Investigation of one-dimensional multi-functional zwitterionic Ag nanowires as a novel modifier for PVDF ultrafiltration membranes
Xinzhen
Zhao†
,
Yongliang
Chen†
,
Huixia
Xuan
and
Chunju
He
*
State Key Lab for Modification of Chemical Fibers and Polymer Materials, College of Material Science and Engineering, Donghua University, Shanghai 201620, China. E-mail: chunjuhe@dhu.edu.cn
First published on 25th September 2015
Abstract
This work presented a novel kind of one-dimensional multi-functional modifier for membrane materials. Cysteine modified silver nanowires (C-AgNWs) were used to modify polyvinylidene fluoride (PVDF) membranes and the comprehensive optimized properties including separation efficiency, mechanical strength and antifouling ability of PVDF membranes demonstrated that monodisperse, orientated and zwitterionic C-AgNWs were an efficient modifier for the PVDF ultrafiltration membrane. The objective of this work was to comprehensively improve the application performance of polymer materials using the special hybrid mechanism of one-dimensional nanomaterials.
1. Introduction
In recent years, membrane technology as an indispensable separation process has been in a rapid development period and has been widely used in the fields of water treatment, industry, pharmaceuticals, seawater desalination, food and energy due to its outstanding features of high efficiency, energy saving and ease of control.1 Ultrafiltration (UF) and microfiltration (MF) technologies with hydrophobic polyvinylidene fluoride (PVDF), polypropylene (PP), polyether sulfone (PES), and polyvinyl chloride (PVC) as membrane materials have been extensively commercialized based on excellent physical and chemical properties and the application mechanism,2–5 and enhancing the application performance of these polymer membranes by efficient modification is the focus of research of membrane technology at this stage. For example, UF technology shows an increasingly important role in the field of water security, and many families have installed UF membrane modules to ensure the quality of domestic water, so the preparation of efficient, antifouling and antibacterial UF membranes has become an urgent demand of the UF membrane industry.High separation efficiency, high membrane strength, and excellent antifouling performance are three main requirements for the applied UF membranes.6 The commercial UF membranes at the present stage can exhibit appropriate separation efficiency and strength, but the fouling resistance is poor. In addition, many literature studies have reported the modification of UF membrane materials, but only one or two parameters of separation efficiency, strength and fouling resistance for UF membranes can be improved at the same time by the variety of modification methods.7–10 For instance, blending an amphiphilic copolymer is capable of achieving fouling resistance with decreased strength, while the surface grafting method with improved antifouling properties and clogged membrane pores is stuck in the experimental stage due to the complex reaction conditions.11–12
Several conditions must be met for the modifier to be effective for the UF membrane: the modifier should be functional, the executed modified method should be simple and easy, and the modifier should interact with the matrix material to exhibit a positive effect.13–15 An efficient organic–inorganic hybrid method is conducive to take full advantage of two types of materials and using functional nanomaterials such as hydrophilic nanoparticles, modified graphene and carbon nanotubes to hybridize polymer membrane materials is a common method for the optimization of membrane properties.16–18 Although the biggest problem limiting the application of nanomaterials is the dispersion problem, agglomerated nanoparticles always exist after hybridization, restricting the efficient functioning of nanomaterials. In recent years, more and more attention has been paid to one-dimensional materials,19,20 which not only possess the characteristics of nanomaterials such as large surface area, high strength and high activity, but also exhibit special advantages of high dispersion and high orientation due to the length–diameter (L/D) ratio. Our previous studies21 found that blending linear chitin nanocrystals was conducive to enhance the overall performance of PVDF membranes, and one-dimensional functional nanomaterials are potential modifiers for hybridization.
In addition, zwitterionic materials22–24 can exhibit highly promising antifouling ability for the antifouling modification of membrane materials. All the betaine based and amino acid based zwitterionic materials exhibit superior ability to inhibit nonspecific adsorption of pollutants, every ion pair of positive and negative groups can bind a vast quantity of water molecules around and form a protective water layer on the material surface, which shields the hydrophobic interactions between the pollutants and the membrane surface. As far as we know, cysteine as a new kind of zwitterionic antifouling coating material is particularly desirable due to the simple and convenient reactions of thiol chemistry.25
In this paper, cysteine modified silver nanowires (C-AgNWs) were prepared though a simple synthetic method, which are extraordinary one-dimensional nanomaterials with multifunctionality such as fouling resistance of zwitterions, antibacterial activity of Ag and high dispersion of nanowires. A trace amount of C-AgNWs was used to hybridize the PVDF membrane, and the separation performance, membrane strength, antifouling properties and antibacterial properties of the modified PVDF membrane were investigated; this paper aims to provide a new idea for hybrid modification.
2. Experimental
2.1 Materials
Polyvinylidene fluoride (PVDF, MG15) was purchased from Arkema. Polyethylene glycol (PEG, Mw ∼ 20![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), polyvinylpyrrolidone (PVP, K30, Mw ∼ 40
000), polyvinylpyrrolidone (PVP, K30, Mw ∼ 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000), silver nitrate (AgNO3) and bovine serum albumin (BSA, Mw ∼ 67
000), silver nitrate (AgNO3) and bovine serum albumin (BSA, Mw ∼ 67![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000) were purchased from Sinopharm Chemical Co. (China). Humic acid (HA, fulvic acid >90%) was purchased from Aladdin. Ethylene glycol (EG), N,N-dimethylacetamide (DMAC) and ethanol were of analytical grade, all aqueous solutions were prepared with deionized water. All the reagents were used as received without further purification.
000) were purchased from Sinopharm Chemical Co. (China). Humic acid (HA, fulvic acid >90%) was purchased from Aladdin. Ethylene glycol (EG), N,N-dimethylacetamide (DMAC) and ethanol were of analytical grade, all aqueous solutions were prepared with deionized water. All the reagents were used as received without further purification.
2.2 Preparation of C-AgNWs
Ag nanowires were prepared according to the reported method26 using a three step reaction method. The first generation AgNWs were prepared by the following modified CuCl2-mediated polyol process. Cu-additive solution (40 μL of a 4 mM CuCl2 solution in EG), 1.5 mL of 140 mM PVP solution and 1.5 mL of 95 mM AgNO3 solution were added sequentially to 5 mL of ethylene glycol (EG) at 151.5 °C to prepare 1st generation AgNWs, which were condensed for the next growth step. The growth reaction steps were executed continuously, and the length of the AgNWs increased with the increasing number of reactions. 5 mL of EG, Cu-additive and 5 mL of PVP solution were mixed and heated for 1 h at 151.5 °C under stirring, then 1 mL of 1st generation AgNW solution was injected into the above solution, and 1.5 mL of the same AgNO3 solution was injected slowly. After the reaction, Ag nanowires were collected by centrifugation for their subsequent use. The cleaned AgNWs with an alcohol–water mixture were added to cysteine solution (10 g L−1, PBS, pH 7.4) under magnetic stirring at room temperature to react for 1 hour. Finally, the product was collected by filtration using a PTFE microporous membrane (0.22 μm).2.3 Preparation of hybrid PVDF membranes
The hybrid PVDF membranes were prepared through a non-solvent induced phase separation (NIPS) method, a certain amount of AgNWs was added to the casting solution of PVDF/PEG/DMAC (10 g/5 g/50 g), and these homogeneous solutions were casted on glass following the same direction and intensity using a steel knife every time. The added mass ratios of AgNWs to PVDF were 0.5%, 1%, 1.5% and 3% and these prepared hybrid PVDF membranes were labeled C-AgNW-0.5%, C-AgNW-1%, C-AgNW-1.5% and C-AgNW-3%, respectively.2.4 Membrane characterization
The mechanical strength of the modified PVDF membrane was tested using a tensile testing machine with the stretching speed of 20 mm min−1. A differential scanning calorimeter (DSC, TA-Q20, America) was employed to investigate the crystallization enthalpy of the modified PVDF membranes with a ramp rate of 10 °C min−1. Surface morphologies of membranes were viewed using a field emission scanning electron microscope (SEM, Hitachi SU8010, Japan), and the cross-sectional morphology of membranes was observed using an environmental scanning electron microscope (ESEM, Quanta-250, FEI). X-ray photoelectron spectroscopy (XPS, Kratos, AXIS UltraDLD) was used to analyse the elemental composition of the modified nanowires. The water contact angles (CAs) on the membranes were measured using an OCA40Micro (Dataphysics Co., Germany) at 25 °C to evaluate the surface wetting ability using a drop shape image analysis system.2.5 Separation experiments
The separation performances of neat and modified PVDF membranes were investigated using a dead-end filtration system with an effective membrane area of 12 cm2. All the filtration experiments were carried out with the operation pressure at 0.1 MPa, and stable water flux J1 (L m−2 h−1) was obtained using pure water as feed and calculated using eqn (1). The rejection was tested with BSA solution (1 g L−1, pH 7.4) as feed solution, the BSA concentrations of the feed and permeate were examined using a UV spectrophotometer (UV-1800, Shimadzu) with a characteristic wavelength at 280 nm and calculated using eqn (2).| J1 = V/A × t | (1) |
| R = (1 − Cp/C) × 100% | (2) |
2.6 Fouling experiments
Dynamic fouling experiments were executed using an alternate feed solution of pure water and pollutants. BSA solution (1 g L−1, pH 7.4) and HA (1 g L−1) were used as pollutant feed, respectively. Firstly, stable water flux was recorded as J1, then the feed was replaced with pollutant solution and stable flux was recorded as Jp, after that, the tested membranes were washed with pure water for 20 minutes and the secondary pure water flux was obtained as J2.The water flux recovery ratio (FRR) value and the irreversible fouling ratio (IFR) value were calculated by the following eqn (3) and (4), respectively.| FRR = J2/J1 × 100% | (3) |
| IFR = 1 − FRR | (4) |
2.7 Antimicrobial assessment
The antibacterial activities of the modified PVDF membranes were investigated using the Gram positive bacterium S. aureus and the Gram negative bacterium E. coli according to the reported method.27 All containers and membranes were previously sterilized. Bacterial colonies were cultivated in 20 mL of a 3.1%yeast-dextrose broth (containing 8 g L−1 beef extract, 10 g L−1 peptone, 5 g L−1 glucose, 5 g L−1 sodium chloride, and 3 g L−1 yeast extract at a pH of 6.8) at 37 °C. 0.1 mL of the bacterial suspension was pipetted out and added to 50 mL of 3.1% yeast-dextrose broth and the resuspended bacterial solution was at a concentration of 106 cells per ml. About 100 mg of modified PVDF membranes were immersed into the 50 mL bacterial suspension and the suspension was shaken at 37 °C for 2 h, 0.1 mL of the bacterial suspension was pipetted out from the flask and 0.9 mL of PBS was added. Then 0.1 mL of the diluted suspension was spread on a triplicate solid agar plate, which was sealed and incubated at 37 °C for 24 h. Finally, the numbers of viable cells were counted. Each antibacterial efficacy in repeated detections was investigated three times and a control experiment was carried out using 100 mg of the neat PVDF membrane.3. Results and discussion
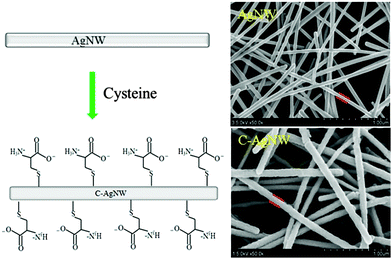
Nanowires, one-dimensional functional nanomaterials, could be used as new types of nanoscale additives,20 and a hybrid method was conducive to demonstrate the functionality of one-dimensional nanomaterials by investigating the change in performance of matrix materials. Because cysteine contained a thiol group and a pair of ionogenic amino and carboxyl groups, which were thought as natural sources of zwitterions, and it was known that cysteine could fabricate an antifouling coating with several nanometer thickness on a gold surface.25 As shown in Fig. 1, the AgNWs were prepared by the reported method,26 and the schematic described that cysteine was used as a coating material and anchored onto the AgNW surface through thiol chemistry. According to the SEM results, the prepared silver nanowires showed a large L/D ratio, smooth surface and uniform diameters (about 85 nm) without significant aggregation. After surface modification with cysteine, the C-AgNW exhibited an increased diameter and a rough surface, indicating that the cysteine coating was fixed onto the surface of the AgNWs through quick and easy thiol chemistry reaction. The detected N and S elements (Table 1) in the modified nanowires were attributable to the featured elemental composition of cysteine. The literature25 reported that when cysteine was anchored onto the material surface in PBS buffer, the majority (about 90%) of amino and carboxy groups were in the ionic state (NH3+ and COO−), so it could be inferred that the C-AgNWs were a one-dimensional material with a cysteine-type zwitterionic surface.| Sample/Element | Atomic concentration (%) | ||||
|---|---|---|---|---|---|
| Ag | C | S | N | O | |
| AgNWs | 99.55 | 0.12 | 0 | 0 | 0.33 |
| C-AgNWs | 91.05 | 3.91 | 1.27 | 1.29 | 2.48 |
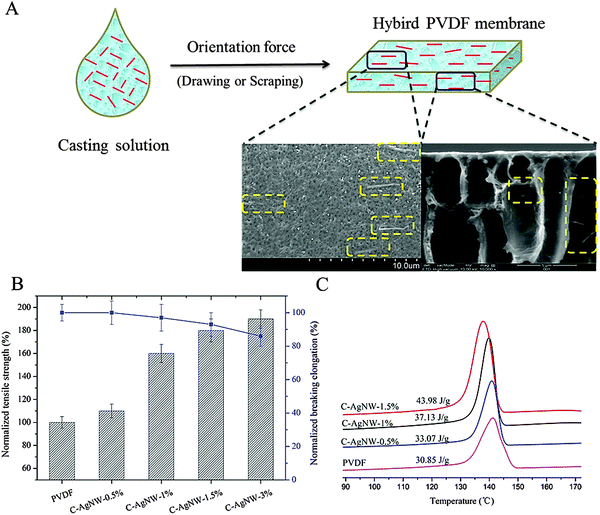
Because the C-AgNWs are a kind of one-dimensional nanomaterial, their unique feature of a high length–diameter ratio not only improved the dispersion by reducing the conventional agglomeration tendency of nanomaterials, but also increased the chances of being oriented. In the preparation of flat membranes and hollow membranes, fixed orientation force of casting or drawing was always additionally used to promote the shaping of the membrane material, one-dimensional C-AgNW and PVDF chains were simultaneously oriented in the orientation process as shown in Fig. 2A. According to the surface and cross-sectional SEM images of the C-AgNW-1% membrane, it was clearly seen that all the added C-AgNWs were arranged in the same direction. Especially in the surface SEM image, all seen C-AgNWs were strictly arranged in accordance with the same direction, exhibited an excellent orientation effect, and all the C-AgNWs were single, showing explicit monodispersity. The tensile strength and breaking elongation of the PVDF membranes before and after hybrid modification are shown in Fig. 2B, and it was clearly seen that a significant increase in the strength of the PVDF membrane after blending C-AgNWs was observed. Compared with the tensile strength value of a neat PVDF membrane, the tensile strength of the C-AgNW-3% membrane was elevated to 190%, and there was no significant decline in the breaking elongation. Fig. 2C shows the significant increase of the crystallization peak intensity of the hybrid PVDF membrane, the crystallization enthalpy of the C-AgNW-1.5% membrane increased from 30.85 J g−1 (PVDF membrane) to 43.98 J g−1, the elevated proportion was more than 40%, which was directly attributed to the increase of membrane strength. Meanwhile, according to Fig. 2A, the C-AgNW directly embedded in the PVDF matrix without visible defects on the membrane surface and came into contact with the PVDF molecular chain. Based on these results, it could be inferred that the increased membrane strength was related to the change in crystallinity of the PVDF membrane, and these two parameters were positively correlated. The increased degree of crystallinity of the modified PVDF membrane should have resulted from the orientated one-dimensional C-AgNW in the PVDF matrix. C-AgNWs, as inorganic–organic hybrid materials with a hard texture and a high L/D ratio, showed a tendency to be easily oriented, and provided the impetus for the orientation of the surrounding polymeric segments during the orientation process. Each orientation of the C-AgNWs would drive the PVDF segments around to be oriented and a large number of PVDF segments with ordered arrangement also resulted in the increase of the crystallinity degree of the modified PVDF membranes, which exhibited increased tensile strength at the macro level. So adding one-dimensional C-AgNW material was beneficial to increase the mechanical strength of the PVDF membrane.
After hybridization of the PVDF membranes with a trace amount of C-AgNWs, the separation efficiency of water flux and rejection of the hybrid PVDF membranes were investigated, and commonly used BSA was selected as the interception media. Because silver is a precious metal, the amount of hybrid AgNWs was no more than 3% due to the perspective of economic benefits. As seen from Fig. 3A and B, more pores were observed on the C-AgNW-1.5% membrane surface than the neat PVDF membrane. As shown in Fig. 3C, the modified PVDF membranes exhibited improved wetting ability with the CA of the C-AgNW-1.5% and C-AgNW-3% membrane decreasing to 71° and 66°, respectively. Fig. 3D showed the water flux and rejection rate of the PVDF membrane was 94 L m−2 h−1 and 75%, nevertheless, the water flux and rejection rate of the C-AgNW-3% membrane increased to 159 L m−2 h−1 and 93%, respectively, Furthermore, the simultaneously increased flux and rejection breaks the conventional trade-off phenomenon, indicating that the added one-dimensional C-AgNWs were conducive to improve the separation efficiency of the PVDF membrane. The improved separation performance of the modified PVDF membrane should be attributed to the enhanced wetting ability on the membrane surface and the matrix to C-AgNWs. The blended hydrophilic C-AgNW in casting solution was conducive to increase the probability of forming membrane pores by delaying the rapid exchange of water and solvent DMAC, which contributed to the flux increase. As zwitterionic nanowires, there were a lot of ionogenic amino and carboxyl groups on the surface of the C-AgNWs, these active amino and carboxyl groups could show strong hydrophilicity though ionic hydration and hydrogen bonding in the aquatic environment, and therefore, a large number of water molecules could be gathered around the C-AgNWs to generate a particular wetting environment. After hybridization, every C-AgNW was uniformly distributed in the membrane surface and the matrix was able to form a slender hydrophilic region in water due to the L/D ratio, the improved microscopic wetting properties of the PVDF membrane surface and the matrix were conducive to the adsorption and permeation of water molecules, and enhance the exclusion and screening ability of hydrophobic BSA molecules to obtain optimized rejection. The more C-AgNWs added, the greater the enhanced proportion of flux and rejection.
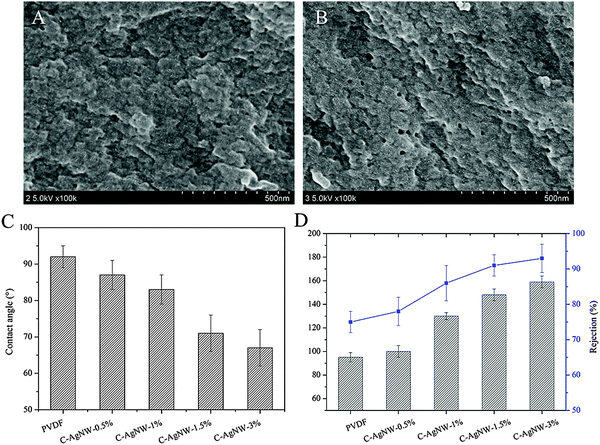 | ||
| Fig. 3 (A) Surface morphology of the PVDF membrane, (B) surface morphology of the C-AgNW-1.5% membrane, (C) water contact angles, (D) and separation performances. | ||
As a kind of hydrophobic material, the PVDF membrane was prone to induce a serious membrane fouling problem during the dynamic separation process, which would reduce the separation efficiency and shorten the service life of the membrane module. The secondary water flux recovery ratio (FRR) values were used to quantify the antifouling ability of modified PVDF membranes. High FRR values (low IFR values) indicated better antifouling performance of the PVDF membranes and less irreversible pollutants remained in the PVDF membranes. As shown in Fig. 4, after the filtration of BSA pollutant solution, the FRR value of the PVDF membrane was only 55%, and the FRR value of the C-AgNW-3% membrane increased to 87%. For the pollutant HA, FRR values of the PVDF membrane, C-AgNW-1%, C-AgNW-1.5% and C-AgNW-3% membranes were 65%, 83%, 91% and 93%, respectively. It was clear that the antifouling performance of the PVDF membranes was significantly increased after adding C-AgNWs. The reason should be attributed to the zwitterionic antifouling characteristics of the C-AgNWs. After the C-AgNWs were evenly distributed in the PVDF membrane, the zwitterionic surface of the C-AgNWs was capable of changing the surrounding microenvironment of the PVDF surface and the matrix due to the super hydrophilicity, wetting properties and antifouling performance of zwitterionic groups, the microenvironment of the modified PVDF membrane could be improved with zwitterionic C-AgNWs and the manifested fouling-resistance properties were conducive to reduce the possibility of adsorption and accumulation of pollutants in the PVDF matrix, whereby original irreversible pollutants were converted to reversible pollutants, which could be washed away by flushing to obtain high FRR values.
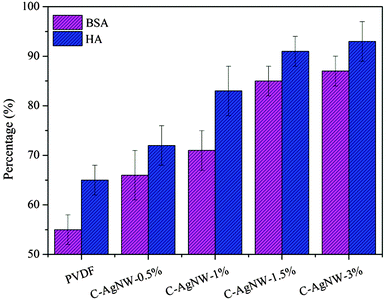 | ||
| Fig. 4 Antifouling performances of neat and modified PVDF membranes with BSA and HA as pollutant feed. | ||
In addition, the antimicrobial properties of the modified PVDF membranes were investigated. Nowadays, silver is considered to be the best antibacterial material, and the bactericidal rates of Ag for various kinds of bacteria are more than 99%; nano-silver is also a potent antimicrobial agent, and could exhibit excellent antibacterial properties in many areas, and AgNWs were able to retain the unique characteristics of sterilization. The antimicrobial experiments of Escherichia coli and Staphylococcus aureus of the modified PVDF membranes were tested through a reported method27 and the results are shown in Table 2; according to the three parallel testing, the bacterial mortalities of the C-AgNW-1.5% membrane were no less than 98%. The modified membrane exhibited significant antibacterial activity for these common Gram bacteria due to the one-dimensional C-AgNWs.
| Bacterial mortality of three samples (%) | |||
|---|---|---|---|
| PVDF | C-AgNW-0.5% | C-AgNW-1.5% | |
| E. coli | 0% | 97% | 99% |
| S. aureus | 0% | 98% | 99% |
4. Conclusions
One-dimensional and multi-functional C-AgNWs were used to prepare a novel PVDF UF membrane. A spot of C-AgNWs was conducive to optimize the performance of the PVDF membrane, and the performance test results of the hybrid C-AgNW-3% membrane showed that the water flux increased about 70% along with improved BSA rejection, the tensile strength was upgraded by 90%, and the FRR values were no less than 85% for BSA and HA pollutants and excellent antibacterial activity of the hybrid PVDF membrane was also observed for S. aureus and E. coli. Although a single performance parameter of the modified PVDF membrane was not as good as the commercial membrane or the reported modified PVDF membranes, the enhanced ratios of comprehensive performance parameters of the modified PVDF membranes demonstrated that C-AgNWs were an effective modifier for the PVDF membrane to enhance the overall application performance, also providing a new idea of using trace one-dimensional materials to modify polymer materials.Acknowledgements
This work was supported by grants from the Program for New Century Excellent Talents in University (No. NCET-12-0827), Program of Introducing Talents of Discipline to Universities (No. 111-2-04) and Innovation Funds for PhD Students of Donghua University (CUSF-DH-D-2015027).Notes and references
- M. A. Shannon, P. W. Bohn, M. Elimelech, J. G. Georgiadis, B. J. Marinas and A. M. Mayes, Nature, 2008, 452, 301 CrossRef CAS PubMed.
- M. Chandler and A. Zydney, J. Membr. Sci., 2004, 237, 181–188 CrossRef CAS.
- Y. Chen, L. Ying, W. Yu, E. T. Kang and K. G. Neoh, Macromolecules, 2003, 36, 9451 CrossRef CAS.
- Y.-K. Wang, G.-P. Sheng, W.-W. Li, Y.-X. Huang, Y.-Y. Yu, R. J. Zeng and H.-Q. Yu, Environ. Sci. Technol., 2011, 45, 9256 CrossRef CAS PubMed.
- B. Verrcht, T. Maere, I. Nopens, C. Brepols and S. Judd, Water Res., 2010, 44, 5274 CrossRef PubMed.
- D. Rana and T. Matsuura, Chem. Rev., 2010, 110, 2448 CrossRef CAS PubMed.
- J. Cho, G. Amy and J. Pellegrino, J. Membr. Sci., 2000, 164, 89 CrossRef CAS.
- A. I. Schafer, A. G. Fane and T. D. Waite, Water Res., 2001, 35, 1509 CrossRef CAS PubMed.
- E. Drioli, A. I. Stankiewicz and F. J. Macedonio, J. Membr. Sci., 2011, 380, 1 CrossRef CAS.
- N. Hilal, O. O. Ogunbiyi, N. J. Miles and R. Nigmatullin, Sep. Sci. Technol., 2005, 40, 1957 CrossRef CAS.
- A. Asatekin and A. M. Mayes, Environ. Sci. Technol., 2009, 43, 4487 CrossRef CAS PubMed.
- Y. Chang, C. Y. Ko, Y. J. Shih, D. Quemener, A. Deratani, T. C. Wei, D. M. Wang and J. Y. Lai, J. Membr. Sci., 2009, 345, 160 CrossRef CAS.
- J. Zlopasa, B. Norder, A. B. Koenders and S. J. Picken, Macromolecules, 2015, 48, 1204 CrossRef CAS.
- W. Stephan, R. D. Noble and C. A. Koval, J. Membr. Sci., 1995, 99, 259 CrossRef CAS.
- F. Lipnizki, R. W. Field and P.-K. Ten, J. Membr. Sci., 1999, 153, 183 CrossRef CAS.
- M. Yu, H. H. Funke, J. L. Falconer and R. D. Noble, Nano Lett., 2008, 9, 225 CrossRef PubMed.
- X. Qu, P. J. J. Alvarez and Q. Li, Water Res., 2013, 47, 3931 CrossRef CAS PubMed.
- Z. Fan, Z. Wang, N. Sun, J. Wang, S. Wang and J. Membr, Science, 2008, 320, 363 CAS.
- Y. Wei, H. Q. Chu, B. Z. Dong, X. Li, S. J. Xia and Z. M. Qiang, Desalination, 2011, 272, 90 CrossRef CAS.
- X. Zhang, T. Zhang, J. Ng and D. D. Sun, Adv. Funct. Mater., 2009, 19, 3731 CrossRef CAS.
- A. W. Qin, X. Li, X. Z. Zhao, D. P. Liu and C. J. He, J. Membr. Sci., 2015, 480, 1 CrossRef CAS.
- O. Azzaroni, A. A. Brown and W. T. S. Huck, Angew. Chem., Int. Ed., 2006, 45, 1770 CrossRef CAS PubMed.
- I. Banerjee, R. C. Pangule and R. S. Kane, Adv. Mater., 2011, 23, 690 CrossRef CAS PubMed.
- Y. Qian and U. Mathias, Chem. Mater., 2012, 24, 2943 CrossRef.
- P. Lin, L. Ding, C. W. Lin and F. Gu, Langmuir, 2014, 30, 6497 CrossRef CAS PubMed.
- J. Lee, P. Lee, H. Lee, D. J. Lee, S. S. Lee and H. V. Ko, Nanoscale, 2012, 4, 6408 RSC.
- Y. Sui, X. L. Gao, Z. N. Wang and C. J. Gao, J. Membr. Sci., 2012, 394, 107 CrossRef.
Footnote |
| † These authors contributed equally. |
| This journal is © The Royal Society of Chemistry and the Centre National de la Recherche Scientifique 2016 |