photographs of a bulk magnetic microplate solution under an applied magnetic field with various orientations (θ) relative to the viewing direction. | ||
The driving force behind the rotation of the magnetic plates is the minimization of potential energy within the applied magnetic field. This potential energy can be described in an equation derived by Studart and coworkers16 (eqn (1)), in which a circular, disc-like ellipsoid is used to approximate a non-magnetic plate coated with magnetic particles:
| Uplate = 2/3π[(a + Δ)(b + Δ)2 − ab2] μ0[χp2/(χp + 1)]H02 sin2ϕ | (1) |
| Uplate ∝ sin2ϕ | (2) |
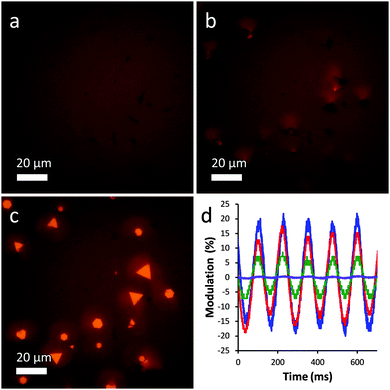
For observation of plate actuation on the microscale, a solution of plates was mixed with a small amount of agarose and placed in a flat 0.4 mm glass capillary under an optical microscope. The agarose serves to stabilize the microplates by preventing their easy migration such as aggregation under magnetic field or sedimentation due to gravity, and also by providing a steric barrier against adhesion to the capillary walls.17 A magnetic field was applied using a handheld rare earth magnet, and the resulting rotation of the microplate sample could readily be observed in bright field mode, as shown in the optical microscopy images in Fig. 3. Confirming the observations of the bulk solution, the plates readily align parallel to the applied field, as shown in Fig. 3a and c, in which the field is oriented parallel and perpendicular to the viewing direction, respectively.
Despite the adsorbed magnetic nanoparticles, the plates retain their high reflectivity and are quite shiny when oriented perpendicular to the observer. The reflectivity of the plates has a strong directional dependence, however, which can be seen in Fig. 3b, in which the plates are rotated at a 45° angle. In this image, the plates appear nearly as dark as in Fig. 3a and scattered light can be seen to one side, indicating that light is being scattered away from the viewer.
The response of the plates to the applied magnetic field was quite rapid and could be quantitatively measured using a simple experimental setup. Briefly, a laser was shone through a cuvette containing a plate sample into a photodetector, which produced an output on a connected oscilloscope. The plate sample was rotated at 4 Hz using a magnetic stir plate, and the resulting fluctuations in transmittance were recorded over time. Microplate samples with varying degrees of magnetic loading were tested for comparison. As shown in Fig. 3d, the plate samples displayed excellent optical modulation properties, with values reaching up to 37%. The optical performance was strongly dependent on the extent of magnetic loading, with higher values leading to enhanced performance and low loadings producing little response. Optical modulation could be detected at frequencies of up to ∼20 Hz, the maximum rotation speed of the stir plate, but accurate measurements were not possible at this frequency due to vibrations from the stir plate motor shaking the cuvette.
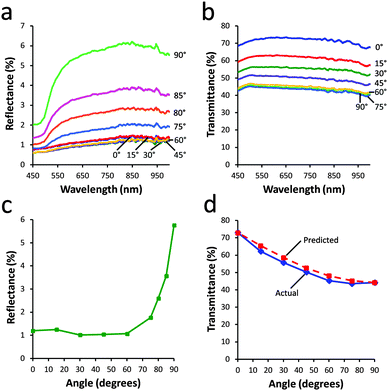
In addition to the optical modulation, the absorbance and transmittance of the plate sample were measured at various applied magnetic field angles. As shown in Fig. 4a, the reflectance of the plate suspension was strongly dependent on the angle of the applied magnetic field, ranging from 1% when θ = 0° to 6% when θ = 90°, which represents a six fold difference between the maximum and minimum reflectance states. Interestingly, the relationship between θ and reflectance, shown in Fig. 4c, was highly nonlinear. Almost no change in reflectance occurred when θ was increased from 0–60°, while a dramatic increase was observed as θ was increased from 60° to 90°. This nonlinear dependence was primarily due to the highly directional nature of the reflectance properties of the microplates; because of the small size of the pinhole on the reflectometer used, when the plates were rotated such that they were only slightly out of perpendicular, they would reflect light away from the pinhole at a small angle, resulting in minimal signal being detected. This result implies that the plates actually behave as true microscale mirrors that can reflect light with precision in a desired direction.
The transmittance of the sample, as shown in Fig. 4b, could be varied from 42% to 72% as θ was decreased from 90° to 0°. This observed increase was largely due to a gradual change in the projected cross section of the plates, which was minimized when the major axis was aligned with the beam path and maximized when the plates were oriented perpendicular to the beam path. In contrast to the reflectance measurements, the relationship between θ and transmittance, as shown in Fig. 4d, exhibited a reverse trend, in which there was a dramatic change in transmittance as θ was varied from 0–60° and almost no change from 60–90°.
This nonlinear relationship can be explained by simple geometric calculations of the projected area of the microplates. Assuming an equilateral triangular plate, which is rotated along the axis of its height, its projected area is simply its geometric area. However, as the plate is rotated along its vertical axis, its projected area becomes dependent on the angle between the viewing direction and its horizontal axis, as shown in eqn (3):
| Aprojected = ½b sin(α) h | (3) |
Conclusions
In summary, we have developed an effective method for the fabrication of novel magnetically responsive microplates by conjugating Au microplates with amine-terminated superparamagnetic maghemite nanoparticles. The superparamagnetic nanoparticles were tightly bound to the Au surface thanks to the strong interactions between the Au and the amine groups, producing a composite possessing both the magnetic response of the maghemite nanoparticles as well as the high degree of anisotropy and reflectance of the Au microplates. The resulting material exhibits a response on the order of Hz when a magnetic field is applied, and will rotate to align with the direction of the applied field in order to minimize the magnetic potential energy of the system. In spite of the adsorbed maghemite particles, the material remains highly reflective, allowing it to retain good contrast between the “on” and “off” states. By applying a magnetic field at various angles, the transmittance of the system can be varied by a factor of ∼2, while the reflectance can be varied by a factor of 6. We believe that this system has potential use as a new type of responsive optical component operating at the microscale. It may also be integrated into a number of current tunable optical applications. In particular, if the Au microplates are replaced by inexpensive alternatives such as mica or many other reflective metal oxides with a similar microstructure, the designed system might be a promising candidate for smart window applications.Acknowledgements
We thank the U.S. National Science Foundation for support of this research (CHE-1308587).Notes and references
- L. He, M. Wang, J. Ge and Y. Yin, Acc. Chem. Res., 2012, 45, 1431 CrossRef CAS PubMed.
- Y. Chen and H. Ming, Photonic Sens., 2012, 2, 37 CrossRef.
- A. Valizadeh, H. Mikaeili, M. Samiei, S.-M. Farkhani, N. Zarghami, M. Kouhi, A. Akbarzadeh and S. Davaran, Nanoscale Res. Lett., 2012, 7, 480 CrossRef PubMed.
- B. Nikoobakht and M. El-Sayed, Chem. Mater., 2003, 15, 1957 CrossRef CAS.
- S. Skrabalak, L. Au, X. Li and Y. Xia, Nat. Protoc., 2007, 2, 2182 CrossRef CAS PubMed.
- C.-C. Chang, H.-L. Wu, C.-H. Kuo and M. H. Huang, Chem. Mater., 2008, 20, 7570 CrossRef CAS.
- J. E. Millstone, S. J. Hurst, G. S. Métraux, J. I. Cutler and C. A. Mirkin, Small, 2009, 5, 646 CrossRef CAS PubMed.
- M. Wang, C. Gao, L. He, Q. Lu, J. Zhang, S. Zorba and Y. Yin, J. Am. Chem. Soc., 2013, 135, 15302 CrossRef CAS PubMed.
- R. M. Erb, K. H. Cherenack, R. E. Stahel, R. Libanori, T. Kinkeldei, N. Münzenrieder, G. Tröster and A. R. Studart, ACS Appl. Mater. Interfaces, 2012, 4, 2860 CAS.
- Y. Mao, J. Liu and J. Ge, Langmuir, 2012, 28, 13112 CrossRef CAS PubMed.
- S. B. Bubenhofer, E. K. Athanassiou, R. N. Grass, F. M. Koehler, M. Rossier and W. J. Stark, Nanotechnology, 2009, 20, 485302 CrossRef CAS PubMed.
- Y. Luo, Mater. Lett., 2007, 61, 1346 CrossRef CAS PubMed.
- J. Lim, A. Eggeman, F. Lanni, R. D. Tilton and S. A. Majetich, Adv. Mater., 2008, 20, 1721 CrossRef CAS PubMed.
- V. Salgueiriño-Maceira, L. M. Liz-Marzán and M. Farle, Langmuir, 2004, 20, 6946 CrossRef PubMed.
- Q. Zhang, J. Ge, J. Goebl, Y. Hu, Y. Sun and Y. Yin, Adv. Mater., 2010, 22, 1905 CrossRef CAS PubMed.
- R. M. Erb, R. Libanori, N. Rothfuchs and A. R. Studart, Science, 2012, 335, 199 CrossRef CAS PubMed.
- Y. Hu, L. He, X. Han, M. Wang and Y. Yin, Nano Res., 2015, 8, 611 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Detailed information on synthesis and videos of microplate manipulation under an optical microscope. See DOI: 10.1039/c5nh00035a |
| This journal is © The Royal Society of Chemistry 2016 |