Achieving stable and efficient water oxidation by incorporating NiFe layered double hydroxide nanoparticles into aligned carbon nanotubes†
Rong
Chen‡
a,
Gengzhi
Sun‡
ab,
Cangjie
Yang
a,
Liping
Zhang
a,
Jianwei
Miao
a,
Huabing
Tao
a,
Hongbin
Yang
a,
Jiazang
Chen
a,
Peng
Chen
*a and
Bin
Liu
*a
aSchool of Chemical and Biomedical Engineering, Nanyang Technological University, 62 Nanyang Drive, 637459, Singapore. E-mail: chenpeng@ntu.edu.sg; liubin@ntu.edu.sg
bInstitute of Advanced Materials (IAM), Jiangsu National Synergetic Innovation Center for Advanced Materials (SICAM), Nanjing Tech University (Nanjing Tech), 30 South Puzhu Road, Nanjing 211816, China
First published on 2nd December 2015
Abstract
A facile and scalable co-precipitation method is developed to prepare stable colloidal NiFe-LDH nanoparticles at room temperature. We further scrolled NiFe-LDH nanoparticles into well-aligned multi-walled carbon nanotube (MWCNT) sheets to form binder-free hybrid microfiber electrodes, which showed excellent OER activity, reaching 180 mA cm−2 at a small overpotential of 255 mV with outstanding durability.
Conceptual insightsFor water electrolysis, the energy loss at the anode is significant because water oxidation requires four-electron transfer. Therefore, it is highly desirable to design efficient oxygen evolution reaction (OER) catalysts and ensure their assembly into practical OER electrodes. In this study, we have developed a facile and scalable co-precipitation method to prepare stable colloidal NiFe-LDH nanoparticles at room temperature. The as-prepared NiFe-LDH nanoparticles showed outstanding water oxidation activity in basic electrolyte with a low Tafel slope of 21.2 mV dec−1 and a high mass activity of 200 mA mg−1 at an overpotential of 260 mV. Furthermore, to overcome poor conductivity of LDH and weak connection between LDH and the conductive support in practical applications, we scrolled NiFe-LDH nanoparticles into well-aligned MWCNT sheets to form binder-free hybrid microfiber electrodes. The microfiber electrode showed excellent OER activity, reaching 180 mA cm−2 at a small overpotential of 255 mV with outstanding durability. Moreover, the microfiber electrodes are highly flexible and could be woven into fabrics with arbitrary patterns. Our study could provide a general strategy to couple active electrocatalysts with porous, flexible and conductive carbon nanotubes for a wide range of electrochemical and catalytic applications. |
The oxygen evolution reaction (OER) is critical for a wide range of renewable electrochemical energy conversion and storage applications such as metal–air batteries and water splitting cells.1–4 However, OER electrocatalysis suffers from sluggish kinetics and high overpotential due to its four-electron transfer process.5 Among the studied OER electrocatalysts, iridium dioxide (IrO2) and ruthenium dioxide (RuO2) show high catalytic activities.6 Unfortunately, the scarcity, high cost and poor stability hamper the widespread application of these noble metal-based electrocatalysts. Alternatively, first row transition metal (e.g. Ni, Co, Fe, and Mn) oxides or hydroxides are emerging as the next-generation water oxidation catalysts owing to their reasonable overpotential, low cost and much improved stability.7–9
Among various OER electrocatalysts made of earth abundant elements, NiFe layered double hydroxide (NiFe-LDH) showed the best OER performance in an alkaline medium.10,11 The LDH structure, which allows easy intercalation of water molecules and anions in-between different layers, is attractive for OER.12–15 However, NiFe-LDH suffers from poor conductivity in OER electrocatalysis. Over the past few years, great efforts have been made to overcome this problem by coupling NiFe-LDH with conductive supports such as carbon nanotubes or graphene.16–18 However, most of the reported NiFe-LDH hybrid catalysts are in the form of powders, which have to be mixed with binders such as Nafion and cast onto conductive substrate to prepare OER electrodes. To some extent, binders can improve the adhesion of the electrocatalyst to the substrate. However, it decreases the electrocatalytic surface area of the catalyst as well as increases dead weight without contributing to catalytic reactions, leading to decreased overall electrocatalytic performance. Furthermore, the stability of the electrode is relatively poor due to loss of contact between electrocatalysts and substrate during electrocatalysis, especially under large current densities.
Herein, we develop a facile and scalable method to prepare stable colloidal NiFe-LDH nanoparticles by rapid co-precipitation of Ni2+ and Fe3+ ions followed by sonication at room temperature. The prepared NiFe-LDH nanoparticles exhibited a high catalytic activity towards OER with a low Tafel slope of 21.2 mV dec−1 and a high mass activity of 200 mA mg−1 at an overpotential of 260 mV. To construct efficient OER electrodes with good durability, we incorporated NiFe-LDH nanoparticles into well-aligned MWCNTs. The NiFe-LDH@MWCNT microfiber electrode obtained exhibited outstanding OER activity and durability owing to the synergistic integration between these two types of functional nanomaterials. Furthermore, the microfiber electrode is highly flexible and could be woven into arbitrary fabric patterns for a large variety of electrochemical and catalytic applications.
Fig. 1a displays the FESEM image of as-prepared NiFe-LDH nanoparticles, which show uniform size distribution of around 33 nm (Fig. 1d). The nanoparticles could be dispersed in DMF to form a stable colloidal suspension with clear Tyndall effect (inset in Fig. 1a). The detailed structure was further examined by transmission electron microscopy (TEM), as shown in Fig. 1b, which confirms the uniform size of NiFe-LDH nanoparticles. HRTEM (inset in Fig. 1b) reveals a clear lattice spacing of around 0.23 nm, corresponding to (015) planes of NiFe-LDH. Fig. 1c shows the XRD pattern with all peaks matching well with the LDH structure.19 The observed peak broadening results from the small crystal domain size of the nanoparticles, particularly in the (00l) direction.20 X-ray photoelectron spectra (XPS) of Ni 2p3/2 and Fe 2p3/2 indicate that the Ni and Fe species exist in the +2 and +3 oxidation states, respectively (Fig. 1e and f). Ultrasonication plays an important role herein not only to confine the size and crystallinity of NiFe-LDH nanoparticles (Fig. S1, ESI†), but also to disperse them in solution to form stable suspensions (Fig. S2, ESI†), which could benefit their subsequent applications in electrocatalysis.
The electrocatalytic activity of as-prepared NiFe-LDH nanoparticles towards OER was evaluated by rotating disk electrode (RDE) measurement in alkaline electrolyte. Fig. 2 depicts the OER polarization curves of the NiFe-LDH nanoparticles and state-of-the-art IrO2 nanoparticles (as a reference), and shows that the overpotential required to drive a 10 mA cm−2 OER current density (η10) for NiFe-LDH nanoparticles (∼260 mV) is significantly lower than that for IrO2 nanoparticles (∼360 mV). In addition, the NiFe-LDH nanoparticles display lower onset potential and smaller Tafel slope (∼21.2 mV dec−1) as compared to IrO2 nanoparticles (Tafel slope ∼42.8 mV dec−1). It is worth mentioning that the Tafel slope of our NiFe-LDH nanoparticles is one of the smallest reported in the literature (Table S1, ESI†). Furthermore, the OER activity could be tuned by changing the Ni to Fe ratio in NiFe-LDH (Fig. S4, ESI†). The water oxidation reaction in basic electrolyte involves four elementary charge transfer steps, as described below:21
| M + OH− → M–OH + e− | (1) |
| M–OH + OH− → M–O + H2O + e− | (2) |
| M–O + OH− → M–OOH + e− | (3) |
| M–OOH + OH− → M + O2 + e− + H2O | (4) |
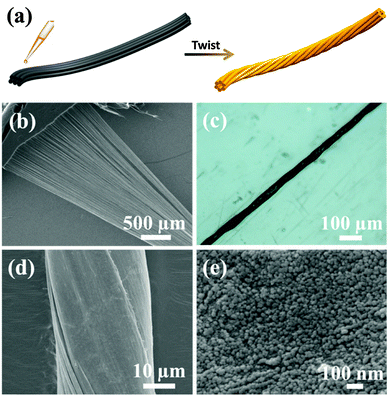
However, insufficient long-term stability (Fig. 5a inset) resulting from the poor electrical conductivity of LDH and weak contact between LDH and the conductive support restricts the practical application of our NiFe-LDH nanoparticles. To overcome this problem and construct an efficient and stable OER electrode, we incorporated NiFe-LDH nanoparticles into well-aligned and highly conductive multi-walled carbon nanotubes (MWCNTs) to form weavable microfiber electrodes. As schematically illustrated in Fig. 3a, this could be achieved by twisting MWCNT sheets with the top surface cast with a thin layer of NiFe-LDH nanoparticles. The MWCNT bundle offers a porous, flexible and conductive support for NiFe-LDH nanoparticles for OER electrocatalysis. In addition, the entangled structure of MWCNT bundles creates numerous intimate contacts between NiFe-LDH nanoparticles and MWCNTs. Fig. 3b shows a MWCNT sheet made of many aligned MWCNTs. The long and aligned MWCNTs could ensure good electrical conductivity and mechanical strength. Fig. 3c displays a typical hybrid microfiber electrode with uniform diameter around 30 μm. After twisting, the NiFe-LDH nanoparticles were uniformly distributed across the entire matrix of MWCNT bundles both inside and outside, resulting in homogeneous and smooth surfaces of MWCNT microfibers (Fig. 3d). The enlarged FESEM image in Fig. 3e clearly shows individual NiFe-LDH nanoparticles that bound firmly on the surface of MWCNT bundles, forming a thin layer. The loading of NiFe-LDH nanoparticles could be adjusted by changing the concentration of NiFe-LDH nanoparticles in the casting solution.
To investigate the OER performance of the microfiber electrode, a single fiber with length of around 5 mm was constructed and applied as the working electrode. The loading amount of NiFe-LDH nanoparticles in the microfiber electrode can significantly affect the OER performance. Thus, we systematically optimized the loading and found that with 90 wt% of NiFe-LDH, the microfiber electrode had the best OER performance (Fig. S6, ESI†). Fig. 4 shows the OER performance of an optimized microfiber electrode at a scan rate of 1 mV s−1. It is found that the overpotential required to drive an anodic current density of 10 mA cm−2 is as low as 215 mV. The current density was calculated based on the geometric area of the microfiber, A = πDh, where D and h are the diameter and length of the microfiber electrode, respectively. The OER current ramps up quickly with increasing applied voltage, reaching 180 mA cm−2 at an overpotential of 255 mV, which to the best of our knowledge is the smallest among all reported values. Video S1 (ESI†) shows the evolution of oxygen bubbles from a single fiber electrode. The OER contribution from bare CNTs is negligible, as shown in the control experiment in Fig. 4a. The microfiber electrode has a Tafel slope of 33.3 mV dec−1 and an onset overpotential of ∼195 mV, which are also among the lowest values reported in the literature. Because of micron-sized diameter, the microfiber electrode showed a large Ohmic loss during electrocatalysis (Fig. S7, ESI†). However, this Ohmic loss could be minimized by connecting a few microfiber electrodes in series for practical applications (Fig. S8, ESI†). Furthermore, the microfiber electrodes are highly flexible and thus they could be woven into fabrics with arbitrary patterns, as illustrated in Video S2 (ESI†), which should be beneficial for a large variety of electrochemical and catalytic applications.
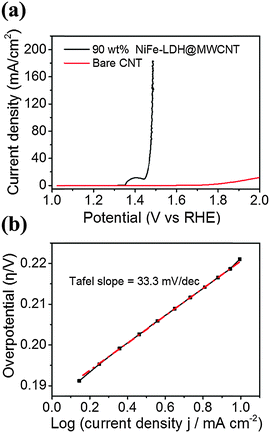 | ||
| Fig. 4 (a) Polarization curves of microfiber electrode with 90 wt% of NiFe-LDH nanoparticles and bare CNT electrode. (b) Tafel slope of microfiber electrode with 90 wt% of NiFe-LDH nanoparticles. | ||
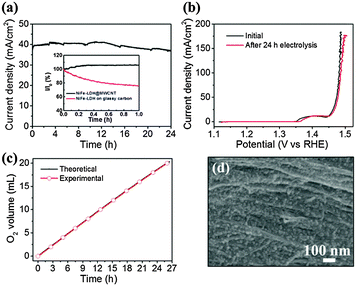
The durability of the microfiber electrode was further tested to highlight its potential applications. For a single microfiber electrode of length of 5 mm at an applied external potential of 1.47 V vs. RHE, the current density remained stable at around 38 mA cm−2 over 24 h of continuous electrolysis (Fig. 5a). The current jump mainly stemmed from bubbles detaching from the electrode. After 24 h of electrolysis, the linear sweep voltammetry curve was obtained again, as shown in Fig. 5b, and almost overlapped with the one obtained before stability testing, indicating the excellent stability of the microfiber electrode. Furthermore, the morphology and elemental chemical states were probed before and after stability testing; these also showed minor changes. The shift of binding energy for Ni 2p, Fe 2p and O 1s towards lower values (Fig. S9, ESI†) resulted from formation of OOH on the surface of NiFe-LDH during OER.22,23 The microfiber electrode had a high selectivity to oxygen by showing a nearly 100% Faradaic efficiency (Fig. 5c). The high activity and durability renders the NiFe-LDH@MWCNT bundle microfiber electrode an excellent candidate for practical OER applications in metal–air batteries and electrolysis cells.
In summary, we have developed a facile and scalable co-precipitation method to prepare stable colloidal NiFe-LDH nanoparticles at room temperature. The as-prepared NiFe-LDH nanoparticles showed outstanding water oxidation activity in basic electrolyte with a low Tafel slope of 21.2 mV dec−1 and a high mass activity of 200 mA mg−1 at an overpotential of 260 mV. To overcome the poor conductivity of LDH and weak connection between LDH and the conductive support in practical OER applications, we incorporated NiFe-LDH nanoparticles into well-aligned MWCNT sheets to form hybrid microfiber electrodes. The microfiber electrode showed excellent OER activity, reaching 180 mA cm−2 at an overpotential of 255 mV with outstanding durability. Furthermore, the microfiber electrodes are highly flexible and could be woven into fabrics with arbitrary patterns. Our study could provide a general strategy to couple active electrocatalysts with porous, flexible and conductive carbon nanotubes for a wide range of electrochemical and catalytic applications.
This study was supported by the Nanyang Technological University startup grant: M4080977.120, Singapore Ministry of Education Academic Research Fund (AcRF) Tier 1: M4011021.120, Singapore A*Star Science and Engineering Research Council – Public Sector Funding (PSF): M4070232.120 and the National Research Foundation (NRF), Prime Minister's Office, Singapore under its Campus for Research Excellence and Technological Enterprise (CREATE) program.
Notes and references
- M.-C. Lin, M. Gong, B. Lu, Y. Wu, D.-Y. Wang, M. Guan, M. Angell, C. Chen, J. Yang, B.-J. Hwang and H. Dai, Nature, 2015, 520, 324–328 CrossRef PubMed.
- Y. Li, M. Gong, Y. Liang, J. Feng, J.-E. Kim, H. Wang, G. Hong, B. Zhang and H. Dai, Nat. Commun., 2013, 4, 1805 CrossRef PubMed.
- R. E. Blankenship, D. M. Tiede, J. Barber, G. W. Brudvig, G. Fleming, M. Ghirardi, M. R. Gunner, W. Junge, D. M. Kramer, A. Melis, T. A. Moore, C. C. Moser, D. G. Nocera, A. J. Nozik, D. R. Ort, W. W. Parson, R. C. Prince and R. T. Sayre, Science, 2011, 332, 805–809 CrossRef PubMed.
- M. Winter and R. J. Brodd, Chem. Rev., 2004, 104, 4245–4270 CrossRef PubMed.
- I. Katsounaros, S. Cherevko, A. R. Zeradjanin and K. J. J. Mayrhofer, Angew. Chem., Int. Ed., 2014, 53, 102–121 CrossRef CAS PubMed.
- E. Fabbri, A. Habereder, K. Waltar, R. Kotz and T. J. Schmidt, Catal. Sci. Technol., 2014, 4, 3800–3821 CAS.
- R. Subbaraman, D. Tripkovic, K.-C. Chang, D. Strmcnik, A. P. Paulikas, P. Hirunsit, M. Chan, J. Greeley, V. Stamenkovic and N. M. Markovic, Nat. Mater., 2012, 11, 550–557 CrossRef CAS PubMed.
- M. W. Kanan and D. G. Nocera, Science, 2008, 321, 1072–1075 CrossRef CAS PubMed.
- D. K. Bediako, Y. Surendranath and D. G. Nocera, J. Am. Chem. Soc., 2013, 135, 3662–3674 CrossRef CAS PubMed.
- C. C. L. McCrory, S. Jung, I. M. Ferrer, S. M. Chatman, J. C. Peters and T. F. Jaramillo, J. Am. Chem. Soc., 2015, 137, 4347–4357 CrossRef CAS PubMed.
- M. Gong and H. Dai, Nano Res., 2015, 8, 23–39 CrossRef CAS.
- Q. Wang and D. O’Hare, Chem. Rev., 2012, 112, 4124–4155 CrossRef CAS PubMed.
- G. Fan, F. Li, D. G. Evans and X. Duan, Chem. Soc. Rev., 2014, 43, 7040–7066 RSC.
- X. Guo, F. Zhang, D. G. Evans and X. Duan, Chem. Commun., 2010, 46, 5197–5210 RSC.
- C. Li, M. Wei, D. G. Evans and X. Duan, Small, 2014, 10, 4469–4486 CrossRef PubMed.
- M. Gong, Y. Li, H. Wang, Y. Liang, J. Z. Wu, J. Zhou, J. Wang, T. Regier, F. Wei and H. Dai, J. Am. Chem. Soc., 2013, 135, 8452–8455 CrossRef CAS PubMed.
- X. Long, J. Li, S. Xiao, K. Yan, Z. Wang, H. Chen and S. Yang, Angew. Chem., Int. Ed., 2014, 53, 7584–7588 CrossRef PubMed.
- C. Tang, H. S. Wang, H. F. Wang, Q. Zhang, G. L. Tian, J. Q. Nie and F. Wei, Adv. Mater., 2015, 27, 4516–4522 CrossRef PubMed.
- J. Han, Y. Dou, J. Zhao, M. Wei, D. G. Evans and X. Duan, Small, 2013, 9, 98–106 CrossRef CAS PubMed.
- D. Evans and R. T. Slade, in Layered Double Hydroxides, ed. X. Duan and D. Evans, Springer Berlin Heidelberg, 2006, vol. 119, ch. 5, pp. 1–87 Search PubMed.
- M. Bajdich, M. García-Mota, A. Vojvodic, J. K. Nørskov and A. T. Bell, J. Am. Chem. Soc., 2013, 135, 13521–13530 CrossRef CAS PubMed.
- M. C. Biesinger, B. P. Payne, L. W. M. Lau, A. Gerson and R. S. C. Smart, Surf. Interface Anal., 2009, 41, 324–332 CrossRef CAS.
- J.-C. Dupin, D. Gonbeau, P. Vinatier and A. Levasseur, Phys. Chem. Chem. Phys., 2000, 2, 1319–1324 RSC.
Footnotes |
| † Electronic supplementary information (ESI) available: SEM, XRD, XPS, polarization curves, stability test, AC impedance spectra, Table S1, Videos S1 and S2. See DOI: 10.1039/c5nh00082c |
| ‡ These authors contributed equally. |
| This journal is © The Royal Society of Chemistry 2016 |