Open Access Article
Open Access ArticleMarine natural products†
John W.
Blunt
*a,
Brent R.
Copp
b,
Robert A.
Keyzers
c,
Murray H. G.
Munro
a and
Michèle R.
Prinsep
d
aDepartment of Chemistry, University of Canterbury, Christchurch, New Zealand. E-mail: john.blunt@canterbury.ac.nz
bSchool of Chemical Sciences, University of Auckland, Auckland, New Zealand
cCentre for Biodiscovery, School of Chemical and Physical Sciences, Victoria University of Wellington, Wellington, New Zealand
dChemistry, School of Science, University of Waikato, Hamilton, New Zealand
First published on 3rd February 2016
Abstract
Covering: 2014. Previous review: Nat. Prod. Rep., 2015, 32, 116–211
This review covers the literature published in 2014 for marine natural products (MNPs), with 1116 citations (753 for the period January to December 2014) referring to compounds isolated from marine microorganisms and phytoplankton, green, brown and red algae, sponges, cnidarians, bryozoans, molluscs, tunicates, echinoderms, mangroves and other intertidal plants and microorganisms. The emphasis is on new compounds (1378 in 456 papers for 2014), together with the relevant biological activities, source organisms and country of origin. Reviews, biosynthetic studies, first syntheses, and syntheses that lead to the revision of structures or stereochemistries, have been included.
1 Introduction
These annual reviews of marine natural products were initiated by the late Professor D. John Faulkner in 19841,2 and continued by the New Zealand group since 2003. A feature of the reviews has been the inclusion of the structures for all new MNPs, and any subsequently revised structures. The number of new MNPs reported each year has steadily grown from 332 in 1984 to 1378 in this present review of the 2014 literature. This has inevitably resulted in an increased size for each review. With the ever-increasing size creating difficulties for preparation of the annual review, the NPR Editorial Board suggested changing the format to focus on a selection of highlighted structures. To maintain the usual comprehensive coverage of all new and revised MNPs, we have prepared a ESI† document with links associated with this review, showing all structures, along with their names, taxonomic origins, locations for collections, and biological activities. The numbers for all highlighted structures in this review (169) are shown in non-italicised bold font, while italicised numbers refer to the remaining structures in the ESI document.† For structures that have their absolute configurations fully described, the compound number in the diagrams is preceded with †. In addition to the highlighted compounds in this review, we have retained the inclusion of reference to first syntheses of MNPs, and comments on new information on ecological aspects, bioactivities or other relevant data for previously reported MNPs, all as non-highlighted material. The Reviews section (Section 2) has also been reformatted to show selected highlights, with all other reviews referenced in a section of the ESI document.†2 Reviews
There continues to be a steady increase in the number of reviews of various aspects of MNP studies. Some of the more significant reviews (16) are given here while a listing of the remainder (71) is given in the ESI section.† A comprehensive review of MNPs reported in 2012 has appeared.3 ‘Marine-sourced anticancer and cancer pain control agents in clinical and late preclinical development’ have been reviewed,4 and ‘New horizons for old drugs and drug leads’ were described.5 The implications of the Convention on Biological Diversity (1999) and its Nagoya Protocol (2010) on the collection of marine genetic resources has been discussed and should be noted by all who collect marine organisms for MNP studies.6 The putative microbial origin of sponge metabolites has been the subject of several reviews and articles.7–11 Developments in chemical ecology for fish and benthic algae and invertebrates for 2010–2012 have been reviewed, with comment on the biosynthesis of bioactive MNPs by symbiotic microorganisms.12 Polyketide biosynthesis in dinoflagellates has been reviewed.13 There have been comprehensive reviews for marine nucleosides,14 saxitoxin,15 and tetrodotoxin.16 A review of ‘Emerging strategies and integrated systems microbiology technologies for biodiscovery of marine bioactive compounds’ provides a very useful oversight of a number of developing techniques.17 ‘AlgaeBase: an on-line resource for algae’ is an article describing a very comprehensive algal database.18 As in previous years, the MarinLit database19 has been updated and used as the literature source for the preparation of this present review.3 Marine microorganisms and phytoplankton
Even considering the trend of recent years that many marine natural products research efforts are directed towards microorganisms, there has been a sharp upward swing in the number of new metabolites reported from marine microorganisms (677 vs. 493 in 2013). Unless otherwise stated, compounds described in this section were obtained from cultures of the named microorganisms.3.1 Marine-sourced bacteria (excluding from mangroves)
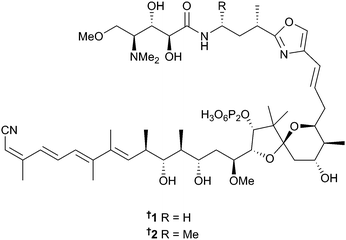
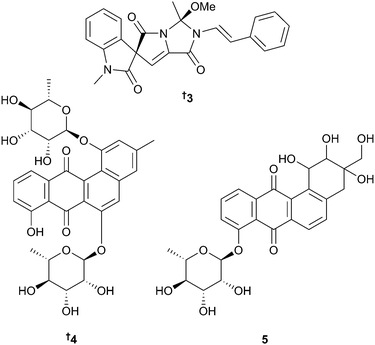
The number of new compounds reported from marine bacteria (164) is similar to the 158 reported in 2013. A metagenomic approach has identified the gene cassette responsible for the biosynthesis of calyculin A, originally isolated from the sponge Discodermia calyx,20 as microbial in origin, arising from Candidatus Entotheonella sp. Functional analysis of the biosynthetic pathway has shown that the end product is actually a diphosphate protoxin, phosphocalyculin A 1 rather than calyculin A, suggesting a phosphorylation/dephosphorylation mechanism for the active chemical defence of the host sponge.21 A further diphosphate, protoxin phosphocalyculin C 2, has been isolated from D. calyx but can be assumed to have arisen from the same microbial source. Phosphocalyculin C, although potent (IC50 = 36 nM vs. P388), is 5000 times less toxic than calyculin C itself.22A Chinese sediment-derived Actinoalloteichus cyanogriseus23 was the source of cyanogramide 3, an unprecedented spirocyclic alkaloid with multidrug-resistance (MDR) reversing activity.24 An interesting dereplication strategy was utilised in the study of sponge-associated Actinokineospora sp. Principal Component Analysis (PCA), hierarchical clustering (HCA) and orthogonal partial least square-discriminant analysis (OPLS-DA) were employed to evaluate HRFTMS and NMR data of crude extracts arising from four different fermentation regimes. Statistical analysis enabled identification of the most suitable one-strain-many-compounds (OSMAC) culture conditions and extraction method resulting in isolation of the O-glycosylated angucyclines actinosporin A 4 and B 5 of which actinosporin A 4 possessed moderate activity against the causative agent of sleeping sickness, Trypanosoma brucei (T. brucei).25
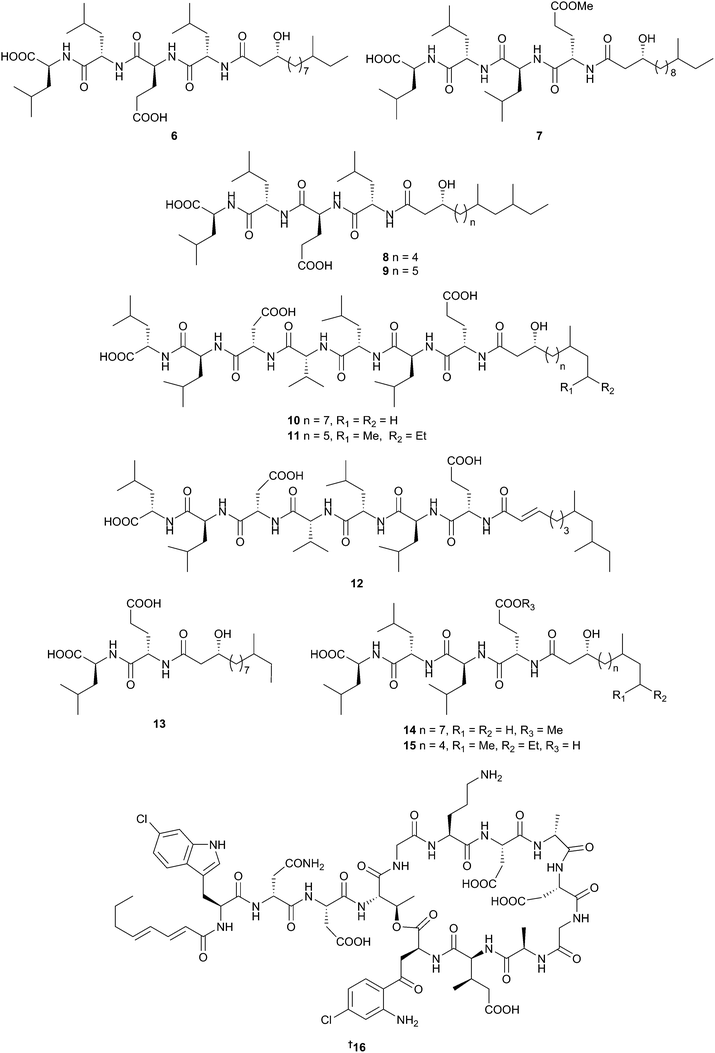
A Korean sediment-derived strain of Bacillus subtilis (B. subtilis) has yielded a variety of linear lipopeptides with differing biological properties, the gageopeptides A–D 6–9,26 gageostatins A–C 10–1227 and gageotetrins A–C 13–15.28 Most are non-cytotoxic with good antibacterial activity, but better antifungal activity especially against the late blight pathogen Phytophthora capsici in the case of gageotetrins A–C. A transformation-associated recombination (TAR) cloning approach was used to capture, activate and express a 67-kb non-ribosomal peptide synthetase (NRPS) biosynthetic gene cluster from Saccharomonospora sp., resulting in isolation of the dichlorinated lipopeptide antibiotic taromycin A 16.29
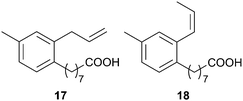
Solwaraspora sp. (ascidian, Trididemnum orbiculatum, Florida Keys, U.S.A.) produced the trialkyl-substituted aromatic acids, solwaric acids A 17 and B 18. Enrichment with 13C-labelled glucose followed by acquisition of a 13C–13C COSY enabled unambiguous determination of the position of the methyl group on the phenyl ring, an approach which could be very useful for structural determination of molecules with multiple quaternary carbons.30
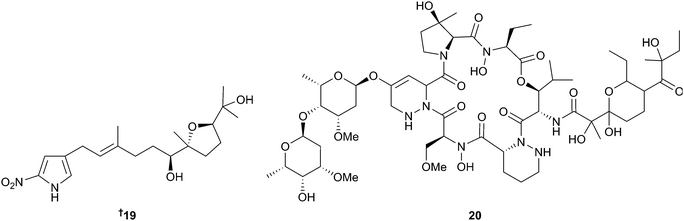
Heronapyrroles A–C are nitropyrrole metabolites with a partially oxidised farnesyl chain appended that were obtained from a Streptomyces sp.31 Biosynthetic considerations prompted the hypothesis that a mono-tetrahydrofuran-diol, heronapyrrole D might be an as yet unidentified metabolite of the bacterium. Following a putative biomimetic synthesis of heronapyrrole D 19 the metabolite was then detected in cultures of the bacterium, thus validating this approach.32 Total synthesis of heronapyrrole C31 has also been reported.33 Mollemycin A 20 is a first-in-class glycol-hexadepsipeptide-polyketide from a Streptomyces sp. (sediment, South Molle Is., Queensland, Australia) and active against certain Gram-positive and Gram negative bacteria, in addition to extremely potent antimalarial activity against drug sensitive and MDR Plasmodium falciparum (P. falciparum) clones.34
A deep-sea strain of Streptomyces, previously a source of spiroindimicins A–D,35 lynamycins A and D35 and piericidins36 has now led to isolation of a number of bisindole alkaloids; indimicins A–E 21–25, lynamycins F 26 and G 2737 and piericidin E1 2836 using the same modified A1BFe + C medium. Piericidin E1 28 was shown to be an intermediate in the biosynthesis of piericidin A138 during identification of the biosynthetic gene cluster.
Growth of the strain in modified ISP3 medium yielded heronamides D–F 29–31.39 Violapyrones H 32, I 33, B 34 and C 3540 were obtained from Streptomyces sp. (starfish, Acanthaster planci, Chuuk, Federated States of Micronesia).41 Violapyrone C 35 has since been synthesised and the absolute configuration determined.42
A study of the biosynthetic pathway for marineosins43 in Streptomyces sp. led to isolation of 23-hydroxyundecylprodiginine 36, 23-ketoundecylprodiginine 37, premarineosin A 38 and 16-ketopremarineosin A 39.44 Syntheses of 23-hydroxyundecylprodiginine 36 (both enantiomers and the perdeuterated version) and of 23-ketoundecylprodiginine 37 were reported, as was the feeding of synthetic prodiginine analogues which led to the production of novel premarineosins, although these were not fully characterised.45
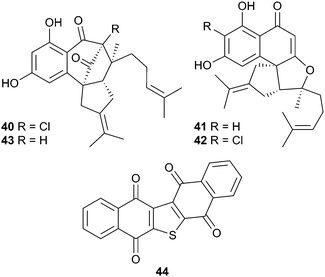
The structure of merochlorin A46,47 has been revised to 4048 and a biomimetic synthesis of (±)-merochlorin B46 has been achieved,49 while a study of the biosynthesis of the merochlorins revealed that just four enzymes are involved50 and that a vanadium-dependent chloroperoxidase mediated a complex series of unprecedented transformations in the biosynthesis. Development of a chlorination method paralleling the biocatalytic process led to the identification of previously undiscovered merochlorins 41–43.51 The known synthetic compound seriniquinone 4452 has now been obtained as a natural product from Serinicoccus sp. and displayed potent and selective antitumour activity.53
Investigation of the biosynthesis of sulfur-containing roseobacticides54,55 produced by Phaeobacter inhibens indicated that these compounds arise from three subunits – dimethylsulfoniopropionate, phenylacetic acid and p-coumaric acid and that roseobacticides regulate the symbiosis between P. inhibens and the microalga Emiliana huxleyi.56 Further new metabolites 45–103 were obtained from the genera Actinoalloteichus, Actinomadura, Actinokineospora, Amycolatopsis, Bacillus, Dermacoccus, Escherichia, Jejua, Micrococcus, Micromonospora, Nocardiopsis, Pelomonas, Pseudoalteromonas, Rapidithrix, Salinispora and Shewanella.57–80 As usual, many new metabolites, and some with revised structures, were also obtained from the genus Streptomyces including 104–172.81–103 The genera Verrucosispora and Vibrio also yielded new metabolites 173 and 174.104,105 Investigation of an octocoral-associated Pseudoalteromonas sp. by MALDI-imaging mass spectrometry (IMS) and molecular network analyses indicated that the strain produces higher levels of the antifungal polyketides, alteramides106,107 in the dark than in the light and also led to revision of the configuration at C-6 for 175 and 176.108 In the light, these compounds were inactivated through a photoinduced intramolecular cyclisation and production of higher levels of these antifungal metabolites in the dark was proposed as a strategy to protect the host corals during night feeding, when they are more exposed.108 Synthesis of the proposed structure of heronamide C109 has indicated that the actual structure of the natural product needs to be re-examined110 and the structure of anthracimycin111 has also been corrected to 177.112 Total syntheses of indoxamycins113 has led to stereochemical revision for the indoxamycins B,114 D113 and E113 to 178–180.115 A number of other total syntheses of bacterial metabolites have been reported. These include syntheses of the depsipeptides, solanamides A116 and B116,117 and arenamides B118 and C,118,119 and synthesis120 of the nucleoside antibiotic A201A.121,122 Total synthesis of dixiamycin B123 was achieved utilising electrochemical oxidation.124 The alkaloid mansouramycin D125,126 was synthesised, as was 5-deoxytetrodotoxin.127,128 New biological activities have been reported for sporolide B129 from Salinispora tropica,130 for some butenolides131,132 and undecylprodigiosin133 from Streptomyces strains.134,135 Biosynthetic studies have been conducted into various bacterial metabolites including arachidonic acid (in Aureispira marina),136,137 macrolactins138 and bacillaene139 (in Bacillus marinus),140 polybrominated aromatics141 (in Marinomonas mediterranea142 and Pseudoalteromonas spp.143), lomaiviticins144 (in Salinispora pacifica145,146 (formerly Micromonospora lomaivitiensis)), tropodithietic acid147 (in Phaeobacter inhibens),148 sulfur volatiles149 (in the Roseobacter clade)150 and avaroferrin151 and putrebactin152 (in Shewanella sp.).153 Biosynthetic studies within the genus Streptomyces include those on thiocoraline154 (in S. albus),155 ikarugamycin,156–158 antimycins159,160 and polyhydroxylated saturated fatty acids,161 marinophenazines162–164 and the isoprenylated phenazines,165,166 JBIR-46, JBIR-47 and JBIR-48.167
3.2 Bacteria from mangroves
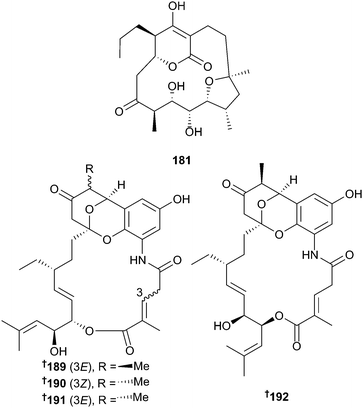
There has been an increase in the number of new metabolites reported from bacteria associated with mangroves (23 in 2014 vs. 10 in 2013). Lechevalieria aerocolonigenes yielded the cyclopentadecane metabolites, mangromicins A 181, B 182168 and D–I 183–188,169 with mangromicin A 181 exhibiting potent antitrypanosomal and radical scavenging (DPPH) activities.168,169 New divergolide congeners 189–192 were obtained from an endophytic Streptomyces strain170 and the biosynthetic gene cluster for divergolides171 identified and characterised.172 Other metabolites 193–203 were isolated from the genera Jishengella, Micromonospora, Streptomyces and Verrucosispora,173–176 and a bio-inspired total synthesis of the indole sesquiterpenoid sespenine177 completed.1783.3 Marine-sourced fungi (excluding from mangroves)
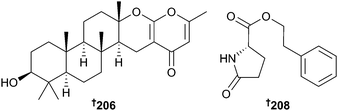
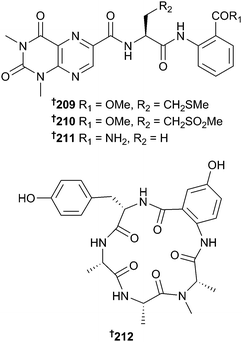
Studies of fungi continue to rise with 318 new compounds reported in 2014 compared to 223 in 2013. Sponge-associated Aspergillus similanensis yielded two isocoumarin derivatives 204 and 205, and a deacetyl analogue of chevalone C,179 chevalone E 206, in addition to pyripyropene S 207.180 While chevalone E 206 itself did not display significant antibacterial activity, it did exhibit synergy with the antibiotics oxacillin and ampicillin against MRSA.181 Ultrasonication of Aspergillus versicolor spores led to the isolation of a mutant with neomycin resistance from which six metabolites not present in the parent strain, including 208 were obtained. Although several patents exist for synthesis of the planar structure of this compound,182–184 this is the first natural product (NP) isolation and establishment of stereochemistry.Cyclo(D-Tyr-D-Pro) was also claimed as a first NP isolation but this has previously been obtained from both terrestrial185 and marine186 sources.187 Lumazine peptides, penilumamide B–D 209–211 and a cyclic pentapeptide, asperpeptide A 212 were obtained from a gorgonian-derived Aspergillus sp. The presence of the sulfone penilumamide188 and the derived sulfoxide penilumamide C 210 led to speculation that these compounds were derived from oxidation of a putative metabolite containing a methionine residue. This led to a feeding experiment with L-methionine resulting in isolation of the sulfide, penilumamide B 209. Yields of penilumamide B 209 and penilumamide increased with increasing concentration of L-methionine and when penilumamide B 209 was exposed to air, penilumamide was detected after a few days whilst penilumamide C 210 was formed several days later.189
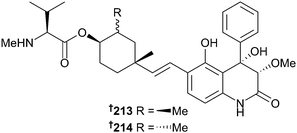
Of the two prenylated hydroquinone derivatives 213 and 214 obtained from a gorgonian-derived Aspergillus sp., 214 exhibited very potent activity against respiratory syncytial virus (RSV).190 These two metabolites differ only in the configuration of a methyl group on a cyclohexane ring yet given that 214 is an extremely potent anti-RSV agent whilst its epimer 213 is completely inactive, indicating the importance of the configuration of this ring for anti RSV-activity.
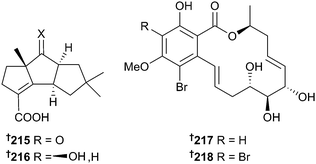
Soft-coral associated Chondrostereum sp. has previously been reported to produce hirsutane-framed sesquiterpenes.191–193 Cultivation of the fungus in a medium with glycerol as the carbon source led to the isolation of the sesquiterpenes chondrosterin I 215 and J 216 of which chondrosterin J 216 displayed potent activity against HTCLs.194 Chemical epigenetic modification of Cochliobolus lunatus (sea anemone Palythoa haddoni) with inhibitors of histone deacetylase (HDAC) resulted in isolation of two brominated 14-membered resorcylic acid lactones 217 and 218, but only in the presence of an HDAC inhibitor.195
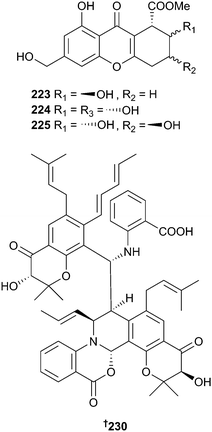
The chromones engyodontiumone A–H 219–226 and the phenol derivatives 227–229 were derived from deep-sea derived Engyodontium album. Of these, engyodontiumones E–G 223–225 were obtained as racemates.196 The known polyketide aspergillus one B197 was also isolated and was a potent inhibitor of settlement of Balanus amphitrite (B. amphitrite) larvae.196 Fermentation of a filamentous fungus of the Eurotiomycetes class (ascidian, Lissoclinum patella, Papua New Guinea) produced the pentacyclic oxazinin A 230, derived from a combination of benzoxazine, isoquinoline and pyran rings. Oxazinin A 230 occurred as a racemate and was antimycobacterial.198
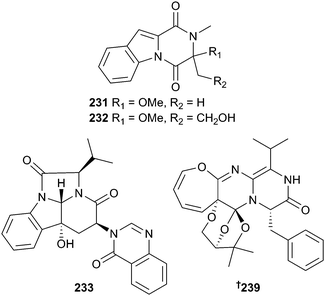
Neosartorya pseudofischeri (starfish Acanthaster planci, Hainan, China) produced different suites of metabolites when cultured in different media. When cultured in glycerol–peptone–yeast extract (GlyPy), the diketopiperazines neosartin A 231 and B 232 were produced along with six known diketopiperazines and a precursor alkaloid but when fermented in glucose–peptone–yeast extract (GluPy), a tetracyclic-fused alkaloid neosartin C 233 was produced along with a known meroterpenoid and five known gliotoxin analogues 234–238, obtained here for the first time as NPs.199 Endophytic Paecilomyces variotii (red alga, Grateloupia turuturu, Qingdao, China) yielded varioxepine A 239, an alkaloid with an unprecedented 3,6,8-trioxabicyclo[3.2.1]octane unit.200
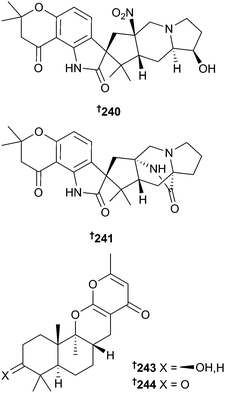
Biosynthetic feeding experiments on Penicillium citrinum using 13C labelled glucose, anthranilic acid and ornithine resulted in isolation of two new citrinalins, 17-hydroxycitrinalin B 240 and citrinalin C 241 and supported the proposition that the citrinalins arise from a bicyclo[2.2.2]diazaoctane precursor. Also in this investigation, synthesis of the enantiomer of citrinalin B201 led to revision of the structure of citrinalin B to 242.202 Penicillipyrones A 243 and B 244 are meroterpenoids obtained from a sediment-derived Penicillium strain and represent a new skeletal class with a unique linkage between the drimane sesquiterpene and pyrone moieties. Penicillipyrone B 244 elicited significant induction of quinone reductase in murine hepatoma cells, indicating a possible cancer preventative role.203
Metabolites 245–254 were also obtained from the genera Acremonium, Arthrinium, Ascotricha and Astrocystis.204–207 As usual, a large number of metabolites were obtained from species of the Aspergillus genus. Two indole diterpenoids 255 and 256, and an isocoumarin 257 were obtained from A. flavus. The known compounds β-aflatrem,208 paspalinine209,210 and leporine B211 were isolated and leporine A212 was prepared from the last of these. Configurations were established for each as 258–261 respectively and β-aflatrem and leporine B were isolated for the first time as MNPs.213 Other metabolites isolated from the genus Aspergillus include 262–327 (the last obtained from co-culture with an unidentified bacterium).214–237 New metabolites 328–371 were also obtained from the genera Beauveria, Cladosporium, Cochliobolus, Curvularia, Dendrodochium, Diaporthaceae, Dichotomomyces, Emericella and Eurotium.238–250 A pyrrolidinoindoline diketopiperazine dimer, cristatumin E, isolated from Eurotium herbariorum,251 appears to be identical to the previously reported eurocristatine.252 The genera Hansfordia, Humicola, Isaria, Neosartorya, Nigrospora, Paecilomyces and Paraconiothyrium also yielded the new metabolites 372–399.179,253–258 The genus Penicillium was the source of many other metabolites 400–461.259–278 Other genera to yield new the metabolites 462–520 were Pseudallescheria, Spicaria, Spiromastix, Stachybotrys, Talaromyces, Trichoderma, Xylaria and Xylariaceae,279–291 while 521 was obtained from a strain of the order Xylariales and also synthesised.292 A number of syntheses of fungal metabolites have resulted in structural revisions of the natural products, including syntheses of (−)-protubonine A 522, (−)-protubonine B 523 (Aspergillus sp.)293,294 and (+)-cristatumin C 524 (Eurotium cristatum).295,296 Synthesis of the racemate of oxalicumone C (Penicillium oxalicum)297 followed by resolution by chiral HPLC and examination of experimental and calculated ECD data, resulted in the configuration of the natural product being assigned as (S)-525.298 Culture of a strain of Aspergillus clavatus isolated from the hydrothermal vent crab Xenograpsus testudinatus in the presence of the abiotic stress agent and hydrothermal vent fluid component zinc (as zinc sulfate), elicited production of a known synthetic cyclic peptide299 that was isolated for the first time from a natural source and named as clavatustide C 526. The fungus did not produce clavatustide C 526 when cultured in the absence of zinc.300 Pericosine E301 (Periconia byssoides) occurs as an enantiomeric mixture in nature and synthesis of the preferred enantiomer (−)-pericosine E 527 has been reported.302 First syntheses of a number of fungal metabolites achieved include those of secalonic acids A303 (Paecilomyces sp.)304 and D305 (Gliocladium sp.)306,307 (±)-sorbiterrin A308 (Penicillium terrestre),309 (−)-auronamide C310 (Penicillium aurantiogriseum),311 calcaripeptides A–C312 (Calcarisporium sp.),313 (−)-aspergilazine A314 (Aspergillus taichungensis),315 aspirin316 (Aspergillus versicolor),317 cochliomycin B318 (Cochliobolus lunatus),319 dendroides320 (Dendrodochium sp.) A,321 B322 and E,322 paecilocin323 (Paecilomyces variotii),324 penicimonoterpene325 (Penicillium chrysogenum)326 and (+)-penostatin E327 (Penicillium sp.).328 The benzaldehyde derivative isotetrahydro-auroglaucin329,330 has been shown to exhibit anti-inflammatory activity, inhibiting the NF-κB pathway through suppressing production of both pro-inflammatory mediators and cytokines.331 Oxirapentyns A332,333 and B333 exhibited growth stimulatory effects on seedling roots of barley and wheat334 while isoechinulin A335,336 was a strong inhibitor of settlement of larvae of the barnacle B. amphitrite.337 Methylpenicinoline338 also displayed anti-inflammatory activity, suppressing expression of pro-inflammatory mediators through the NF-κB and MAPK pathways.339 Heavy metal stress of two strains of hydrothermal vent fungi (Aspergillus sclerotiorum and A. clavatus) induced biosynthesis of metabolites that were not produced under normal culture conditions: A. sclerotiorum produced aspochracin340,341 when stressed with copper and produced stephacidin A342 and notoamides B342 and F343 under normal culture conditions whilst A. clavatus produced the acetophenone derivative clavatol344,345 under stress conditions and deoxytryptoquivaline346 and tryptoquivaline A346 in metal-free culture.347
3.4 Fungi from mangroves
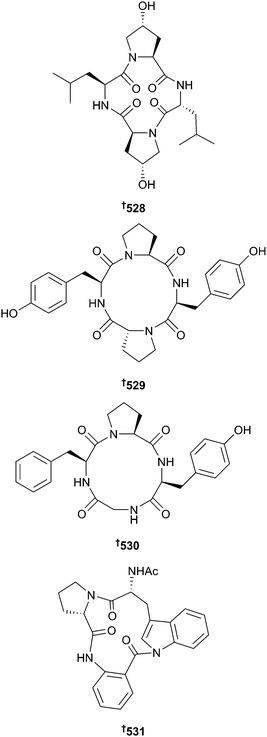
There has been a considerable increase in the number of new metabolites reported from mangrove-associated fungi (108 in 2014 vs. 75 in 2013), with the majority coming from endophytic species. Co-culture of Alternaria and Phomopsis species led to isolation of three cyclic peptides 528–530, all of which exhibited significant activity against a range of plant pathogenic fungi,348,349 whilst co-culture of two brown alga (Sargassum)-derived Aspergillus species also produced a cyclic peptide, psychrophilin E 531.350
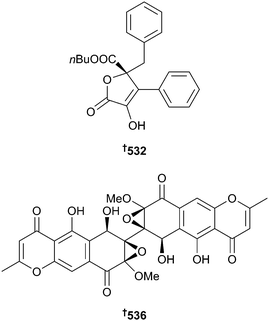
Aspergillus flavipes was the source of the aromatic butyrolactones, flavipesin A 532 and B 533 and the previously synthesised 534351 and 535,352 of which flavipesin A 532 exhibited moderate to good antibacterial activity. Unlike penicillin, it was able to penetrate the biofilm matrix to kill live bacteria inside mature Staphylococcus aureus biofilm.353 An endophytic Diaporthe sp. was the source of diaporine 536, an unprecedented symmetric polyketide which induces conversion of tumour associated macrophages from the M2 to the M1 phenotype in both cellular and animal models.354
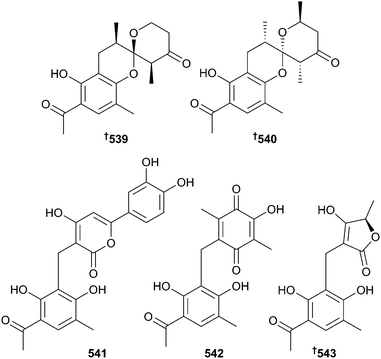
The polyketides dothiorelone F 537 and I 538 were obtained from endophytic Dothiorella sp. along with three known analogues.355 Of these analogues, dothiorelone G356 is actually the same as the previously reported cytosporone R,357 also obtained from a mangrove associated species (Leucostoma persoonii). Peniphenones A–D 539–543 were obtained from Penicillium dipodomyicola. Of these, peniphenone A 539, 540 occurs as a racemate and was separated into its enantiomers by chiral HPLC while peniphenones B 541 and C 542 strongly inhibited Mycobacterium tuberculosis protein tyrosine phosphatase B (MptpB).358
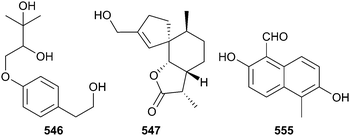
The polyketides 544 and 545 were obtained from co-culture of mangrove soil derived Penicillium sp. with the sediment derived bacterium Streptomyces fradiae and were not produced in discrete bacterial and fungal control cultures, suggesting the activation of silent gene clusters by co-cultivation. These also occurred as a racemate and were separated by chiral chromatography into 544359 a known terrestrial fungal metabolite (9R,14S)-epoxy-11-deoxyfunicone, but now isolated as a first-time MNP, and the enantiomer (9S,14R)-epoxy-11-deoxyfunicone 545.360 An endophytic Penicillium sp. was the source of a phenyl ether derivative 546 and a spiroax-4-ene-12-one derivative 547 with the spiroax-4-ene-12-one derivative 547 more potent to the MG-63 cell line with in vivo activity and significant inhibition of human osteosarcoma in nude mice upon oral administration.361 The prenylated phenols vaccinol A–G, 548–554 and the naphthalene derivative vaccinal A 555 were isolated from Pestalotiopsis vaccinii. Vaccinal A 555 exhibited potent COX-2 inhibition.362 Other genera or families of fungi associated with mangroves to yield the new metabolites 556–635 were Acremonium, Alternaria, Daldinia, Guignardia, Penicillium, Pestalotiopsis, Phoma, Phomopsis, Pseudolagarobasidium, Rhytidhysteron, Stemphylium and Xylariaceae.363–388
3.5 Cyanobacteria
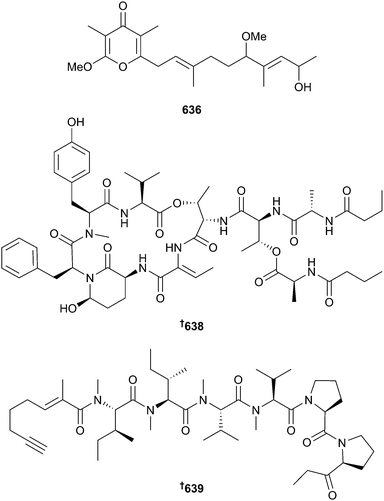
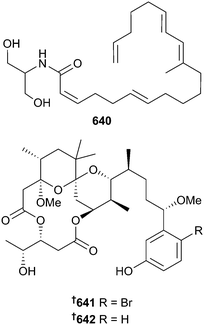
There has been an increase in the number of new metabolites reported from cyanobacteria since 2013, but the total numbers are still low overall (20 in 2014 vs. 9 in 2013), seemingly continuing an overall downward trend. Typical of the phylum, the vast majority of metabolites reported were peptidic in nature. Although not peptidic, yoshinone A 636 and the diastereoisomers yoshinone B1 and B2 637 were isolated from Leptolyngbya sp. Yoshinone A 636 inhibited differentiation of 3T3-L1 cells into adipocytes without accompanying cytotoxicity suggesting potential as an anti-obesity lead.389 A cyanobacterial assemblage, consisting mostly of Lyngbya sp. (now renamed as Moorea sp.) yielded the dolastatin 13 analogue, kurahamide 638, a strong inhibitor of the proteases elastase and chymotrypsin390 and the acetylenic lipopeptide kurahyne 639 an inhibitor of the HeLa cell line and inducer of apoptosis.391Mooreamide A 640 is a cannabinomimetic lipid obtained from Moorea bouillonii and is the most potent marine-derived inhibitor of the neuroreceptor CB1 reported to date.392 Two new aplysiatoxin analogues, 3-methoxyaplysiatoxin 641 and 3-methoxydebromoaplysiatoxin 642 were isolated from Trichodesmium erythraeum and 642, along with the co-isolated known debromo analogues debromoaplysiatoxin393 and anhydrodebromoaplysiatoxin,394 were inhibitors of the Chikungunya virus.395
Other new metabolites 643–655 were obtained from the genera Anabaena, Lyngbya, Moorea, Oscillatoria and Symploca.396–402 The absolute configurations of coibacins A and B403 have been determined as 656 and 657 respectively by total synthesis.404 Syntheses of biselyngbyolide A405 (Lyngbya (Moorea) sp.),406 apratoxin C407 (Lyngbya (Moorea) sp.),408 malevamide D409 (Symploca hydnoides),410 micromide411 (Symploca sp.),412 and 12-epi-hapalindole Q isonitrile413 (Hapalosiphon laingii),414 have also been achieved. Total syntheses of the proposed structures for itralamide B415 and coibamide A416 have indicated that the structures of these natural products require revision.417,418 Grassypeptolides A–C419 were shown to selectively inhibit the dipeptidyl peptidase (DPP8) protease and molecular docking studies indicated that grassypeptolide A binds to the enzyme at two different sites.420 Gallinamide A421 was shown to be a potent and selective inhibitor of the human cysteine protease cathepsin L.422 A genome mining approach was utilised to identify three proteusin rSAM epimerases, enzymes which install multiple D-amino acids in genetically encoded peptide chains, from three strains of cyanobacteria including a marine-derived Pleurocapsa strain.423 A genome mining approach was also used to show that LanA peptides, linear precursor peptides of lanthionine-containing peptides (lanthipeptides),424 are highly diverse among different systems and that closely related lanthipeptide synthetases can be associated with quite different substrate sets.425
3.6 Dinoflagellates
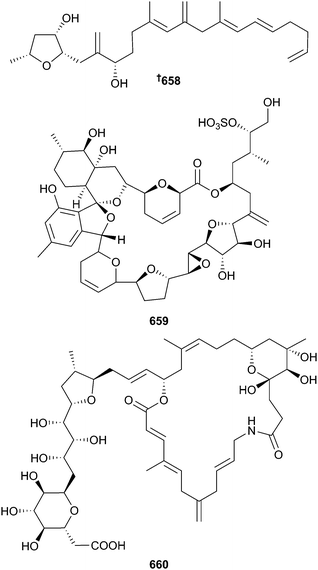
The number of new metabolites reported from dinoflagellates has remained about the same as for 2013 with 19 compounds reported in 2014. The linear polyketide, amphirionin-4 658, from an Amphidinium species displayed very potent and selective growth promotion activity on murine bone marrow stromal ST-2 cells. Feeding experiments with 13C single and double labelled acetate indicated that polyketide 658 comprises a linear C22 chain with two irregular C1 sites (in the terahydrofuran moiety) and four C1 branches.426Dinophysis acuminata was the source of the macrolide acuminolide A 659 which was non-toxic to human tumour cell lines (HTCLs) but a potent stimulator of actomyosin ATPase activity.427 Belizentrin 660, a 25-membered macrolactam obtained from Prorocentrum belizeanum is the first member of its class of polyunsaturated and polyhydroxylated macrocycles and displayed potent effects on neuronal network integrity in cerebellar cells, ultimately resulting in cell death.428Other genera of dinoflagellates from which the new metabolites 661–675 were isolated include Amphidinium, Azadinium, Karenia and Vulcanodinium.429–436 Tentative structures were assigned to ovatoxin-g 676 and an isomer of palytoxin, the so-called isobaric palytoxin.437 The absolute configuration of amphidinin A438 was determined as 677.439 Synthesis of genetically predicted saxitoxin intermediates with identification and quantification in the dinoflagellate Alexandrium tamarense and the cyanobacterium Anabaena cicinalis supports the genetically proposed biosynthetic route440 to saxitoxin.441 Metabolomic and proteomic analyses indicated that Karenia brevis exhibited allelopathy against two competitor diatoms Asterionellopsis glacialis and Thalassiosira pseudonana. The former, which co-occurs with K. brevis, exhibited somewhat more robust metabolism while in the latter, energy metabolism was disrupted and cellular protection mechanisms were impeded.442 Other studies examined the biosynthesis of brevetoxin,443,444 brevisamide445,446 and the genetics of toxin production in Alexandrium catenella.447
4 Green algae
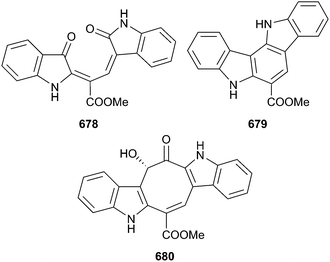
Research into green algal chemistry continues at low ebb with just 13 relevant articles published and just three new compounds published for 2014. The new compounds were the racemosines A–C 678–680,448,449 bisindole alkaloids isolated from Caulerpa racemosa which are biosynthetically related to the well-established green algal metabolite caulerpin, also from a Caulerpa sp.450 Caulerpin featured in studies of antinociception mechanisms451 and the antituberculosis activities of caulerpin and synthetic analogues.452 Included in the green algal literature for 2014 was a study on the effect of natural lycopene (E/Z mixture) on a human prostate cancer cell line,453 while a thought-provoking paper tackled a problem all marine natural product chemists should be alert to – the misuse of taxonomic descriptors and the implications that this has. Misuse of such descriptors appears to be a particular problem with marine algae.4545 Brown algae
The output of new compounds (17) from brown algae in 2014 was again relatively low and although dominated by terpenoid chemistry saw the emergence of a new class of brown algal metabolite. This was the characterisation of a 1-deoxysphingoid, 3-epi-xestoaminol C 681, from a New Zealand collection of Xiphophora chondrophylla following a M. tuberculosis-guided fractionation. A genome-wide screening against a library of non-essential gene deletion mutants of Saccharomyces cerevisiae established the cellular processes that 681 disrupted.455Some 27 diterpenoids ranging across six classes were isolated from a Chinese collection of Dictyota plectens. These included seven new diterpenes, belonging to the dolabellanes 682–684, prenylated guaianes 685–687, and a xenicane 688, 19 known analogues and an ethoxylated artifact as well.456 Three new diterpenoids, a dolabellane 689 a xenicane 690, and a prenylated guaiane 691 with five previously characterised compounds were characterised from Mediterranean collections of several Dictyota spp.457 Three new, 692–694, and three known dolabellane diterpenoids,458,459 were isolated from a Brazilian D. pfaffi, and a single crystal X-ray analysis established the absolute configuration of the known 10,18-diacetoxy-8-hydroxy-2,6-dolabelladiene.458,460 The meroterpenoids sargachromanol Q 695 and R 696 along with the known sargachromanol J461 resulted from re-examination of the original extract from Sargassum siliquastrum,462 while thunberol, 697, is the only new sterol of five isolated from the Chinese Sargassum thunbergii.463 Typical brown algal phlorotannins such as eckol and dieckol have stimulated research into a wide range of topics – heme oxygenase-1 expression,464 oxidative stress,465 anti-adipogenic activity,466 anti-HIV-1467 and antibacterial activity.468 Among the studies on the anti-inflammatory properties of phlorotannins469,470 was the synthesis of a rhodamine-labelled dieckol. Confocal laser microscopy determined that the labeled dieckol was mainly located in the endoplasmic reticulum and studies showed that the anti-inflammatory activity of the conjugate was considerably greater than that of dieckol itself.471 The potential therapeutic properties of fucoxanthin and derivatives have been studied,472,473 as have those of polyphenols such as octaphlorethol,474–476 and mono- and diacylglycerols.477
6 Red algae
There continues to be a marked variation in the number of new compounds obtained from red algae and reported each year, with 42 for 2014 compared to 9 for 2013. There was the usual range of structural types with glycolipids 698478 and 699–701,479 halogenated allenes and alkynes 702–704,480705–709,481710482 and 711,482 halogenated monoterpenes 712–718,483 sesquiterpenes 719–725,484726,485727,485 and 728–730,486 (including the unusual seco-laurokamurone 719), diterpenes 731–733,487 brominated aromatics 734488 and 735–737489 and the mahorones 738–739, unusual 2,3-dibrominated 2-cyclopentenones.490 The use of rapid dereplication tools (UHPLC–PDA–HRMS, 2D HSQC NMR, software tools and databases) for identification of the allenes 710 and 711482 and LC-UV-MS-SPE-NMR for the monoterpenes 712–718483 were notable features.A synthesis491 of the brominated sesquiterpene aldingenin C492 showed that the original structure was incorrect, and it was suggested that aldingenin C was probably the known compound caespitol.493 A synthesis494 of the proposed structure of prevezol B495 has shown that the original structure was incorrect. Of the ∼70 polyhalogenated acyclic monoterpenes isolated from Plocamium spp., surprisingly none had been synthesised until the use of a broadly applicable approach generated four of the naturally occurring compounds and a number of analogues.496 An asymmetric total synthesis of (+)-bermudenynol497 has been accomplished,498 while a total synthesis of the snyderane (+)-luzofuran499 has also been made.500 The anti-inflammatory potential of 5β-hydroxypalisadin B501 from Laurencia snackeyi has been demonstrated in LPS-stimulated RAW 264.7 macrophages502 and LPS-induced zebrafish embryo.503
7 Sponges
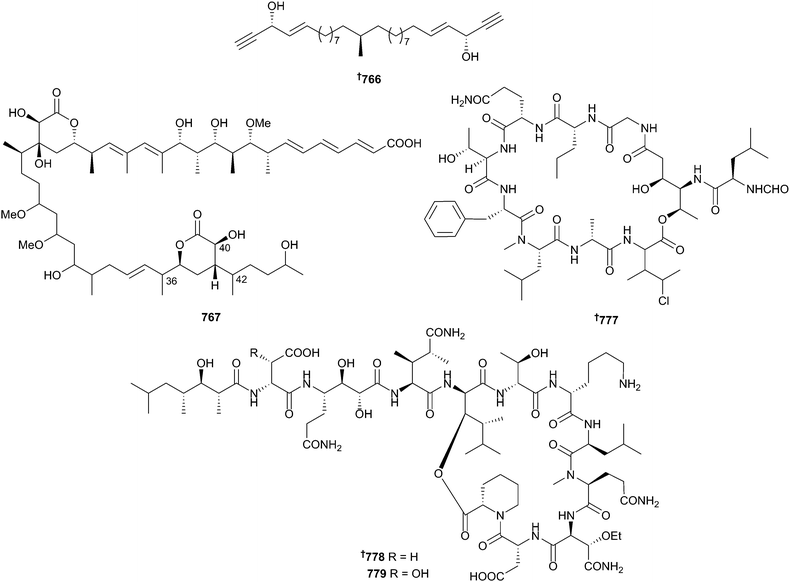
With 283 new structures reported from the phylum Porifera in 2014, sponges have returned again to be a dominant source of new bioactive metabolites, although the growing realisation that microbial symbionts are the real producers of “sponge” specialised metabolites highlights the need for more detailed metagenomic and biosynthetic analyses of sponge matrices. Sphingoids 740504 and 741,505 taurinated fatty acids 742–745506,507 and a large number of polyacetylenes 746–765508–513 have been reported from the genera Callyspongia, Coscinoderma, Echinoclathria, Petrosia, Placospongia, Siphonochalina and Xestospongia. The synthesis of a series of miyakosyne A (Petrosia sp.)514 diastereomers followed by RP-HPLC separation at −56 °C has revealed that the natural product 766 is actually a mixture of two stereoisomers in ∼96![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 4 (14R)
4 (14R)![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
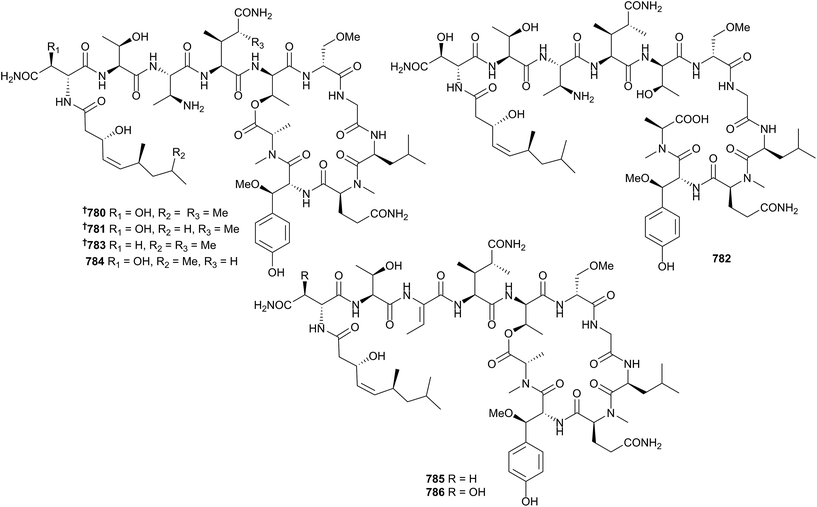
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) (14S) ratio.515 A comprehensive blend of synthesis with NMR, IR and vibrational circular dichroism (VCD) spectroscopy has allowed for the relative configurational assignment of the C-36–C-42 segment of hemicalide 767 (Hemimycale sp.), as well as securing the absolute configuration of C-42. This potent (sub-nM) inhibitor of mitosis was sourced from a deep water sponge collected at the Torres Islands, Vanuatu, but to date has only been reported in a patent.516,517 A series of peroxides 768–773,518 halogenated alkenes 774 and 775,519 and an amide 776520 have been reported from Plakortis simplex, Dysidea sp. and Anoxycalyx (Scolymastra) joubini, respectively. Sponges continue to be a prolific source of peptide natural products. Reisolation of cyclolithistide A from a deep sea Discodermia japonica (−200 m, Sagami Bay, Japan), originally reported in 1998,521 allowed in-depth LC-MS/MS and Marfey's analyses that necessitated a revision of the amino acid sequence and configurations as shown here 777.522 The simple substitution of a side-chain Asp for hydroxy-Asp in pipecolidepsins A 778 and B 779 (Homophymia lamellosa, Saint Marie Is., Madagascar) resulted in a greater than ten-fold increase in activity against three HTCLs.523 The synthesis of 778 had already been reported in 2013.524
(14S) ratio.515 A comprehensive blend of synthesis with NMR, IR and vibrational circular dichroism (VCD) spectroscopy has allowed for the relative configurational assignment of the C-36–C-42 segment of hemicalide 767 (Hemimycale sp.), as well as securing the absolute configuration of C-42. This potent (sub-nM) inhibitor of mitosis was sourced from a deep water sponge collected at the Torres Islands, Vanuatu, but to date has only been reported in a patent.516,517 A series of peroxides 768–773,518 halogenated alkenes 774 and 775,519 and an amide 776520 have been reported from Plakortis simplex, Dysidea sp. and Anoxycalyx (Scolymastra) joubini, respectively. Sponges continue to be a prolific source of peptide natural products. Reisolation of cyclolithistide A from a deep sea Discodermia japonica (−200 m, Sagami Bay, Japan), originally reported in 1998,521 allowed in-depth LC-MS/MS and Marfey's analyses that necessitated a revision of the amino acid sequence and configurations as shown here 777.522 The simple substitution of a side-chain Asp for hydroxy-Asp in pipecolidepsins A 778 and B 779 (Homophymia lamellosa, Saint Marie Is., Madagascar) resulted in a greater than ten-fold increase in activity against three HTCLs.523 The synthesis of 778 had already been reported in 2013.524The stellatolides A–G 780–786 are a family of cyclodepsipeptides from a Madagascan Ecionemia acervus. The full stereochemical analyses of several variants were established by Marfey's analysis, and the solid-phase peptide total synthesis of 780 was also achieved. The unexplained racemisation of L-Thr during the Marfey's analysis is a salient warning to all experimentalists using this technique. Several of the stellatolides were cytotoxic in the nM range against three HTCLs.525
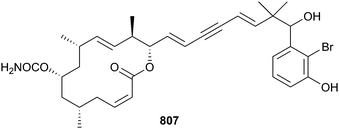
The genera Asteropus, Discodermia, Pipestela, Reniochalina, Stylissa, Suberites and Theonella have also yielded a large number of peptides 787–806.526–532 Callyspongiolide 807 is an unusual carbamate macrolide with an unprecedented conjugated dienyne side chain isolated from Callyspongia sp. (Ambon, Indonesia). Evaluation of 807 against three HTCLs indicated potent cytotoxicity (IC50 = 60–320 nM). Notably, the viability of cell lines treated with callyspongiolide was not affected by QVD-OPh, a known caspase-inhibitor, suggesting the test compound induces cellular toxicity in a caspase-independent manner.533
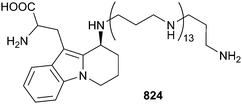
The total synthesis of halichondrin A has been accomplished. Halichondrin A is a “phantom” natural product, viz. it is the only member of the norhalichondrin/halichondrin/homohalichondrin family not to have been detected from a natural source, typically Halichondria okadai,534,535 even though concerted efforts have been made to try and detect this compound for more than 20 years. Use of the synthetic material to guide studies searching for the presence of halichondrin A in legacy extracts of H. okadai were unsuccessful.536 A diketopiperazine 808,537 a 3-alkylpiperidine 809,538 seven manzamine-type alkaloids 810–816539,540 and seven 3-alkylpyridines 817–823541 have been isolated from the sponge genera Callyspongia, Acanthostrongylophora and Topsentia. Axinyssa aculeata (Okinawa) was the source of protoaculeine B 824 that is a putative novel N-terminal residue for the peptidic toxins aculeines A–C. The structure of this polyamine compound, including identifying its attachment to the peptide toxins themselves, was determined from detailed synthetic and mass spectrometric studies.542
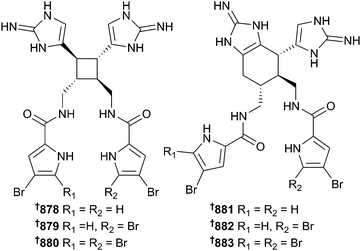
Other indole 825–829543,544 and β-carboline 830–837544–546 alkaloids were reported from Hyrtios, and Luffariella sponges. A biomimetic total synthesis of (±)-dictazole B, isolated from the shallow water sponge Smenospongia cerebriformis,547 has been performed using artificial light to facilitate the [2 + 2] photochemical cycloaddition reaction between aplysinopsin monomers, under the presumed high local concentration of the parent reactant that would be found in the relevant sponge organelle. This biomimetic route is a viable alternative to the [4 + 2] Diels–Alder cycloaddition that has been presumed to take place in the biosynthesis of the cycloaplysinopsins.548 Fifteen new aaptamine alkaloids 838–852549–551 have been reported from Aaptos sponges while a new polycyclic aromatic alkaloid 853 came from an Ancorina sp.552 An unbiased screen of a library of 480 sponge extracts identified the surprisingly simple compound girolline553 from Stylissa carteri (Mont Pass, Pohnpei, Micronesia) as a potent inhibitor of signalling of MyD88-dependent and -independent Toll-Like Receptors (TLR) 2, 3, 4, 5 and 7, reducing cytokine production in peripheral blood mononuclear cells and macrophages, therefore imbuing the molecule with potent anti-inflammatory activity. In-depth chemical genetics profiling identified elongation factor 2 as the molecular target of girolline implying inhibition of protein synthesis as its mode of action.554 Guanidine-derived alkaloids 854–863 were reported from Monanchora pulchra,555Acanthella cavernosa556 and Biemna laboutei,557 while several brominated hymenialdisine-type 864–867 and oroidin/oroidin dimer-type 868–877 came from Callyspongia sp.558 and Agelas sp.,559–561 respectively. A highly scalable, multi-gram synthesis of axinellamines A and B (Axinella sp.)562 and analogues has been achieved. Evaluation of the antibacterial properties of racemic synthetic products highlighted their potent inhibition of both Gram positive and negative bacteria (MIC 0.5–8 μg mL−1vs. eight bacterial cell lines), even though the initial isolation study reported minimal activity. Further detailed investigation could not determine a specific mode of action but did suggest secondary membrane-disruption as the likely route to activity.563 The comprehensive total synthesis of sceptrin, ageliferin and massadine congeners via single electron cycloaddition reactions has several wide-ranging consequences. Firstly, it necessitates the revision of the absolute configuration of sceptrin and its brominated analogues 878–880564–566 and ageliferin and congeners 881–883567 as shown here, based upon X-ray crystal structures. Moreover, the massadines had been thought to be rearrangement products from the ageliferins,568,569 however the absolute configurations of the two series of compounds are antipodal, hence indicating enantiodivergent biosynthetic pathways in vivo to these bromopyrrole alkaloids and invalidating the so-called “Scheuer-hypothesis” for their formation.570,571
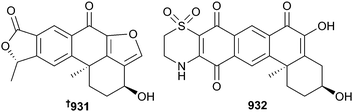
Bromotyrosine metabolites 884–893 have been isolated from Callyspongia,572Aplysinella573 and Dendrilla574 sponges, while Haliclona, Petrosia, Phoriospongia and Dragmacidon sponges have yielded substituted purine/pyrimidine compounds 894–898.504,575–577 As usual, sponges continue to be a valuable reservoir of new terpenoid metabolites. Merosesquiterpenoids 899–930 came from the sponge genera Aka (Siphonodictyon), Dysidea, Dactylospongia and Xestosopngia.578–584 In particular, xestolactone A 931 and xestosaprol O 932 were isolated from Xestospongia vansoesti (Palawan Is., Philippines). Xestosaprol O is twenty times more potent as an inhibitor of indoleamine 2,3-dioxygenase (IDO; IC50 = 4 μM) than 931 (IC50 = 81 μM), making it a lead towards inhibitors of tumour immune escape. The total syntheses of 932 and two analogues were achieved using the rarely applied silver-catalysed Sato photochemical coupling, leading to a short and efficient synthesis of the natural product. Comparative analysis of 932 and its analogues showed that the 3-OH functional group is highly detrimental for IDO-inhibitory activity.585
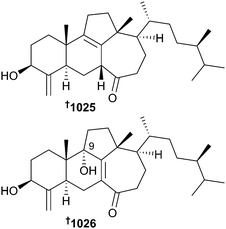
A merotetraterpenoid 933 has been reported from Sarcotragus spinosulus.586 Similarly, Axinyssa, Dysidea, Ircinia and Topsentia sponges were the sources of a number of sesquiterpenoids 934–964,587–591 some of which had been cleaved, while diterpenoids were obtained from Diacarnus megaspinorhabdosa965–970,592Spongia sp. 971–973593 and Darwinella oxeata974–982.594 A large number of sesterterpenoids 983–1011 were reported in 2014 from the sponge genera Clathria, Coscinoderma, Hippospongia, Hyrtios, Petrosaspongia, Phorbas and Scalarispongia.506,595–601 As always, sponges yielded a variety of steroids 1012–1019,504,505,602,603 steroidal amines 1020 and 1021,604 and degraded steroids 1022–1024605,606 from species of Echinoclathria, Corticium, Haliclona, Ircinia, Petrosia, Plakortis and Theonella. T. swinhoei (Xisha Is., S. China Sea) yielded swinhoeisterols A 1025 and B 1026 with unprecedented 6/6/5/7 tetracyclic frameworks. The absolute configurations of the compounds were secured by a combination of X-ray diffraction, TDDFT ECD calculations and Mosher's method. Detailed in silico analysis of both compounds against 211 potential protein targets of relevance for cancer therapy revealed 1025 should exhibit activity against the histone acetyltransferase (h)p300. This was validated in vitro with IC50 = 2.9 μM while 1026 was almost 100 times less potent (IC50 = 240 μM), indicating the steric importance of the 9-OH in abrogating activity.607
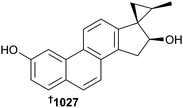
Cinanthrenol A 1027 is the first example of a phenanthrene-containing steroid. It was sourced from Cinachyrella sp. collected by dredging at −160 m (Oshima-Shinsone, Japan) where the unique phenanthrene-fluorescence signature was used to select the sponge from amongst detritus. As well as moderate activity against various HTCLs, 1027 was a potent estrogen-receptor binder, displacing estradiol in a competitive manner with IC50 = 10 nM, as well as altering estrogen-responsive gene expression.608
A series of triterpenoids 1028–1032 have been reported from Jaspis stellifera609 and Siphonochalina siphonella.610 An artificial “sponge” has been developed for the non-destructive concentration of marine natural products in sensitive marine ecosystems. A pump is used to suction sponge-matrix particles that are filtered into a hollow-fibre bioreactor. This is used to cultivate microbial symbionts that produce secondary metabolites which are, in turn, trapped on reversed-phase cartridges for concentration. The artificial sponge system was used in three expeditions across 11 locations, two in the Pacific and one in the South China Sea, with the deployment at Pulau Lakei (Sarawak, Malaysia) yielding jasplakinolide, jasplakinolide B and C in a 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, the same proportions as found from Jaspis splendens collected near Vanuatu.611,612 Metagenomic analyses continue to rise in their importance to sponge natural product biosyntheses. Detailed metagenomic profiling of the “High Microbial Abundance” (38–57% microbial biomass) sponge Plakortis halichondrioides (Little San Salvador Is., Caribbean Sea) found characteristic gene sequences of poribacteria (a bacterial phylum found exclusively in sponges) but the known supA and swfA biosynthetic clusters observed were absent from the poribacteria genomes. Further investigation showed similarity between these clusters and protist type I polyketide synthetase (PKS) genes hence protists could be a previously overlooked reservoir of novel bioactive metabolites.613 A comprehensive metagenomic survey of the microbial community associated with the metabolite-rich sponge Theonella swinhoei has uncovered a new bacterial genus, Entotheonella. This genus is a member of the new candidate phylum Tectomicrobia and appears to be a widespread sponge endosymbiont across many diverse sponge species. Moreover, biosynthetic gene cassettes suitable for production of many “Theonella swinhoei” NRPS and PKS compounds, including the onnamide,614 polytheonamide,615 keramamide616 and cyclotheonamide617 classes, have been identified in the microbial genome.8,9 A metagenomic approach identified the gene cassette for calyculin A (Discodermia calyx)20 biosynthesis as being microbial in origin, as described in 3.1 Bacteria, and resulted in the identification of two diphosphate protoxins (see 1 and 2),21,22 again underscoring the importance of symbiotic microbial communities in the production of “sponge” metabolites. The microtubule-stabilising mode of action of laulimalide618 and peloruside A619 has been established through the use of high-resolution crystal structures of the compounds bound to tubulin,620 while aaptamine621 has been shown to have protective effects against cisplatin-induced kidney damage,622 and also prevents photoageing.623 A polybrominated diphenylether624 (Dysidea granulosa) has been shown to inhibit hepatitis C non-structural protein NS3 helicase, a validated antiviral target.625 The four bromotyrosine compounds ianthelliformisamine A–C (Suberea ianthelliformis)626 and spermatinamine (Pseudoceratina sp.)627 were selective and potent inhibitors of human carbonic anhydrase IX (IC50 = 0.20–0.36 μM), with implications for maintaining cellular pH homeostasis and hence application in a variety of disease states.628 The sesterterpenoid heteronemin (Hyrtios sp.)629 inhibited trans-activation response DNA-binding protein 43 kDa (TDP-43), a key factor of several neurodegenerative states. Heteronemin binds to TDP-43 (ki = 270 nM) and altered the aggregation state and localisation of this important neurochemical target.630 The first total syntheses of an all-(Z) octadeca-pentene-3-one (Callyspongia sp.),631,632 strongylodiol G (Strongylophora sp.),633,634 bitungolide B (Theonella swinhoei),635,636 plakortone L (Plakortis clathrata),637,638 epiplakinic acid F (Plakinastrella sp.),639 and its methyl ester (Plakortis halichondrioides),640,641 gracilioether F (Plakinastrella mamilaris),642,643 taumycins A and B (revised 1033 and 1034, Fascaplysinopsis sp.),644,645 polydiscamides B–D (Ircinia sp.),646,647 callipeltin M (Latrunculia sp.),648,649 topsentolide A2 (revised 1035, Topsentia sp.),650,651 penarolide sulfate A2 (Penares sp.),652,653 haliclorensin C (Haliclona tulearensis),654,655 nakinadine A (revised 1036, Amphimedon sp.),656,657 thiaplakortone A (Plakortis lita),658,659 (+)-cylindradine A (Axinella cylindratus),660,661 ianthelliformisamines A–C (Suberea ianthelliformis),626,662,663 tokaradine C (Pseudoceratina purpurea),663,664 (+)-hemifistularin 3 (revised 1037, Verongia sp.),665,666 trachycladines A and B (Trachycladus laevispirulifer),667–669 cyclospongiaquinone-1 (Stelospongia conulata),670,671 deoxyspongiaquinol (revised 1038) and deoxyspongiaquinone (revised 1039, Euryspongia sp.),672,673 plakinamine B (Plakina sp.)674,675 and clionamine D (Cliona celata)676,677 have all been completed. The total syntheses of the putative structures of 15-oxopuupehenoic acid (Hyrtios sp.)678,679 and astakolactin (Cacospongia scalaris)680,681 have also been achieved but the spectroscopic data call into question the original identified structures, with no alternative suggestions provided.
1 ratio, the same proportions as found from Jaspis splendens collected near Vanuatu.611,612 Metagenomic analyses continue to rise in their importance to sponge natural product biosyntheses. Detailed metagenomic profiling of the “High Microbial Abundance” (38–57% microbial biomass) sponge Plakortis halichondrioides (Little San Salvador Is., Caribbean Sea) found characteristic gene sequences of poribacteria (a bacterial phylum found exclusively in sponges) but the known supA and swfA biosynthetic clusters observed were absent from the poribacteria genomes. Further investigation showed similarity between these clusters and protist type I polyketide synthetase (PKS) genes hence protists could be a previously overlooked reservoir of novel bioactive metabolites.613 A comprehensive metagenomic survey of the microbial community associated with the metabolite-rich sponge Theonella swinhoei has uncovered a new bacterial genus, Entotheonella. This genus is a member of the new candidate phylum Tectomicrobia and appears to be a widespread sponge endosymbiont across many diverse sponge species. Moreover, biosynthetic gene cassettes suitable for production of many “Theonella swinhoei” NRPS and PKS compounds, including the onnamide,614 polytheonamide,615 keramamide616 and cyclotheonamide617 classes, have been identified in the microbial genome.8,9 A metagenomic approach identified the gene cassette for calyculin A (Discodermia calyx)20 biosynthesis as being microbial in origin, as described in 3.1 Bacteria, and resulted in the identification of two diphosphate protoxins (see 1 and 2),21,22 again underscoring the importance of symbiotic microbial communities in the production of “sponge” metabolites. The microtubule-stabilising mode of action of laulimalide618 and peloruside A619 has been established through the use of high-resolution crystal structures of the compounds bound to tubulin,620 while aaptamine621 has been shown to have protective effects against cisplatin-induced kidney damage,622 and also prevents photoageing.623 A polybrominated diphenylether624 (Dysidea granulosa) has been shown to inhibit hepatitis C non-structural protein NS3 helicase, a validated antiviral target.625 The four bromotyrosine compounds ianthelliformisamine A–C (Suberea ianthelliformis)626 and spermatinamine (Pseudoceratina sp.)627 were selective and potent inhibitors of human carbonic anhydrase IX (IC50 = 0.20–0.36 μM), with implications for maintaining cellular pH homeostasis and hence application in a variety of disease states.628 The sesterterpenoid heteronemin (Hyrtios sp.)629 inhibited trans-activation response DNA-binding protein 43 kDa (TDP-43), a key factor of several neurodegenerative states. Heteronemin binds to TDP-43 (ki = 270 nM) and altered the aggregation state and localisation of this important neurochemical target.630 The first total syntheses of an all-(Z) octadeca-pentene-3-one (Callyspongia sp.),631,632 strongylodiol G (Strongylophora sp.),633,634 bitungolide B (Theonella swinhoei),635,636 plakortone L (Plakortis clathrata),637,638 epiplakinic acid F (Plakinastrella sp.),639 and its methyl ester (Plakortis halichondrioides),640,641 gracilioether F (Plakinastrella mamilaris),642,643 taumycins A and B (revised 1033 and 1034, Fascaplysinopsis sp.),644,645 polydiscamides B–D (Ircinia sp.),646,647 callipeltin M (Latrunculia sp.),648,649 topsentolide A2 (revised 1035, Topsentia sp.),650,651 penarolide sulfate A2 (Penares sp.),652,653 haliclorensin C (Haliclona tulearensis),654,655 nakinadine A (revised 1036, Amphimedon sp.),656,657 thiaplakortone A (Plakortis lita),658,659 (+)-cylindradine A (Axinella cylindratus),660,661 ianthelliformisamines A–C (Suberea ianthelliformis),626,662,663 tokaradine C (Pseudoceratina purpurea),663,664 (+)-hemifistularin 3 (revised 1037, Verongia sp.),665,666 trachycladines A and B (Trachycladus laevispirulifer),667–669 cyclospongiaquinone-1 (Stelospongia conulata),670,671 deoxyspongiaquinol (revised 1038) and deoxyspongiaquinone (revised 1039, Euryspongia sp.),672,673 plakinamine B (Plakina sp.)674,675 and clionamine D (Cliona celata)676,677 have all been completed. The total syntheses of the putative structures of 15-oxopuupehenoic acid (Hyrtios sp.)678,679 and astakolactin (Cacospongia scalaris)680,681 have also been achieved but the spectroscopic data call into question the original identified structures, with no alternative suggestions provided.
8 Cnidarians
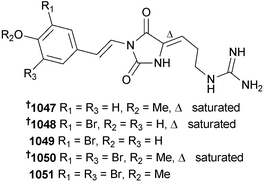
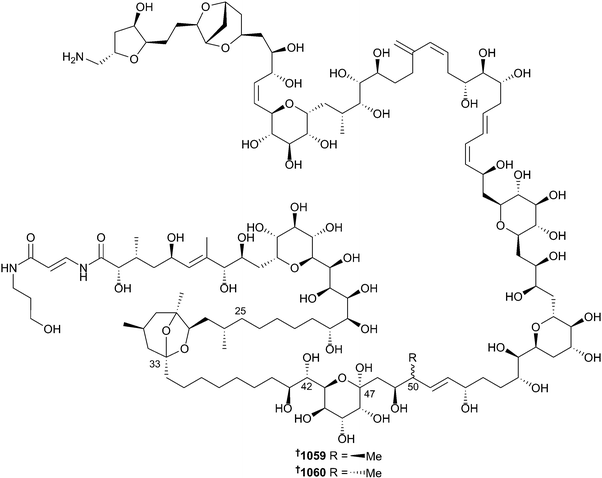
The number of new compounds reported from cnidarians in 2014 (201) is similar to the previous decadal average. The importance of chemical cues in coral reef remediation have been highlighted in a study that found that coral and fish juveniles were repelled from reefs that were overfished and seaweed-dominated.682 A method of recovery of such degraded habitats was proposed to involve reduction in fishing harvest of critical species of herbivorous fishes. The chemistry of both hard and soft corals are dominated by terpenes – alkaloids are only rarely isolated. In 2014 there were seven examples of alkaloids isolated from soft corals, comprised of an unusual diketopiperazine 1040 from Menella kanisa,683 and six tetraprenylated purines, malonganenone L–Q 1041–1046, from Echinogorgia pseudossapo.684 In contrast, cnidarians of the order Zoantharia (zoanthids) were the sources of five new parazoanthine congeners 1047–1051 (Parazoanthus axinellae).685 These structures were established using a metabolomics model, with LCMS/MS fragment analysis being used to define structures and LC-ECD employed to assign absolute configuration (to 1047, 1048 and 1050).Other zoanthamine analogues, 1052–1058, were isolated from Zoanthus sp.686,687 and Zoanthus kuroshio688 as well as a new 42-hydroxypalytoxin diastereomer 1059 from Palythoa tuberculosa.689 Previous studies of an extract of Palythoa toxica identified the major toxin to be a 42-hydroxy analogue of palytoxin 1060 however the configuration at positions C-41 and C-42 remained unresolved.690 More recent J-based analysis has defined the configuration at these stereocentres as (41S) and (42S). The same study also established the absolute configuration of a related palytoxin analogue isolated from P. tuberculosa as being (42S)-hydroxy-(50S)-palytoxin 1059. The relative affinity of palytoxin, 1059 and 1060 towards a mouse anti-palytoxin monoclonal antibody identified palytoxin to have the most potent KD (nM), while 1059 and 1060 were approximately one and three orders of magnitude less potent respectively. Similar relative levels of cytotoxicity towards keratinocytes were also observed, with palytoxin being the most potent. Palytoxin is known to be capable of disrupting mechanisms of cellular ion homeostasis: NMR studies have defined the C-25–C-33 and C-47–C-53 fragments of palytoxin as being involved with Ca2+ coordination.691
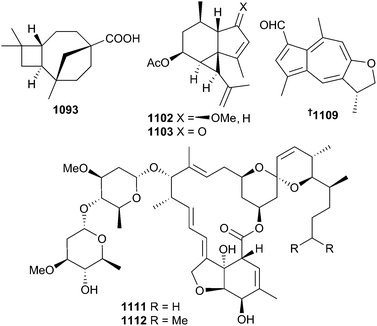
A series of butenolide and cyclopentenones 1061–1072 were reported from Subergorgia suberosa and Sinularia sp. soft corals.692–694 A comprehensive investigation of a Bahamian collection of Pseudopterogorgia rigida identified a chamigrane 1073 and seven bisabolanes 1074–1079 as new sesquiterpenes.695 The study also reported an extensive number of co-metabolites 1080–1083 that had been previously reported from terrestrial plants or as semi-synthetic derivatives 1084–1090. Other sesquiterpenes 1091–1110 have been reported from Rumphella antipathies,696–698Lemnalia philippinensis,699Menella kanisa,700Nephthea erecta,701 an unidentified gorgonian,702Dendronephthya sp.,703Sinularia kavarattiensis,704Echinogorgia sassapo reticulata705 and Anthrogorgia ochracea.706 Of these sesquiterpenes, rumphellaoic acid A 1093697 contains a unique skeleton, while shagenes A 1102 and B 1103702 and ochracenoid A 1109706 contain rarely reported skeletons. In the case of the ochracenoid A, absolute configuration was assigned by use of TDDFT calculated ECD data.
A South China Sea collection of Anthrogorgia caerulea yielded, in addition to two known avermectin macrolides, avermectin B2a and 22,23-dihydroavermectin A1a,707 two new congeners B1c1111 and B1e1112.708 All four MNPs exhibited moderate antifouling activities.
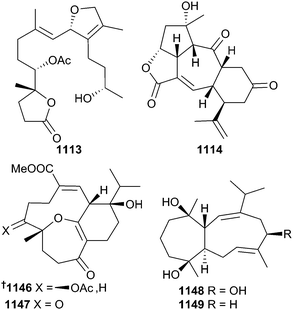
Of the thirty-six cembrane related metabolites 1113–1149 reported from cnidarians in 2014,709–722 secocrassumol 1113 (Lobophytun crassum)709 is notable as it is derived from the cembrane skeleton via an unusual C-11–C-12 bond cleavage, sinugyrosanolide A 1114 (Sinularia gyrosa) is an unprecedented C-4 norcembranoid,710 tortuosenes A 1146 and B 1147 (Sarcophyton tortuosum)721 represent the first examples of cembranoids containing a C-2–C-20 ring closure, and sinularbols A 1148 and B 1149 (Sinularia arborea) contain a rare C-3–C-9 ring closure.722
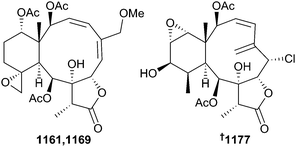
Cnidarian chemistry is dominated by metabolites derived from terpene biosynthesis, with particularly large numbers falling into the diterpenoid classification. Thirty-eight new briarane-skeletoned MNPs (1150, gemmacolides AS–AY 1151–1157, briarenolide J 1158, junceellolides M–P 1159–1162, fragilisinins A–L 1163–1174, dollfusilins A 1175 and B 1176, briaviolides A–J 1177–1186 and anthrogonoid A 1187) were reported from octocoral species Pennatula aculeata, Dichotella gemmacea, Junceella gemmacea, J. fragilis, Ellisella dollfusi, Briareum violacea, and Anthrogorgia caerulea.723–730 As usually happens each year, there is duplication of structure/trivial name amongst a small number of MNPs. In the set of briaranes reported from Junceella gemmacea (South China Sea) and J. fragilis (also South China Sea), junceellolide O 1161726 and fragilisinin G 1169727 share the same assigned structure, however spectroscopic and photometric data reported for the two compounds are quite different clearly indicating that one of the structures is in need of revision. The structure and absolute configuration of briaviolide A 1177729 was secured by single crystal X-ray analysis.
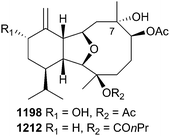
The second dominant class of diterpenoid metabolites reported from soft corals contain the eunicellin skeleton. Examples of eunicellins were reported from Anthrogorgia caerulea (antsimplexin A, 1188),730Muricella sibogae (sibogins A 1189 and B 1190),731Cladiella sp. (cladieunicellin J, 1191),732Klyxum molle (klymollins T–X, 1192–1196),733Cladiella sp. (cladieunicellin M–Q, 1197–1201),734Cladiella krempfi (krempfielins N–R, 1202–1206),735,736 and Cladiella hirsuta (hirsutalin N–R, 1207–1211).737 Readers should note that cladieunicellin N 1198 is a structural duplicate of the previously reported Klyxum molle metabolite klymollin Q,738 exhibiting identical spectroscopic, spectrometric and chiroptical properties and that the structure elucidation of cladieunicellin M–Q also led to revision of configuration at C-7 of the structure of litophynin I diacetate to that shown in 1212.
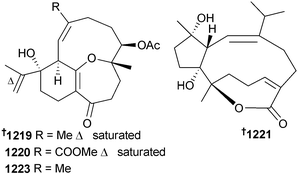
Further examples of diterpenoids, 1213 and 1214,7391215 and 1216,740 cespitulones A 1217 and B 1218,741 dihydrosarsolenone 1219, methyldihydrosarsolenoneate 1220, and sarsolilides B 1221 and C 1222,742 have been isolated from soft corals of the genera Cespitularia, Xenia, and Sarcophyton. Structure elucidation and determination of absolute configuration of 1219 and 1220742 led the authors to propose a revision of the relative configuration at C-2 of the previously reported MNP sarsolenone (to that shown 1223).743 Absolute configuration was assigned to 1219 and 1220 using TDDFT calculations of ECD data.
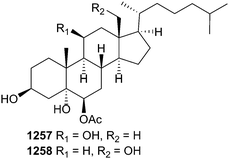
Sarcophyton ehrenbergi was the source of a number of prostaglandins including sarcoehrendins A–J 1224–1233.744 In addition, three prostaglandins previously reported as synthetic compounds 1234–1236 were reported as natural products for the first time. Relatively potent activity was observed towards phosphodiesterase-4, a target for CNS, inflammatory and respiratory diseases. Soft corals also yielded a variety of steroids including pregnanes 1237 and 1238,745seco-sterols 1239–1244746,747 and hydroxylated/polyhydroxylated sterols 1245–1265747–753 from species of Schleronephthya, Subergorgia, Sarcophyton, Sinularia, Verrucella, Echinogorgia, and Leptogorgia. Noteworthy amongst these compounds, was the enhanced (synergistic) cytotoxicity observed for paclitaxel in the presence of punicinols A 1257 and B 1258 (Leptogorgia punicea) and that the two sterols were also able to inhibit A549 tumour cell growth in a clonogenic assay over a sustained period of ten days.752
In addition to the mildly antiproliferative epoxyergosterols 1266–1271 (Anthopleura midori),754 investigation of MNP chemistry of sea anemones and hydrozoa has identified two new cytolysins (3013 and 3375 Da) from the tentacles of the hydrozoan Olindias sambaquiensis755 and PhcrTx1, a 32 amino acid residue acid-sensing ion channel inhibiting peptide from the sea anemone Phymanthus crucifer756 that represents the first example of a peptide containing an Inhibitor Cysteine Knot scaffold to be isolated from a cnidarian. Further study of the biological activities of previously reported anemone toxins has identified Av3 (Anemonia viridis) to show specificity towards arthropod voltage-gated sodium channels by binding to one of the transmembrane clefts of the channel α-subunit,757 while AdE-1 (Aiptasia diasphana) prolongs cardiomyocyte action potential duration while lowering peak amplitude via slowing inactivation of sodium channels and enhancing the transient K+ current.758 Mutation of the pore-forming toxin sticholysin I (Stichodactyla helianthus) residue tryptophan 111 to cysteine reduced the toxins affinity for membranes by an order of magnitude,759 cysteine mutants of phenylalanine 15 or arginine 52 did not alter pore-forming activity but did protect the toxin from peroxynitrite oxidative damage,760 while a third study, using monolayers of phosphatidycholine and sphingomyelin, determined that the toxin preferentially binds and penetrates membranes which have moderate enrichment in sphingomyelin and membrane fluidity.761 The first total syntheses of alkaloids N-(3-guanidinopropyl)-2-(4-hydroxyphenyl)-2-oxoacetamide (Campanularia sp.)762,763 and (±)-tubastrindole B (Tubastraea sp.),764,765 butenolide (+)-hydroxyancepsenolide (Pterogorgia anceps),766,767 and diterpenes sandresolide B,768 amphilectolide769 and (+)-ileabethoxazole770 (Pseudopterogorgia elisabethae)771,772 have been completed. Conversion of bipinnatin J (Pseudopterogorgia bipinnata)773 to intricarene (Pseudopterogorgia kallos)774via a photochemical pathway has been demonstrated,775 while a photochemical (E/Z) olefin isomerisation was a critical step in the total synthesis of the natural product analog 17-deoxyprovidencin.776 Clarification of the structure of the cladiellin diterpene sclerophytin F (Sclerophytum capitalis)777 has been an ongoing issue, with Friedrich and Paquette in 2002 proposing the structure should be revised to be the (3S) diastereomer of sclerophyton A.778 Synthesis of this proposed revised structure gave a product whose spectroscopic data differed from those reported for the natural product, suggesting further investigation is needed to resolve this issue.779 A modular approach to a number of cladiellin MNPs using a gold-catalysed tandem reaction of 1,7-diynes has been reported.780 Hippuristanol (Isis hippuris)781 analogues have been evaluated as inhibitors of eukaryotic translation initiation,782 cembranoid and ergosterol MNPs from Vietnamese cnidarians were found to exhibit selective in vitro activity against T. brucei,783 a number of diterpenes (Sinularia maxima) were found to be modest to poor inhibitors of NF-κB transcriptional activation,784 structurally simpler analogues of fuscol/eunicol exhibit comparable or better anti-inflammatory activity in the mouse ear edema assay,785 semi-synthetic oxygenated dolabellane diterpenes exhibit in vitro anti-HIV activity,786 further semi-synthetic examples of hydoxysterols were found to induce pregnane X receptor transactivation,787 flexibilide788 exhibits in vivo anti-neuroinflammatory activity in rats,789 11-epi-sinulariolide acetate inhibits carcinoma cell migration and invasion by suppressing a number of phosphorylation-dependent pathways,790 13-acetoxysarcocrassolide induces apoptosis in carcinoma cells by activation of p38/JNK and suppression of PI3K/AKT pathways,791 and cembranoids from Sarcophyton glaucum exhibit cytotoxicity towards a murine melanoma cell line.792,793
9 Bryozoans
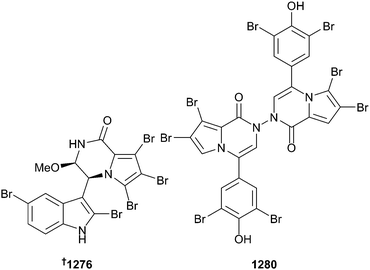
There were only three reports (containing 19 compounds) of new metabolites isolated from bryozoans in 2014, which is a large increase on 2013 when only one new metabolite was reported from this understudied phylum. The bromopyrrole alkaloids aspidostomides A–H 1272–1279 and aspidazide A 1280 were isolated from the Patagonian bryozoan Aspidostoma giganteum. Aspidostomide E 1276 exhibited moderate inhibition of the 786-O renal carcinoma cell line. Aspidazide A 1280 is a rare asymmetrical diacylazide and NOE NMR correlations and chemical transformations were utilised in determination of the structure.794 A series of ceramides neritinaceramide A–E 1281–1285 were obtained from Bugula neritina and exhibited selective but weak activity against two HTCLs.795 The same sample yielded several sterols 1286–1290.796 Convolutamydine A797 displayed significant anti-inflammatory activity both in vitro and in vivo.79810 Molluscs
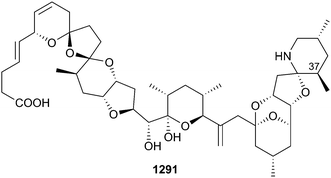
The 23 new metabolites reported from molluscs is the average number reported per year over the past decade. 37-epi-Azaspiracid-1 1291 was isolated from contaminated raw shellfish – the epimer forms spontaneously via an equilibrium and formation, as expected, is accelerated by heating.799 Implication of the latter in relationship to the cooking of shellfish is important as 37-epi-AZA1 was 5-fold more toxic towards Jurkat T lymphocyte cells in vitro than AZA1. Corresponding epimers of the related toxins AZA2 and AZA3 were also detected.Specimens of the Mediterranean sacoglossan mollusc Thuridilla hopei afforded new nor-diterpene aldehydes 1292–1294800 in addition to known congeners thuridillin A, B and C.801 The mollusc feeds on the green alga Derbesia tenuissima, extracts of which only contain a known epoxylactone,802 supporting the assumption that 1292–1294 are mollusc transformation products. Acetylenic alcohols 1295–1299 were isolated from both the Mediterranean dorid nudibranch Peltodoris atromaculata and one of the nudibranch's common dietary prey, the sponge Haliclona fulva.803 In a similar manner, renieramycin-related alkaloids fennebricin A 1300 and B 1301 were isolated from both the nudibranch Jorunna funebris and the sponge Xestospongia sp.804 The substructurally-related isoquinoline 1302 was only identified in the sponge extract. New diketopiperazines 1303–1306 were reported from Pleurobranchus areolatus – related metabolites have been reported from the ascidian Didemnum sp., a suspected prey of P. areolatus.805 Further investigation into the origin of tetrodotoxin in Pleurobranchaea maculata demonstrated that the mollusc can accumulate the toxin through its diet however there was no identifiable tetrodotoxin source in the molluscs local environment.806 Two studies of sea hares identified two moderately cytotoxic sesquiterpenes oculiferane 1307 and epi-obtusane 1308 (Aplysia oculifera)807 and an anti-neuroinflammatory diterpene dactyloditerpenol acetate 1309 (A. dactylomela).808 6-Bromoisatin, typically isolated from muricid molluscs, is weakly antiproliferative towards HT29 cells, and enhances apoptosis in an in vivo colon cancer model.809 Further structure–activity relationship studies of the cyclic peptide sanguinamide B (Hexabranchus sanguineaus)810 have found that antiproliferative activity is dependent upon both the location of specific amino acids in the macrocycle and their configuration.811,812 New synthetic auristatin analogues, being related to the original MNP dolastatin 10 (Dolabella auricularia),813 bearing changes at the N-terminus showed pronounced antitumour activity.814 The binding of three examples to tubulin was investigated by co-crystal X-ray studies, identifying an interesting structural feature whereby in the solid state the valine-dolaisoleucine fragment exists in the cis-configuration, whereas in solution it exists solely in the trans-configuration. In further studies, cholesterol and other simple sterols purified from mussels showed anti-aging and neuroprotective properties,815 synthetic tambjamine analogues show enhanced tumour cell antiproliferative and chloride transport properties,816 and lamellarin D (Lamellaria sp.)817 induces senescence in cancer cells, in a process that includes the generation of intracellular ROS and requires the presence of topoisomerase I.818 Biosynthesis of long chain polyunsaturated fatty acids in the scallop Chlamys nobilis was investigated, revealing the presence of a new elongase, that can elongate 20:4n-6 and 20:5n-3 to C22 and C24 acids, and a Δ8-desaturase.819 A new example of an α4/7-conotoxin, α-BnIA 1310 was isolated from crude venom of the molluscivorous cone snail Conus bandanus.820 Peptides with the same sequence, Mr1.1 and Bn1.1, were previously identified by PCR amplification of venom duct cDNA from molluscs C. marmoreus821 and C. bandanus.822 The peptide reversibly inhibited the human α7 nicotinic acetylcholine receptor (nAChR) and blocked nerve-evoked skeletal muscle contractions. Conus bandanus (Vietnam) was also the source of BnIIID 1311, a 15 residue M–1 family peptide containing six cysteines (disulfide connectivity not determined) and three post-translational modifications comprised of a bromotryptophan, γ-carboxy glutamate and amidated aspartic acid residues.823 An unusual α5/5 conotoxin AusIA 1312 was purified from the venom of C. australis – both synthetic globular (natural) and ribbon (different disulfide linkages) configurations inhibited the α7 nAChR.824 A short cyclic hexapeptide Vi804 1313 was isolated from crude venom of C. virgo and the solution structure of it and the DW3 synthetic analogue explored by NMR spectroscopy.825 The high hydrophobicity of γ-conotoxins make them difficult to synthesise by standard peptide synthesis techniques. A recent report describes the use of a Lys4 solubilising C-terminus tag to enable the synthesis and NaV subtype selectivity of three previously reported C. consors γ-conotoxins, γ-CnVIB, γ-CnVIC and γ-CnVID.826 Further studies have reported on the structure and activity of dicarba analogues of α-RgIA,827 the influence of disulfide connectivity on structure and bioactivity of α-TxIA,828 the influence of acetylcholine to affect the binding of α-MII at nAChRs,829 the neuronal target selectivity of the Conus textile T-superfamily peptide TxVC,830 and the Ca2+-activated K+ (BK) channel selectivity of the unusual M superfamily conotoxin Vt3.1.831
11 Tunicates (ascidians)
The 22 new tunicate-derived natural products presented in this review is the second lowest number reported in one year over the last decade. A structurally-diverse range of metabolites were reported, with examples of glycerides 1314 and 1315,832 amino alcohols 1316–1322,833 new didemnaketal congeners 1323, 1324834 and 1325, 1326,835 halogenated alkaloids 1327 and 1328,836 a new rubrolide (R) 1329837 (that unfortunately shares the same letter designation as a related metabolite reported from Aspergillus terreus217), pyridoacridine cnemidine A 1330552 and sulfated sterols 1331 and 1332.838 Noteworthy was the isolation, structure elucidation and synthesis of a rare example of a modified nucleoside bearing a 5′-thiomethyl substituent 1333.839 The same study also led to the reassignment of structure of a known sponge metabolite hamiguanosinol840 from the enol tautomer to the guanosine keto tautomer 1334. The tanjungides A 1335 and B 1336,841 novel dibromoindole enamide alkaloids isolated from Diazona cf. formosa exhibit potent cytotoxicity (<1–2 μM) towards a panel of HTCLs. An eleven-step linear synthesis utilising Buchwald vinyliodide amidation and peptide synthesis established the absolute configuration of the alkaloids.Over the years a number of cyclic ribosomal peptides, now known as the cyanobactins, have been reported from ascidians, typically of the genus Lissoclinum. The true producers of these natural products are photosynthetic symbiotic cyanobacteria of the genus Prochloron. The apparent random distribution of cyanobactins isolated from ascidians has now been credited to host phylogeny, with genetic analysis revealing that ‘Lissoclinum patella’ falls into three phylogenetic groups that in turn may contain further cryptic species.842 The implications that local extinctions of such cryptic species may reduce marine natural product diversity is indeed food-for-thought in the context of climate change. A very interesting demonstration of the use of the biosynthetic machinery of cyanobactin production was recently reported, whereby engineered enzymes of the patellamide pathway, in combination with enzymes from other cyanobactin-related pathways, were used for the in vitro production of a small library of cyclic peptides.843 This proof of principle allowed for the preparation of 1–2 mg of each peptide. Total synthesis has led to revision of the original structure proposed for didemnaketal B (Didemnum sp.)844 to 1337, requiring stereochemical inversion of the C-10–C-20 spiroacetal domain of the MNP.845 Such a revision may be of relevance to the ongoing revision of the (stereo)structure of didemnaketal A.846 Syntheses of the reported structures of polycitorols A and B (unidentified ascidian)847,848 and (+)-didemniserinolipid C (Didemnum sp.)849,850 suggest that the structures of all three MNPs require revision. Total synthesis has also led to revision of the structure of mandelalide A (Lissoclinum sp.)851 to 1338852 and confirmation of the revised structure of haouamine B (Aplidium haouarianum),853–855 while the structures of distomadines A and B (Pseudodistoma aureum)856,857 and synoxazolidinones A and B (Synoicum pulmonaria)858,859 have been confirmed by synthesis. Synoxazolidinones A and C and S. pulmonaria co-metabolites pulmonarins A and B exhibited variable levels of anti-biofouling activity against a panel of test organisms, with synoxazolidinone C being particularly potent as both a growth and adhesion inhibitor.860 The effects of ascidian extracts on the estrogen receptor-negative breast cancer cell line MDA-MB-231 were investigated by content-rich screening, leading to the identification of eusynstyelamide B (Didemnum candidum, originally isolated from Eusynstyela latericus861) as a moderate cytotoxin (IC50 5 μM) causing cell cycle arrest in G2/M and inducing apoptosis.862 The potently cytotoxic macrolide iejimalide C (Eudistoma cf. rigida)863 joins congeners iejimalides A and B as being identified as an inhibitor of the vacuolar-type ATP-driven proton pump (H+-ATPase).864 After 24 h treatment, cells also exhibited actin aggregates, but as the MNP does not inhibit actin polymerisation in vitro, it was concluded that actin activity was a consequence of disruption of pH homeostasis. Preliminary data suggesting that trabectedin (Et 743) exhibits anti-angiogenic activity towards breast cancer cell lines has been reported.865
12 Echinoderms
The 35 new metabolites reported from echinoderms in this review is 25% lower than the average number reported per annum over the last decade. Beyond the simple sulfonic acid derivative 1339 isolated from the sea urchin Brisaster latifrons866 and the highly substituted unsymmetrical binaphthoquinone mirabiquinone 1340 (sea urchin Scaphechinus mirabilis),867 the natural product chemistry of echinoderms is dominated by steroidal tri- (1341868 and 1342869), tetra- (1343–1352870–873), penta- and hexaoses (1353–1371).873–875 The aglycone 1372 was also isolated.874 In many cases these MNPs exhibited biological activity including anti-inflammatory,866 cytotoxic,870,872,874 hemolytic870 and antifungal873,875 properties.A number of new saponins were identified in extracts of the Australian sea cucumber Holothuria lessoni using solely LC-MS/MS metabolomic techniques.876 In a conceptually similar manner, the chemical diversity of saponins present in different organs of the starfish Asterias rubens was investigated by combinations of MALDI-TOF and LC-MS(/MS) techniques.877 The latter study concluded that different organs are characterised by different saponin mixtures and inter-specimen variability exists suggesting influence of sex and/or collecting season on saponin profile. A short octapeptide echinometrin 1373 (sea urchin Echinometra lucunter) was found to exhibit ability to degranulate mast cells leading to an inflammatory reaction.878 The sequence of the peptide is an internal fragment of vitellogenin, a nutrient protein present in sea urchin gametogenic cells, suggesting the possibility that echinometrin is a cryptide.879
The first synthesis of a pyranonaphthazarin pigment isolated from the sea urchin Echinothrix diadema880 has been reported.881 Further studies using purified pentahydroxynaphthoquinone echinochrome A882,883 have identified it to enhance mitochondrial biogenesis,884 to protect cardiomyocyte mitochondria from the effects of cardiotoxic drugs885 and to inhibit acetylcholinesterase in an irreversible and uncompetitive mode.886 The mechanisms of antioxidant reactivity of the structurally related echinamines A and B887 has been investigated using DFT methods.888 The benzochromenone comaparvin (Comanthus bennetti)889 exhibits anti-inflammatory activity in the carrageenan-induced rat model.890 A simple synthesis of ovothiol A891 from L-hisidine has been reported,892 the role of a non-heme iron oxidase enzyme in the biosynthesis of ovothiol A investigated,893 NMR-pH titrations used to investigate the microspeciation of the amino acid,894 and the antiproliferative activity towards Hep-G2 cell line via an autophagy mechanism identified.895 Sterols and fatty acids from the spiny sea-star Marthasterias glacialis exhibit both anti-inflammatory896 and cell cycle arrest properties,897 while sterols from an urchin and a starfish exhibit selective antiparasitic activity towards T. brucei.783 Investigation of the structure and bioactivity of chimeric analogues of the starfish cardiac-stomach relaxing SALMFamide neuropeptides S1 and S2 has highlighted the importance of the C-terminus for bioactivity and that the N-terminus confers structural stability.898 Treatment of pancreatic cancer in vivo in athymic mice using a combination of frondoside A (Cucumaria frondosa)899 and the nucleoside anticancer agent gemcitabine was significantly more effective than with either drug alone.900 The cytotoxic and apoptotic-inducing abilities of steroids from the cold water starfish Ctenodiscus crispatus901 and the triterpene glycosides, including echinoside A, from Holothuria scabra and Cucumaria frondosa have been reported.902 Echinoside A and related saponin holothurin A inhibit dietary fat absorption and decrease the adipose tissue accumulation in mice,903 and typicosides (Actinocucumis typica)904 exhibit a strong immunostimulatory effect on mouse macrophages with marked increase in lysosomal activity and ROS formation.905 Complete NMR assignments have been reported for four pentaoside asterosaponins: thornasteroside A, versicoside A, anasteroside B and asteronylpentaglycoside sulfate (Asterias amurensis).906
13 Mangroves
In addition to a series of mildly antioxidant glycosides, marinoids F–M 1374–1381, reported from the whole fruits of the mangrove Avicennia marina,907,908seco-diterpene 1382 (Ceriops decandra),909 cinnamides 1383–1387 (Micromelum falcatum, mangrove associate),910 protolimonoids 1388–1394 (Xylocarpus granatum),911 and limonoids 1395–1401,9121402–1404,9131405–1408,9141409–1418,9151419–1422916 and 1423917 (X. rumphii and X. granatum) were also isolated from mangroves or their associates. The absolute configuration of the unusual spiro-seco-abietane decandrinin 1382 was secured via analysis of experimental and calculated ECD and ORD chiroptical data909 while of the limonoids reported, the structures of xylorumphiin G 1398,912 xylogranatopyridine A 1402,913 granatumin L 1409915 and granatumin Y 1422916 were established by single crystal X-ray diffraction studies.Further investigation of previously reported mangrove MNPs has identified avicequinone C (Avicennia marina)918 as a 5α-reductase-type 1 inhibitor,919 catunaregin (Micromelum falcatum)920 as an angiogenesis inhibitor,921 the cardiac glycoside neriifolin (Cerbera manghas)922 as the acaricidal component of Panonychus citri,923 and that leaf extracts and purified components of Avicennia marina exhibit antibacterial activity with relevance to urinary tract infections.924 Finally, a new biomimetic synthesis925 and a structure–activity relationship study of the protein tyrosine phosphatase 1B inhibiting properties926 of the unusual dimeric alkylbutenolide paracaseolide A (Sonneratia paracaseolaris)927 have been reported.
14 Miscellaneous
Two studies of sea grass from the Egyptian Red Sea led to the identification of flavone xyloside 1424 (Thalassia hemprichii)928 and dihydrochalcone diglycoside 1425 (Thalassodendron ciliatum)929 as antimicrobial and anti-influenza A virus MNPs respectively. Thelepamide 1426 is an unusual ketide-amino acid isolated from the annelid worm Thelepus crispus.930 The relative configuration of the alkaloid was determined by a combination of heteronuclear J-based configurational analysis and GIAO calculated chemical shifts and DP4 probability analysis. Mild cytotoxicity towards a leukemia cell line was also observed. The arenicins, antimicrobial peptides produced by the polychaete worm Arenicola marina,931 are constitutively expressed in a range of tissues in the organism suggestive that the peptides play a front-line role in defense against infections.932 Cypridina luciferyl sulfate 1427 appears to be a more stable storage form of cypridina luciferin, the luminescence precursor of the ostracod Cypridina (Vargula) hilgendorfii.933 The luciferin could be converted to luciferyl sulfate by action of crude extract of the organism, presumably containing a sulfotransferase, in the presence of 3′-phosphoadenosine 5′-phosphosulfate (PAPS, a sulfate donor), while the reverse reaction took place in the presence of adenosine 3′,5′-diphosphate, a sulfate acceptor.Extracts of ovary tissue from Takifugu pardalis yielded a new tetrodotoxin analogue, 6-deoxyTTX 1428.934 The potent voltage-gated sodium channel blocker was also detected by LC-MSMS in other marine animals including snail and octopus. Alanine scanning of the hagfish (Myxine glutinosa)935 12-amino acid residue antimicrobial peptide myxinidin has identified a number of critical residues for the observed biological activities and that judicious substitution with arginine led to the identification of more potent analogues.936 The 33-amino-acid residue peptide pardaxin (flatfish Pardachirus marmoratus) exhibited in vivo activity towards MRSA dermal infection and enhanced wound healing.937 A range of arsenic-containing lipids have been synthesised, providing valuable reference standards for future studies directed towards understanding the uptake, biotransformation and toxicity of these unusual MNPs.938
15 Conclusion
In a recent review the number of new MNPs reported from a range of countries was analysed based on the location of the principal author for each publication.3 What is not apparent from this analysis is the location of the collecting sites for the organisms. This can be problematical as shown in an extreme example: one prominent MNP chemist based in the Mid-West of the USA, thousands of km from any coastal resource, collected widely (see Fig. 1).With the biogeographic aspect of the MarinLit database19 now widely available an alternative perspective is available for viewing the collection data. It is now possible to search by region showing who is collecting where and hence the interests that MNP chemists have in the various regions of the globe. The 50 years of collecting effort globally is depicted in Fig. 2. The red stars in Fig. 2 represent the collecting sites, described in ∼9000 papers, from which ∼25![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 700 new compounds have been obtained. The actual number of unique collection sites is less than the number of papers as multiple collections have often been made at the same sites. Fig. 2 displays those areas that have had high collecting pressures and those where there has been, for whatever reason, lower collecting pressures. An analysis has been made of the collecting effort globally by semi-decade since 1965. For convenience the globe was divided up into 22 regions or countries (see Table 1) and the published papers for each region compiled. Totals for papers (citing collection sites) describing new compounds from 1965 are also listed in Table 1 while Fig. 3 shows the incidence of discovery of new compounds in each region or country by semi-decade since 1965. It is worth noting that since 1965–280 papers contained no biogeographic data, not even a country or an ocean, to describe the origin of new compounds being reported. Fortunately, recent years have seen the increasing use of exact coordinates. The Japanese region, including Okinawa, has been the most productive region with 3877 compounds described. This was followed closely by China with 2915 compounds from the mainland with a further 525 compounds to be included if the regions of the South China Sea and Yellow Sea are also considered. But what is remarkable here is the rapid emergence of MNP chemistry using Chinese-based collections as seen in Fig. 3. Other regions where there have been extensive collections are in the Mediterranean (2358) (which includes the Mediterranean coasts of Spain and France, Italy, the islands of the Mediterranean, Greece, Turkey, Israel, Egypt, the Arabian Peninsula, Black Sea and the Mediterranean African coastlines), Australia (1854), the North (1539) and South (1548) Pacific islands and atolls, and the Gulf of Mexico, Caribbean Sea and islands (1524). Other regions that have been well explored include Taiwan (1350) and Maritime SE-Asia and Papua-New Guinea (1340).
700 new compounds have been obtained. The actual number of unique collection sites is less than the number of papers as multiple collections have often been made at the same sites. Fig. 2 displays those areas that have had high collecting pressures and those where there has been, for whatever reason, lower collecting pressures. An analysis has been made of the collecting effort globally by semi-decade since 1965. For convenience the globe was divided up into 22 regions or countries (see Table 1) and the published papers for each region compiled. Totals for papers (citing collection sites) describing new compounds from 1965 are also listed in Table 1 while Fig. 3 shows the incidence of discovery of new compounds in each region or country by semi-decade since 1965. It is worth noting that since 1965–280 papers contained no biogeographic data, not even a country or an ocean, to describe the origin of new compounds being reported. Fortunately, recent years have seen the increasing use of exact coordinates. The Japanese region, including Okinawa, has been the most productive region with 3877 compounds described. This was followed closely by China with 2915 compounds from the mainland with a further 525 compounds to be included if the regions of the South China Sea and Yellow Sea are also considered. But what is remarkable here is the rapid emergence of MNP chemistry using Chinese-based collections as seen in Fig. 3. Other regions where there have been extensive collections are in the Mediterranean (2358) (which includes the Mediterranean coasts of Spain and France, Italy, the islands of the Mediterranean, Greece, Turkey, Israel, Egypt, the Arabian Peninsula, Black Sea and the Mediterranean African coastlines), Australia (1854), the North (1539) and South (1548) Pacific islands and atolls, and the Gulf of Mexico, Caribbean Sea and islands (1524). Other regions that have been well explored include Taiwan (1350) and Maritime SE-Asia and Papua-New Guinea (1340).
| Country or Oceanic region | #Compounds | #Papers |
|---|---|---|
| Pacific Russia, sea of Japan | 377 | 167 |
| China | 2915 | 942 |
| South China sea and Yellow sea | 525 | 203 |
| Taiwan | 1350 | 376 |
| Japan, including Okinawa | 3877 | 1570 |
| South Korea | 848 | 239 |
| North Pacific islands and atolls | 1539 | 576 |
| South Pacific islands and atolls | 1548 | 522 |
| Maritime SE Asia, Papua-New Guinea | 1340 | 502 |
| Australia | 1854 | 677 |
| Mainland SE Asia (including East Malaysia) | 457 | 173 |
| South Asia | 714 | 333 |
| Indian ocean and islands | 326 | 155 |
| Mediterranean, Arabian Peninsula, Black sea | 2358 | 876 |
| Other African countries | 456 | 154 |
| Atlantic Europe and the Baltic sea | 476 | 210 |
| Atlantic ocean and islands | 361 | 140 |
| South American countries | 538 | 210 |
| Central America | 245 | 86 |
| Gulf of Mexico, Caribbean sea and islands | 1524 | 571 |
| North America | 1382 | 520 |
| Arctic and Antarctica | 330 | 105 |
The graph shows very clearly those countries that were most closely studied in the early years. Compounds of Japanese origin were prominent from the 1960s, but the search for MNPs was quickly taken up with compounds from the Mediterranean, North Pacific and North American regions appearing in the early 1970s, followed by Australian and Maritime SE-Asian compounds later in that decade. Prospecting activity in other parts of Asia was relatively slow to start and it was not really until the 1990s that compounds of Mainland SE-Asian, South Korean, Taiwan and Chinese origin started to appear. The output from these regions has rapidly accelerated since, particularly so in the case of compounds of Chinese origin. Fig. 2 and 3, taken in combination, provide a snapshot of the past efforts in marine natural products as well as current endeavours and highlights those areas of the globe that are currently under-explored.
16 Acknowledgements
We thank Dr Serin Dabb and Dr Helen Potter (Royal Society of Chemistry) for the provision of data used in this review, from the MarinLit database.1917 References
- D. J. Faulkner, Nat. Prod. Rep., 1984, 1, 251–280, 10.1039/np9840100251
.
- D. J. Faulkner, Nat. Prod. Rep., 1984, 1, 551–598, 10.1039/np9840100551
.
- J. W. Blunt, B. R. Copp, R. A. Keyzers, M. H. G. Munro and M. R. Prinsep, Nat. Prod. Rep., 2014, 31, 160–258, 10.1039/c3np70117d
.
- D. J. Newman and G. M. Cragg, Mar. Drugs, 2014, 12, 255–278, DOI:10.3390/md12010255
.
- G. M. Cragg, P. G. Grothaus and D. J. Newman, J. Nat. Prod., 2014, 77, 703–723, DOI:10.1021/np5000796
.
- L. E. Lallier, O. McMeel, T. Greiber, T. Vanagt, A. D. W. Dobson and M. Jaspars, Nat. Prod. Rep., 2014, 31, 612–616, 10.1039/c3np70123a
.
- C. Hardoim and R. Costa, Mar. Drugs, 2014, 12, 5089–5122, DOI:10.3390/md12105089
.
- M. C. Wilson, T. Mori, C. Rückert, A. R. Uria, M. J. Helf, K. Takada, C. Gernert, U. A. E. Steffens, N. Heycke, S. Schmitt, C. Rinke, E. J. N. Helfrich, A. O. Brachmann, C. Gurgui, T. Wakimoto, M. Kracht, M. Crüsemann, U. Hentschel, I. Abe, S. Matsunaga, J. Kalinowski, H. Takeyama and J. Piel, Nature, 2014, 506, 58–62, DOI:10.1038/nature12959
.
- M. Jaspars and G. Challis, Nature, 2014, 506, 38–39, DOI:10.1038/nature13049
.
- U. R. Abdelmohsen, K. Bayer and U. Hentschel, Nat. Prod. Rep., 2014, 31, 381–399, 10.1039/c3np70111e
.
- P. C. Still, T. A. Johnson, C. M. Theodore, S. T. Loveridge and P. Crews, J. Nat. Prod., 2014, 77, 690–702, DOI:10.1021/np500041x
.
- M. P. Puglisi, J. M. Sneed, K. H. Sharp, R. Ritson-Williams and V. J. Paul, Nat. Prod. Rep., 2014, 31, 1510–1553, 10.1039/c4np00017j
.
- R. M. Van Wagoner, M. Satake and J. L. C. Wright, Nat. Prod. Rep., 2014, 31, 1101–1137, 10.1039/c4np00016a
.
- R.-M. Huang, Y.-N. Chen, Z. Zeng, C.-H. Gao, X. Su and Y. Peng, Mar. Drugs, 2014, 12, 5817–5838, DOI:10.3390/md12125817
.
- A. P. Thottumkara, W. H. Parsons and J. Du Bois, Angew. Chem., Int. Ed., 2014, 53, 5760–5784, DOI:10.1002/anie.201308235
.
- V. Bane, M. Lehane, M. Dikshit, A. O'Riordan and A. Furey, Toxins, 2014, 6, 693–755, DOI:10.3390/toxins6020693
.
- J. Rocha-Martin, C. Harrington, A. Dobson and F. O'Gara, Mar. Drugs, 2014, 12, 3516–3559, DOI:10.3390/md12063516
.
- M. D. Guiry, G. M. Guiry, L. Morrison, F. Rindi, S. V. Miranda, A. C. Mathieson, B. C. Parker, A. Langangen, D. M. John, I. Bárbara, C. F. Carter, P. Kuipers and D. J. Garbary, Cryptogam.: Algol., 2014, 35, 105–115, DOI:10.7872/crya.v35.iss2.2014.105
.
- http://rsc.66557.net/marinlit, accessed 4 December 2015.
- Y. Kato, N. Fusetani, S. Matsunaga, K. Hashimoto, S. Fujita and T. Furuya, J. Am. Chem. Soc., 1986, 108, 2780–2781, DOI:10.1021/ja00270a061
.
- T. Wakimoto, Y. Egami, Y. Nakashima, Y. Wakimoto, T. Mori, T. Awakawa, T. Ito, H. Kenmoku, Y. Asakawa, J. Piel and I. Abe, Nat. Chem. Biol., 2014, 10, 648–655, DOI:10.1038/nchembio.1573
.
- Y. Egami, T. Wakimoto and I. Abe, Bioorg. Med. Chem. Lett., 2014, 24, 5150–5153, DOI:10.1016/j.bmcl.2014.10.002
.
- P. Fu, S. X. Wang, K. Hong, X. Li, P. P. Liu, Y. Wang and W. M. Zhu, J. Nat. Prod., 2011, 74, 1751–1756, DOI:10.1021/np200258h
.
- P. Fu, F. Kong, X. Li, Y. Wang and W. Zhu, Org. Lett., 2014, 16, 3708–3711, DOI:10.1021/ol501523d
.
- U. R. Abdelmohsen, C. Cheng, C. Viegelmann, T. Zhang, T. Grkovic, S. Ahmed, R. J. Quinn, U. Hentschel and R. Edrada-Ebel, Mar. Drugs, 2014, 12, 1220–1244, DOI:10.3390/md12031220
.
- F. S. Tareq, M. A. Lee, H.-S. Lee, Y.-J. Lee, J. S. Lee, C. M. Hasan, M. T. Islam and H. J. Shin, J. Agric. Food Chem., 2014, 62, 5565–5572, DOI:10.1021/jf502436r
.
- F. S. Tareq, M. A. Lee, H.-S. Lee, J.-S. Lee, Y.-J. Lee and H. J. Shin, Mar. Drugs, 2014, 12(2), 871–885, DOI:10.3390/md12020871
.
- F. S. Tareq, M. A. Lee, H.-S. Lee, Y.-J. Lee, J. S. Lee, C. M. Hasan, M. T. Islam and H. J. Shin, Org. Lett., 2014, 16, 928–931, DOI:10.1021/ol403657r
.
- K. Yamanaka, K. A. Reynolds, R. D. Kersten, K. S. Ryan, D. J. Gonzalez, V. Nizet, P. C. Dorrestein and B. S. Moore, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 1957–1962, DOI:10.1073/pnas.1319584111
.
- G. A. Ellis, T. P. Wyche, C. G. Fry, D. R. Braun and T. S. Bugni, Mar. Drugs, 2014, 12, 1013–1022, DOI:10.3390/md12021013
.
- R. Raju, A. M. Piggott, L. X. B. Diaz, Z. Khalil and R. J. Capon, Org. Lett., 2010, 12, 5158–5161, DOI:10.1021/ol102162d
.
- J. Schmidt, Z. Khalil, R. J. Capon and C. W. B. Stark, Beilstein J. Org. Chem., 2014, 10, 1228–1232, DOI:10.3762/bjoc.10.121
.
- X.-B. Ding, D. P. Furkert, R. J. Capon and M. A. Brimble, Org. Lett., 2014, 16, 378–381, DOI:10.1021/ol403246j
.
- R. Raju, Z. G. Khalil, A. M. Piggott, A. Blumenthal, D. L. Gardiner, T. S. Skinner-Adams and R. J. Capon, Org. Lett., 2014, 16, 1716–1719, DOI:10.1021/ol5003913
.
- W. J. Zhang, Z. Liu, S. M. Li, T. T. Yang, Q. B. Zhang, L. Ma, X. P. Tian, H. B. Zhang, C. G. Huang, S. Zhang, J. H. Ju, Y. M. Shen and C. S. Zhang, Org. Lett., 2012, 14, 3364–3367, DOI:10.1021/ol301343n
.
- Y. Chen, W. Zhang, Y. Zhu, Q. Zhang, X. Tian, S. Zhang and C. Zhang, Org. Lett., 2014, 16, 736–739, DOI:10.1021/ol4034176
.
- W. Zhang, L. Ma, S. Li, Z. Liu, Y. Chen, H. Zhang, G. Zhang, Q. Zhang, X. Tian, C. Yuan, S. Zhang, W. Zhang and C. Zhang, J. Nat. Prod., 2014, 77, 1887–1892, DOI:10.1021/np500362p
.
- S. Tamura, N. Takahashi, S. Miyamoto, R. Mori, S. Suzuki and J. Nagatsu, Agric. Biol. Chem., 1963, 27, 576–582, DOI:10.1271/bbb1961.27.576
.
- W. Zhang, S. Li, Y. Zhu, Y. Chen, Y. Chen, H. Zhang, G. Zhang, X. Tian, Y. Pan, S. Zhang, W. Zhang and C. Zhang, J. Nat. Prod., 2014, 77, 388–391, DOI:10.1021/np400665a
.
- J. Zhang, Y. Jiang, Y. Cao, J. Liu, D. Zheng, X. Chen, L. Han, C. Jiang and X. Huang, J. Nat. Prod., 2013, 76, 2126–2130, DOI:10.1021/np4003417
.
- H. J. Shin, H.-S. Lee, J. S. Lee, J. Shin, M. A. Lee, H.-S. Lee, Y.-J. Lee, J. Yun and J. S. Kang, Mar. Drugs, 2014, 12, 3283–3291, DOI:10.3390/md12063283
.
- J. S. Lee, J. Shin, H. J. Shin, H.-S. Lee, Y.-J. Lee, H.-S. Lee and H. Won, Eur. J. Org. Chem., 2014, 2014, 4472–4476, DOI:10.1002/ejoc.201402524
.
- C. Boonlarppradab, C. A. Kauffman, P. R. Jensen and W. Fenical, Org. Lett., 2008, 10, 5505–5508, DOI:10.1021/ol8020644
.
- S. M. Salem, P. Kancharla, G. Florova, S. Gupta, W. Lu and K. A. Reynolds, J. Am. Chem. Soc., 2014, 136, 4565–4574, DOI:10.1021/ja411544w
.
- P. Kancharla, W. Lu, S. M. Salem, J. Xu Kelly and K. A. Reynolds, J. Org. Chem., 2014, 79, 11674–11689, DOI:10.1021/jo5023553
.
- L. Kaysser, P. Bernhardt, S. J. Nam, S. Loesgen, J. G. Ruby, P. Skewes-Cox, P. R. Jensen, W. Fenical and B. S. Moore, J. Am. Chem. Soc., 2012, 134, 11988–11991, DOI:10.1021/ja305665f
.
- G. Sakoulas, S.-J. Nam, S. Loesgen, W. Fenical, P. R. Jensen, V. Nizet and M. Hensler, PLoS One, 2012, 7, e29439, DOI:10.1371/journal.pone.0029439
.
- L. Kaysser, P. Bernhardt, S.-J. Nam, S. Loesgen, J. G. Ruby, P. Skewes-Cox, P. R. Jensen, W. Fenical and B. S. Moore, J. Am. Chem. Soc., 2014, 136, 14626, DOI:10.1021/ja509209d
.
- R. Meier, S. Strych and D. Trauner, Org. Lett., 2014, 16, 2634–2637, DOI:10.1021/ol500800z
.
- R. Teufel, L. Kaysser, M. T. Villaume, S. Diethelm, M. K. Carbullido, P. S. Baran and B. S. Moore, Angew. Chem., Int. Ed., 2014, 53, 11019–11022, DOI:10.1002/anie.201405694
.
- S. Diethelm, R. Teufel, L. Kaysser and B. S. Moore, Angew. Chem., Int. Ed., 2014, 53, 11023–11026, DOI:10.1002/anie.201405696
.
- R. Wilputte and R. H. Martin, Bull. Soc. Chim. Belg., 1956, 65, 874–898, DOI:10.1002/bscb.19560650908
.
- L. Trzoss, T. Fukuda, L. V. Costa-Lotufo, P. Jimenez, J. J. La Clair and W. Fenical, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 14687–14692, DOI:10.1073/pnas.1410932111
.
- M. R. Seyedsayamdost, G. Carr, R. Kolter and J. Clardy, J. Am. Chem. Soc., 2011, 133, 18343–18349, DOI:10.1021/ja207172s
.
- M. R. Seyedsayamdost, R. J. Case, R. Kolter and J. Clardy, Nat. Chem., 2011, 3, 331–335, DOI:10.1038/nchem.1002
.
- M. R. Seyedsayamdost, R. Wang, R. Kolter and J. Clardy, J. Am. Chem. Soc., 2014, 136, 15150–15153, DOI:10.1021/ja508782y
.
- P. Fu, Y. Zhu, X. Mei, Y. Wang, H. Jia, C. Zhang and W. Zhu, Org. Lett., 2014, 16, 4264–4267, DOI:10.1021/ol5019757
.
- T. P. Wyche, J. S. Piotrowski, Y. Hou, D. Braun, R. Deshpande, S. McIlwain, I. M. Ong, C. L. Myers, I. A. Guzei, W. M. Westler, D. R. Andes and T. S. Bugni, Angew. Chem., Int. Ed., 2014, 53, 11583–11586, DOI:10.1002/anie.201405990
.
- T. Grkovic, U. R. Abdelmohsen, E. M. Othman, H. Stopper, R. Edrada-Ebel, U. Hentschel and R. J. Quinn, Bioorg. Med. Chem. Lett., 2014, 24, 5089–5092, DOI:10.1016/j.bmcl.2014.08.068
.
- Y. Dashti, T. Grkovic, U. R. Abdelmohsen, U. Hentschel and R. J. Quinn, Mar. Drugs, 2014, 12, 3046–3059, DOI:10.3390/md12053046
.
- Y. Kwon, S.-H. Kim, Y. Shin, M. Bae, B.-Y. Kim, S. K. Lee, K.-B. Oh, J. Shin and D.-C. Oh, Mar. Drugs, 2014, 12, 2326–2340, DOI:10.3390/md12042326
.
- Z. Ma, J. Hu, X. Wang and S. Wang, J. Antibiot., 2014, 67, 175–178, DOI:10.1038/ja.2013.89
.
- C.-H. Gao, Y.-N. Chen, L.-X. Pan, F. Lei, B. Long, L.-Q. Hu, R.-C. Zhang, K. Ke and R.-M. Huang, J. Antibiot., 2014, 67, 541–543, DOI:10.1038/ja.2014.27
.
- Z. Ma and J. Hu, Appl. Biochem. Biotechnol., 2014, 173, 705–715, DOI:10.1007/s12010-014-0879-1
.
- K. Chakraborty, B. Thilakan and V. K. Raola, J. Agric. Food Chem., 2014, 62, 12194–12208, DOI:10.1021/jf504845m
.
- M. A. M. Mondol and H. J. Shin, Mar. Drugs, 2014, 12, 2913–2921, DOI:10.3390/md12052913
.
- J. Bai, D. Liu, S. Yu, P. Proksch and W. Lin, Tetrahedron Lett., 2014, 55, 6286–6291, DOI:10.1016/j.tetlet.2014.09.100
.
- M. Wagner, W. M. Abdel-Mageed, R. Ebel, A. T. Bull, M. Goodfellow, H.-P. Fiedler and M. Jaspars, J. Nat. Prod., 2014, 77, 416–420, DOI:10.1021/np400952d
.
- X. Yan, X.-X. Tang, L. Chen, Z.-W. Yi, M.-J. Fang, Z. Wu and Y.-K. Qiu, Mar. Drugs, 2014, 12, 2156–2163, DOI:10.3390/md12042156
.
- N. Takatani, K. Nishida, T. Sawabe, T. Maoka, K. Miyashita and M. Hosokawa, Appl. Microbiol. Biotechnol., 2014, 98, 6633–6640, DOI:10.1007/s00253-014-5702-y
.
- E. E. Eltamany, U. R. Abdelmohsen, A. K. Ibrahim, H. A. Hassanean, U. Hentschel and S. A. Ahmed, Bioorg. Med. Chem. Lett., 2014, 24, 4939–4942, DOI:10.1016/j.bmcl.2014.09.040
.
- T. Kawahara, M. Itoh, M. Izumikawa, I. Kozone, N. Sakata, T. Tsuchida and K. Shin-ya, J. Antibiot., 2014, 67, 261–263, DOI:10.1038/ja.2013.124
.
- S. Sato, F. Iwata, T. Fukae and M. Katayama, J. Antibiot., 2014, 67, 479–482, DOI:10.1038/ja.2014.17
.
- M. C. Kim, E. Hwang, T. Kim, J. Ham, S. Y. Kim and H. Cheol Kwon, J. Nat. Prod., 2014, 77, 2326–2330, DOI:10.1021/np400888e
.
- Y. Kim, H. Ogura, K. Akasaka, T. Oikawa, N. Matsuura, C. Imada, H. Yasuda and Y. Igarashi, Mar. Drugs, 2014, 12, 4110–4125, DOI:10.3390/md12074110
.
- X.-X. He, X.-J. Chen, G.-T. Peng, S.-Y. Guan, L.-F. Lei, J.-H. Yao, B.-X. Liu and C.-X. Zhang, Nat. Prod. Res., 2014, 28, 680–682, DOI:10.1080/14786419.2014.891591
.
- J. Tebben, C. Motti, D. Tapiolas, P. Thomas-Hall and T. Harder, Mar. Drugs, 2014, 12, 2802–2815, DOI:10.3390/md12052802
.
- Y. Sangnoi, A. Plubrukarn, V. Arunpairojana and A. Kanjana-Opas, World J. Microbiol. Biotechnol., 2014, 30, 1135–1139, DOI:10.1007/s11274-013-1531-x
.
- M. Xu, M. L. Hillwig, A. L. Lane, M. S. Tiernan, B. S. Moore and R. J. Peters, J. Nat. Prod., 2014, 77, 2144–2147, DOI:10.1021/np500422d
.
- Y. Wang, X. Tang, Z. Shao, J. Ren, D. Liu, P. Proksch and W. Lin, J. Antibiot., 2014, 67, 395–399, DOI:10.1038/ja.2014.3
.
- A. L. Kunz, A. Labes, J. Wiese, T. Bruhn, G. Bringmann and J. F. Imhoff, Mar. Drugs, 2014, 12, 1699–1714, DOI:10.3390/md12041699
.
- D. Sun, W. Sun, Y. Yu, Z. Li, Z. Deng and S. Lin, Nat. Prod. Res., 2014, 28, 1602–1606, DOI:10.1080/14786419.2014.928877
.
- W. Ai, X.-P. Lin, Z. Tu, X.-P. Tian, X. Lu, F. Mangaladoss, Z.-L. Zhong and Y. Liu, Nat. Prod. Res., 2014, 28, 1219–1224, DOI:10.1080/14786419.2014.891204
.
- X. Zhou, H. Huang, J. Li, Y. Song, R. Jiang, J. Liu, S. Zhang, Y. Hua and J. Ju, Tetrahedron, 2014, 70, 7795–7801, DOI:10.1016/j.tet.2014.02.007
.
- D. Liu, A. Yang, C. Wu, P. Guo, P. Proksch and W. Lin, Bioorg. Med. Chem. Lett., 2014, 24, 5288–5293, DOI:10.1016/j.bmcl.2014.09.049
.
- Y. Song, Q. Li, X. Liu, Y. Chen, Y. Zhang, A. Sun, W. Zhang, J. Zhang and J. Ju, J. Nat. Prod., 2014, 77, 1937–1941, DOI:10.1021/np500399v
.
- A. Tripathi, M. M. Schofield, G. E. Chlipala, P. J. Schultz, I. Yim, S. A. Newmister, T. D. Nusca, J. B. Scaglione, P. C. Hanna, G. Tamayo-Castillo and D. H. Sherman, J. Am. Chem. Soc., 2014, 136, 1579–1586, DOI:10.1021/ja4115924
.
- H. Huang, Y. Cao, L. Tian, W. Lin and K. Zhang, Chem. Nat. Compd., 2014, 50, 402–404, DOI:10.1007/s10600-014-0970-4
.
- S. Huang, L. Ma, M. H. Tong, Y. Yu, D. O'Hagan and H. Deng, Org. Biomol. Chem., 2014, 12, 4828–4831, 10.1039/c4ob00970c
.
- J. Deng, C. Lu, S. Lia, H. Hao, Z. Li, J. Zhu, Y. Li and Y. Shen, Bioorg. Med. Chem. Lett., 2014, 24, 1362–1365, DOI:10.1016/j.bmcl.2014.01.037
.
- Z. Lin, M. Koch, C. D. Pond, G. Mabeza, R. A. Seronay, G. P. Concepcion, L. R. Barrows, B. M. Olivera and E. W. Schmidt, J. Antibiot., 2014, 67, 121–126, DOI:10.1038/ja.2013.115
.
- R. Sugiyama, S. Nishimura, N. Matsumori, Y. Tsunematsu, A. Hattori and H. Kakeya, J. Am. Chem. Soc., 2014, 136, 5209–5212, DOI:10.1021/ja500128u
.
- L. Farnaes, N. G. Coufal, C. A. Kauffman, A. L. Rheingold, A. G. DiPasquale, P. R. Jensen and W. Fenical, J. Nat. Prod., 2014, 77, 15–21, DOI:10.1021/np400466j
.
- E. Harunari, C. Imada, Y. Igarashi, T. Fukuda, T. Terahara and T. Kobayashi, Mar. Drugs, 2014, 12, 491–507, DOI:10.3390/md12010491
.
- Y. Sun, K. Takada, Y. Nogi, S. Okada and S. Matsunaga, J. Nat. Prod., 2014, 77, 1749–1752, DOI:10.1021/np500337m
.
- P. Fu, M. Johnson, H. Chen, B. A. Posner and J. B. MacMillan, J. Nat. Prod., 2014, 77, 1245–1248, DOI:10.1021/np500207p
.
- J. Lee, H. Kim, T. G. Lee, I. Yang, D. H. Won, H. Choi, S.-J. Nam and H. Kang, J. Nat. Prod., 2014, 77, 1528–1531, DOI:10.1021/np500285a
.
- K. Moon, C.-H. Ahn, Y. Shin, T. H. Won, K. Ko, S. K. Lee, K.-B. Oh, J. Shin, S.-I. Nam and D.-C. Oh, Mar. Drugs, 2014, 12, 2526–2538, DOI:10.3390/md12052526
.
- M. W. Mullowney, E. Ó. hAinmhire, A. Shaikh, X. Wei, U. Tanouye, B. D. Santarsiero, J. E. Burdette and B. T. Murphy, Mar. Drugs, 2014, 12, 3574–3586, DOI:10.3390/md12063574
.
- K. Ko, S.-H. Lee, S.-H. Kim, E.-H. Kim, K.-B. Oh, J. Shin and D.-C. Oh, J. Nat. Prod., 2014, 77, 2099–2104, DOI:10.1021/np500500t
.
- H. Kim, I. Yang, R. S. Patil, S. Kang, J. Lee, H. Choi, M.-S. Kim, S.-J. Nam and H. Kang, J. Nat. Prod., 2014, 77, 2716–2719, DOI:10.1021/np500558b
.
- Y.-Y. Bu, H. Yamazaki, K. Ukai and M. Namikoshi, Mar. Drugs, 2014, 12, 6102–6112, DOI:10.3390/md12126102
.
- M. C. Duncan, W. R. Wong, A. J. Dupzyk, W. M. Bray, R. G. Linington and V. Auerbuch, Antimicrob. Agents Chemother., 2014, 58, 1118–1126, DOI:10.1128/AAC.02025-13
.
- N. Yi-Lei, W. Yun-Dan, W. Chuan-Xi, L. Ru, X. Yang, F. Dong-Sheng, J. Hong and L. Yun-Yang, Nat. Prod. Res., 2014, 28, 2134–2139, DOI:10.1080/14786419.2014.926350
.
- H. K. Zane, H. Naka, F. Rosconi, M. Sandy, M. G. Haygood and A. Butler, J. Am. Chem. Soc., 2014, 136, 5615–5618, DOI:10.1021/ja5019942
.
- H. Shigemori, M. A. Bae, K. Yazawa, T. Sasaki and J. Kobayashi, J. Org. Chem., 1992, 57, 4317–4320, DOI:10.1021/jo00041a053
.
- C. Olano, I. Garcia, A. Gonzalez, M. Rodriguez, D. Rozas, J. Rubio, M. Sanchez-Hidalgo, A. F. Brana, C. Mendez and J. A. Salas, Microb. Biotechnol., 2014, 7, 242–256, DOI:10.1111/1751-7915.12116
.
- W. J. Moree, O. J. McConnell, D. D. Nguyen, L. M. Sanchez, Y.-L. Yang, X. Zhao, W.-T. Liu, P. D. Boudreau, J. Srinivasan, L. Atencio, J. Ballesteros, R. G. Gavilán, D. Torres-Mendoza, H. M. Guzmán, W. H. Gerwick, M. Gutiérrez and P. C. Dorrestein, ACS Chem. Biol., 2014, 9, 2300–2308, DOI:10.1021/cb500432j
.
- R. Raju, A. M. Piggott, M. M. Conte and R. J. Capon, Org. Biomol. Chem., 2010, 8, 4682–4689, 10.1039/c0ob00267d
.
- K. Sakanishi, S. Itoh, R. Sugiyama, S. Nishimura, H. Kakeya, Y. Iwabuchi and N. Kanoh, Eur. J. Org. Chem., 2014, 2014, 1376–1380, DOI:10.1002/ejoc.201301487
.
- K. H. Jang, S. J. Nam, J. B. Locke, C. A. Kauffman, D. S. Beatty, L. A. Paul and W. Fenical, Angew. Chem., Int. Ed., 2013, 52, 7822–7824, DOI:10.1002/anie.201302749
.
- K. H. Jang, S.-J. Nam, J. B. Locke, C. A. Kauffman, D. S. Beatty, L. A. Paul and W. Fenical, Angew. Chem., Int. Ed., 2014, 53, 621, DOI:10.1002/anie.201310144
.
- S. Sato, F. Iwata, T. Mukai, S. Yamada, J. Takeo, A. Abe and H. Kawahara, J. Org. Chem., 2009, 74, 5502–5509, DOI:10.1021/jo900667j
.
- O. F. Jeker and E. M. Carreira, Angew. Chem., Int. Ed., 2012, 51, 3474–3477, DOI:10.1002/anie.201109175
.
- C. He, C. Zhu, B. Wang and H. Ding, Chem.–Eur. J., 2014, 20, 15053–15060, DOI:10.1002/chem.201403986
.
- M. Mansson, A. Nielsen, L. Kjaerulff, C. H. Gotfredsen, M. Wietz, H. Ingmer, L. Gram and T. O. Larsen, Mar. Drugs, 2011, 9, 2537–2552, DOI:10.3390/md9122537
.
- B. Kitir, M. Baldry, H. Ingme and C. A. Olsen, Tetrahedron, 2014, 70, 7721–7732, DOI:10.1016/j.tet.2014.05.107
.
- R. N. Asolkar, K. C. Freel, P. R. Jensen, W. Fenical, T. P. Kondratyuk, E.-J. Park and J. M. Pezzuto, J. Nat. Prod., 2009, 72, 396–402, DOI:10.1021/np800617a
.
- S. Chandrasekhar, K. Sathish, G. P. K. Reddy and P. S. Mainkar, Tetrahedron: Asymmetry, 2014, 25, 348–355, DOI:10.1016/j.tetasy.2014.01.004
.
- S. Nie, W. Li and B. Yu, J. Am. Chem. Soc., 2014, 136, 4157–4160, DOI:10.1021/ja501460j
.
- H. A. Kirst, D. E. Dorman, J. L. Occolowitz, N. D. Jones, J. W. Paschal, R. L. Hamill and E. F. Szymanski, J. Antibiot., 1985, 38, 575–586, DOI:10.7164/antibiotics.38.575
.
- Q. H. Zhu, J. Li, J. Y. Ma, M. H. Luo, B. Wang, H. B. Huang, X. P. Tian, W. J. Li, S. Zhang, C. S. Zhang and J. H. Ju, Antimicrob. Agents Chemother., 2012, 56, 110–114, DOI:10.1128/aac.05278-11
.
- Q. B. Zhang, A. Mandi, S. M. Li, Y. C. Chen, W. J. Zhang, X. P. Tian, H. B. Zhang, H. X. Li, W. M. Zhang, S. Zhang, J. H. Ju, T. Kurtan and C. S. Zhang, Eur. J. Org. Chem., 2012, 5256–5262, DOI:10.1002/ejoc.201200599
.
- B. R. Rosen, E. W. Werner, A. G. O'Brien and P. S. Baran, J. Am. Chem. Soc., 2014, 136, 5571–5574, DOI:10.1021/ja5013323
.
- U. W. Hawas, M. Shaaban, K. A. Shaaban, M. Speitling, A. Maier, G. Kelter, H. H. Fiebig, M. Meiners, E. Helmke and H. Laatsch, J. Nat. Prod., 2009, 72, 2120–2124, DOI:10.1021/np900160g
.
- K. S. Prakash and R. Nagarajan, Org. Lett., 2014, 16, 244–246, DOI:10.1021/ol4032396
.
- M. Yotsu-Yamashita, B. Schimmele and T. Yasumoto, Biosci., Biotechnol., Biochem., 1999, 63, 961–963, DOI:10.1271/bbb.63.961
.
- Y. Satake, M. Adachi, S. Tokoro, M. Yotsu-Yamashita, M. Isobe and T. Nishikawa, Chem.–Asian J., 2014, 9, 1922–1932, DOI:10.1002/asia.201402202
.
- G. O. Buchanan, P. G. Williams, R. H. Feling, C. A. Kauffman, P. R. Jensen and W. Fenical, Org. Lett., 2005, 7, 2731–2734, DOI:10.1021/ol050901i
.
- K. Dineshkumar, V. Aparna, K. Z. Madhuri and W. Hopper, Chem. Biol. Drug Des., 2014, 83, 350–361, DOI:10.1111/cbdd.12252
.
- V. J. R. V. Mukku, M. Speitling, H. Laatsch and E. Helmke, J. Nat. Prod., 2000, 63, 1570–1572, DOI:10.1021/np0001676
.
- J. S. Dickschat, T. Martens, T. Brinkhoff, M. Simon and S. Schulz, Chem. Biodiversity, 2005, 2, 837–865, DOI:10.1002/cbdv.200590062
.
- D. Kim, J. S. Lee, Y. K. Park, J. F. Kim, H. Jeong, T. Oh, B. S. Kim and C. H. Lee, J. Appl. Microbiol., 2007, 102, 937–944, DOI:10.1111/j.1365-2672.2006.03172.x
.
- M. Strand, M. Carlsson, H. Uvell, K. Islam, K. Edlund, I. Cullman, B. Altermark, Y.-F. Mei, M. Elofsson, N.-P. Willassen, G. Wadell and F. Almqvist, Mar. Drugs, 2014, 12, 799–821, DOI:10.3390/md12020799
.
- M. Leirós, E. Alonso, J. A. Sanchez, M. E. Rateb, R. Ebel, W. E. Houssen, M. Jaspars, A. Alfonso and L. M. Botana, ACS Chem. Neurosci., 2014, 5, 71–80, DOI:10.1021/cn4001878
.
- S. Hosoya, V. Arunpairojana, C. Suwannachart, A. Kanjana-Opas and A. Yokota, Int. J. Syst. Evol. Microbiol., 2006, 56, 2931–2935, DOI:10.1099/ijs.0.64504-0
.
- T. Ujihara, M. Nagano, H. Wada and S. Mitsuhashi, FEBS Lett., 2014, 588, 4032–4036, DOI:10.1016/j.febslet.2014.09.023
.
- K. Gustafson, M. Roman and W. Fenical, J. Am. Chem. Soc., 1989, 111, 7519–7524, DOI:10.1021/ja00201a036
.
- J. Moldenhauer, D. C. G. Götz, C. R. Albert, S. K. Bischof, K. Schneider, R. D. Süssmuth, M. Engeser, H. Gross, G. Bringmann and J. Piel, Angew. Chem., Int. Ed., 2010, 49, 1465–1467, DOI:10.1002/anie.200905468
.
- W. Qin, Y. Liu, P. Ren, J. Zhang, H. Li, L. Tian and W. Li, ChemBioChem, 2014, 15, 2747–2753, DOI:10.1002/cbic.201402384
.
- E. L. Teuten, L. Xu and C. M. Reddy, Science, 2005, 307, 917–920, DOI:10.1126/science.1106882
.
- V. Agarwal and B. S. Moore, ACS Chem. Biol., 2014, 9, 1980–1984, DOI:10.1021/cb5004338
.
- V. Agarwal, A. A. El Gamal, K. Yamanaka, D. Poth, R. D. Kersten, M. Schor, E. E. Allen and B. S. Moore, Nat. Chem. Biol., 2014, 10, 640–647, DOI:10.1038/nchembio.1564
.
- H. He, J. Am. Chem. Soc., 2001, 123, 5362–5363, DOI:10.1021/ja010129o
.
- A. J. Waldman and E. P. Balskus, Org. Lett., 2014, 16, 640–643, DOI:10.1021/ol403714g
.
- J. E. Janso, B. A. Haltli, A. S. Eustáquio, K. Kulowski, A. J. Waldman, L. Zha, H. Nakamura, V. S. Bernan, H. He, G. T. Carter, F. E. Koehn and E. P. Balskus, Tetrahedron, 2014, 70, 4156–4164, DOI:10.1016/j.tet.2014.03.009
.
- A. Penesyan, J. Tebben, M. Lee, T. Thomas, S. Kjelleberg, T. Harder and S. Egan, Mar. Drugs, 2011, 9, 1391–1402, DOI:10.3390/md9081391
.
- N. L. Brock, A. Nikolay and J. S. Dickschat, Chem. Commun., 2014, 50, 5487–5489, 10.1039/c4cc01924e
.
- A. R. J. Curson, J. D. Todd, M. J. Sullivan and A. W. B. Johnston, Nat. Rev. Microbiol., 2011, 9, 849–859, DOI:10.1038/nrmicro2653
.
- N. L. Brock, M. Menke, T. A. Klapschinski and J. S. Dickschat, Org. Biomol. Chem., 2014, 12, 4318–4323, 10.1039/c4ob00719k
.
- T. Boettcher and J. Clardy, Angew. Chem., Int. Ed., 2014, 53, 3510–3513, DOI:10.1002/anie.201310729
.
- C. Z. Soe and R. Codd, ACS Chem. Biol., 2014, 9, 945–956, DOI:10.1021/cb400901j
.
- M. Fujita and R. Sakai, Mar. Drugs, 2014, 12, 4799–4809, DOI:10.3390/md12094799
.
- F. Romero and F. Espliego, J. Antibiot., 1997, 50, 734–737, DOI:10.7164/antibiotics.50.734
.
- A. H. Al-Mestarihi, G. Villamizar, J. Fernández, O. E. Zolova, F. Lombó and S. Garneau-Tsodikova, J. Am. Chem. Soc., 2014, 136, 17350–17354, DOI:10.1021/ja510489j
.
- K. Jomon, Y. Kuroda, M. Ajisaka and H. Sakai, J. Antibiot., 1972, 25, 271–280, DOI:10.7164/antibiotics.25.271
.
- J. Antosch, F. Schaefers and T. A. M. Gulder, Angew. Chem., Int. Ed., 2014, 53, 3011–3014, DOI:10.1002/anie.201310641
.
- G. Zhang, W. Zhang, Q. Zhang, T. Shi, L. Ma, Y. Zhu, S. Li, H. Zhang, Y.-L. Zhao, R. Shi and C. Zhang, Angew. Chem., Int. Ed., 2014, 53, 4840–4844, DOI:10.1002/anie.201402078
.
- L. L. Yan, N. N. Han, Y. Q. Zhang, L. Y. Yu, J. Chen, Y. Z. Wei, Q. P. Li, L. Tao, G. H. Zheng, S. E. Yang, C. X. Jiang and X. D. Zhang, J. Antibiot., 2010, 63, 259–261, DOI:10.1038/ja.2010.21
.
- Z. Han, Y. Xu, O. McConnell, L. L. Liu, Y. X. Li, S. H. Qi, X. Z. Huang and P. Y. Qian, Mar. Drugs, 2012, 10, 668–676, DOI:10.3390/md10030668
.
- C. Viegelmann, L. Margassery, J. Kennedy, T. Zhang, C. O'Brien, F. O'Gara, J. Morrissey, A. Dobson and R. Edrada-Ebel, Mar. Drugs, 2014, 12, 3323–3351, DOI:10.3390/md12063323
.
-
A. P. D. d. M. Espindola, Ph.D. Thesis, University of California, San Diego, USA, 2008
.
- Y. Song, H. Huang, Y. Chen, J. Ding, Y. Zhang, A. Sun, W. Zhang and J. Ju, J. Nat. Prod., 2013, 76, 2263–2268, DOI:10.1021/np4006025
.
- P. Zeyhle, J. S. Bauer, M. Steimle, F. Leipoldt, M. Rösch, J. Kalinowski, H. Gross and L. Heide, ChemBioChem, 2014, 15, 2385–2392, DOI:10.1002/cbic.201402394
.
- M. Izumikawa, S. T. Khan, M. Takagi and K. Shin-ya, J. Nat. Prod., 2010, 73, 208–212, DOI:10.1021/np900747t
.
- S. T. Khan, M. Izumikawa, K. Motohashi, A. Mukai, M. Takagi and K. Shin-ya, FEMS Microbiol. Lett., 2010, 304, 89–96, DOI:10.1111/j.1574-6968.2009.01886.x
.
- P. Zeyhle, J. S. Bauer, J. Kalinowski, K. Shin-ya, H. Gross and L. Heide, PLoS One, 2014, 9, 99–122, DOI:10.1371/journal.pone.0099122
.
- T. Nakashima, M. Iwatsuki, J. Ochiai, Y. Kamiya, K. Nagai, A. Matsumoto, A. Ishiyama, K. Otoguro, K. Shiomi, Y. Takahashi and S. Ōmura, J. Antibiot., 2014, 67, 253–260, DOI:10.1038/ja.2013.129
.
- T. Nakashima, Y. Kamiya, M. Iwatsuki, Y. Takahashi and S. Ōmura, J. Antibiot., 2014, 67, 533–539, DOI:10.1038/ja.2014.34
.
- Z. Xu, M. Baunach, L. Ding, H. Peng, J. Franke and C. Hertweck, ChemBioChem, 2014, 15, 1274–1279, DOI:10.1002/cbic.201402071
.
- L. Ding, A. Maier, H. H. Fiebig, H. Gorls, W. H. Lin, G. Peschel and C. Hertweck, Angew. Chem., Int. Ed., 2011, 50, 1630–1634, DOI:10.1002/anie.201006165
.
- S.-R. Li, G.-S. Zhao, M.-W. Sun, H.-G. He, H.-X. Wang, Y.-Y. Li, C.-H. Lu and Y.-M. Shen, Gene, 2014, 544, 93–99, DOI:10.1016/j.gene.2014.04.052
.
- P. Wang, F. Kong, J. Wei, Y. Wang, W. Wang, K. Hong and W. Zhu, Mar. Drugs, 2014, 12, 477–490, DOI:10.3390/md12010477
.
- K. Kyeremeh, K. S. Acquah, A. Sazak, W. Houssen, J. Tabudravu, H. Deng and M. Jaspars, Mar. Drugs, 2014, 12, 999–1012, DOI:10.3390/md12020999
.
- J. Zhang, Z. Qian, X. Wu, Y. Ding, J. Li, C. Lu and Y. Shen, Org. Lett., 2014, 16, 2752–2755, DOI:10.1021/ol501072t
.
- K. Kyeremeh, K. Acquah, M. Camas, J. Tabudravu, W. Houssen, H. Deng and M. Jaspars, Mar. Drugs, 2014, 12, 5197–5208, DOI:10.3390/md12105197
.
- L. Ding, A. Maier, H. H. Fiebig, W. H. Lin and C. Hertweck, Org. Biomol. Chem., 2011, 9, 4029–4031, 10.1039/c1ob05283g
.
- Y. Sun, P. Chen, D. Zhang, M. Baunach, C. Hertweck and A. Li, Angew. Chem., Int. Ed., 2014, 53, 9012–9016, DOI:10.1002/anie.201404191
.
- N. M. Gomes, L. J. Bessa, S. Buttachon, P. M. Costa, J. Buaruang, T. Dethoup, A. M. S. Silva and A. Kijjoa, Mar. Drugs, 2014, 12, 822–839, DOI:10.3390/md12020822
.
- R. Obata, T. Sunazuka, Z. Li, Z. Tian, Y. Harigaya, N. Tabata, H. Tomoda and S. Omura, J. Antibiot., 1996, 49, 1133–1148, DOI:10.7164/antibiotics.49.1133
.
- C. Prompanya, T. Dethoup, L. Bessa, M. Pinto, L. Gales, P. Costa, A. Silva and A. Kijjoa, Mar. Drugs, 2014, 12, 5160–5173, DOI:10.3390/md12105160
.
-
M. Hoffmann and M. Molenda, Eur. Pat. Appl., EP 2090291 A1 20090819, 2009
.
-
A. Furukawa, T. Yoshimoto, O. Tsuru, K. Ajisawa and Y. Kinoshita, Jpn. Kokai Tokkyo Koho, JP 03056462 A 19910312, 1991
.
-
H. Eberhardt, Ger. Offen., DE 2456634 A1 19760812, 1976
.
- A. Gusterova, V. Ivanova, K. Aleksieva, M. Kolarova, B. Schlegel and U. Graefe, Biotechnol. Biotechnol. Equip., 2004, 18, 72–76, DOI:10.1080/13102818.2004.10819233
.
- B. Solomon and E. Rosenberg, PCT Int. Appl., WO 2003104441 A2 20031218, 2003.
- Y. Dong, C.-B. Cui, C.-W. Li, W. Hua, C.-J. Wu, T.-J. Zhu and Q.-Q. Gu, Mar. Drugs, 2014, 12, 4326–4352, DOI:10.3390/md12084326
.
- S. W. Meyer, T. F. Mordhorst, C. Lee, P. R. Jensen, W. Fenical and M. Kock, Org. Biomol. Chem., 2010, 8, 2158–2163, 10.1039/b910629d
.
- M. Chen, C.-L. Shao, X.-M. Fu, C.-J. Kong, Z.-G. She and C.-Y. Wang, J. Nat. Prod., 2014, 77, 1601–1606, DOI:10.1021/np5001686
.
- M. Chen, C.-L. Shao, H. Meng, Z.-G. She and C.-Y. Wang, J. Nat. Prod., 2014, 77, 2720–2724, DOI:10.1021/np500650t
.
- H. J. Li, Y. L. Xie, Z. L. Xie, Y. Chen, C. K. Lam and W. J. Lan, Mar. Drugs, 2012, 10, 627–638, DOI:10.3390/md10030627
.
- H. J. Li, T. Chen, Y. L. Xie, W. D. Chen, X. F. Zhu and W. J. Lan, Mar. Drugs, 2013, 11, 551–558, DOI:10.3390/md11020551
.
- H. J. Li, W. J. Lan, C. K. Lam, F. Yang and X. F. Zhu, Chem. Biodiversity, 2011, 8, 317–324, DOI:10.1002/cbdv.201000036
.
- H.-J. Li, W.-H. Jiang, W.-L. Liang, J.-X. Huang, Y.-F. Mo, Y.-Q. Ding, C.-K. Lam, X.-J. Qian, X.-F. Zhu and W.-J. Lan, Mar. Drugs, 2014, 12, 167–175, DOI:10.3390/md12010167
.
- W. Zhang, C.-L. Shao, M. Chen, Q.-A. Liu and C.-Y. Wang, Tetrahedron Lett., 2014, 55, 4888–4891, DOI:10.1016/j.tetlet.2014.06.096
.
- Q. Yao, J. Wang, X. Zhang, X. Nong, X. Xu and S. Qi, Mar. Drugs, 2014, 12, 5902–5915, DOI:10.3390/md12125902
.
- K. Trisuwan, V. Rukachaisirikul, M. Kaewpet, S. Phongpaichit, N. Hutadilok-Towatana, S. Preedanon and J. Sakayaroj, J. Nat. Prod., 2011, 74, 1663–1667, DOI:10.1021/np200374j
.
- Z. Lin, M. Koch, M. H. A. Aziz, R. Galindo-Murillo, M. D. Tianero, T. E. Cheatham, L. R. Barrows, C. A. Reilly and E. W. Schmidt, Org. Lett., 2014, 16, 4774–4777, DOI:10.1021/ol502227x
.
- W.-L. Liang, X. Le, H.-J. Li, X.-L. Yang, J.-X. Chen, J. Xu, H.-L. Liu, L.-Y. Wang, K.-T. Wang, K.-C. Hu, D.-P. Yang and W.-J. Lan, Mar. Drugs, 2014, 12, 5657–5676, DOI:10.3390/md12115657
.
- P. Zhang, A. Mándi, X.-M. Li, F.-Y. Du, J.-N. Wang, X. Li, T. Kurtán and B.-G. Wang, Org. Lett., 2014, 16, 4834–4837, DOI:10.1021/ol502329k
.
- E. F. Pimenta, A. M. Vita-Marques, A. Tininis, M. H. R. Seleghim, L. D. Sette, K. Veloso, A. G. Ferreira, D. E. Williams, B. O. Patrick, D. S. Dalisay, R. J. Andersen and R. G. S. Berlinck, J. Nat. Prod., 2010, 73, 1821–1832, DOI:10.1021/np100470h
.
- E. V. Mercado-Marin, P. Garcia-Reynaga, S. Romminger, E. F. Pimenta, D. K. Romney, M. W. Lodewyk, D. E. Williams, R. J. Andersen, S. J. Miller, D. J. Tantillo, R. G. S. Berlinck and R. Sarpong, Nature, 2014, 509, 318–324, DOI:10.1038/nature13273
.
- L. Liao, J.-H. Lee, M. You, T. J. Choi, W. Park, S. K. Lee, D.-C. Oh, K.-B. Oh and J. Shin, J. Nat. Prod., 2014, 77, 406–410, DOI:10.1021/np400826p
.
- N. Khamthong, V. Rukachaisirikul, C. Pakawatchai, S. Saithong, S. Phongpaichit, S. Preedanon and J. Sakayaroj, Phytochem. Lett., 2014, 10, 50–54, DOI:10.1016/j.phytol.2014.08.002
.
- J.-F. Wang, F.-Q. Xu, Z. Wang, X. Lu, J.-T. Wan, B. Yang, X.-F. Zhou, T.-Y. Zhang, Z.-C. Tu and Y. Liu, Nat. Prod. Res., 2014, 28, 1070–1074, DOI:10.1080/14786419.2014.905935
.
- W.-J. Wang, D.-Y. Li, Y.-C. Li, H.-M. Hua, E.-L. Ma and Z.-L. Li, J. Nat. Prod., 2014, 77, 1367–1371, DOI:10.1021/np500110z
.
- J. Kornsakulkarn, S. Saepua, S. Komwijit, P. Rachtawee and C. Thongpanchang, Tetrahedron, 2014, 70, 2129–2133, DOI:10.1016/j.tet.2014.02.004
.
- M. R. TePaske, J. B. Gloer, D. T. Wicklow and P. F. Dowd, J. Nat. Prod., 1992, 55, 1080–1086, DOI:10.1021/np50086a008
.
- R. J. Cole, J. W. Dorner, J. A. Lansden, R. H. Cox, C. Pape, B. Cunfer, S. S. Nicholson and D. M. Bedell, J. Agric. Food Chem., 1977, 25, 1197–1201, DOI:10.1021/jf60213a061
.
- H. Sun, S. Gao, X. Li, C. Li and B. Wang, Chin. J. Oceanol. Limnol., 2013, 31, 464–470, DOI:10.1007/s00343-013-2106-2
.
- C. Zhang, L. Jin, B. Mondie, S. S. Mitchell, A. L. Castelhano, W. Cai and N. Bergenhem, Bioorg. Med. Chem. Lett., 2003, 13, 1433–1435, DOI:10.1016/s0960-894x(03)00153-7
.
- M. R. TePaske, J. B. Gloer, D. T. Wicklow and P. F. Dowd, Tetrahedron Lett., 1991, 32, 5687–5690, DOI:10.1016/s0040-4039(00)96814-x
.
- K. Sun, Y. Li, L. Guo, Y. Wang, P. Liu and W. Zhu, Mar. Drugs, 2014, 12, 3970–3981, DOI:10.3390/md12073970
.
- J. Xu, S. Zhao and X. Yang, Nat. Prod. Res., 2014, 28, 994–997, DOI:10.1080/14786419.2014.902945
.
- W. Fang, X. Lin, X. Zhou, J. Wan, X. Lu, B. Yang, W. Ai, J. Lin, T. Zhang, Z. Tu and Y. Liu, Med. Chem. Commun., 2014, 5, 701–705, 10.1039/c3md00371j
.
- X. Hu, Q.-W. Xia, Y.-Y. Zhao, Q.-H. Zheng, Q.-Y. Liu, L. Chen and Q.-Q. Zhang, Heterocycles, 2014, 89, 1662–1669, DOI:10.3987/COM-14-13004
.
- T. Zhu, Z. Chen, P. Liu, Y. Wang, Z. Xin and W. Zhu, J. Antibiot., 2014, 67, 315–318, DOI:10.1038/ja.2013.135
.
- X. Hu, Q.-W. Xia, Y.-Y. Zhao, Q.-H. Zheng, Q.-Y. Liu, L. Chen and Q.-Q. Zhang, Chem. Pharm. Bull., 2014, 62, 942–946, DOI:10.1248/cpb.c14-00312
.
- J.-F. Wang, X.-P. Lin, C. Qin, S.-R. Liao, J.-T. Wan, T.-Y. Zhang, J. Liu, M. Fredimoses, H. Chen, B. Yang, X.-F. Zhou, X.-W. Yang, Z.-C. Tu and Y.-H. Liu, J. Antibiot., 2014, 67, 581–583, DOI:10.1038/ja.2014.39
.
- X. Ma, T. Zhu, Q. Gu, R. Xi, W. Wang and D. Li, J. Ocean Univ. China, 2014, 13, 1067–1070, DOI:10.1055/s-0033-1338558
.
- X.-H. Nong, Y.-F. Wang, X.-Y. Zhang, M.-P. Zhou, X.-Y. Xu and S.-H. Qi, Mar. Drugs, 2014, 12, 6113–6124, DOI:10.3390/md12126113
.
- L. Koch, A. Lodin, I. Herold, M. Ilan, S. Carmeli and O. Yarden, Mar. Drugs, 2014, 12, 4713–4731, DOI:10.3390/md12094713
.
- X.-H. Liu, F.-P. Miao, X.-R. Liang and N.-Y. Ji, Nat. Prod. Res., 2014, 28, 1182–1186, DOI:10.1080/14786419.2014.923996
.
- F. Song, B. Ren, C. Chen, K. Yu, X. Liu, Y. Zhang, N. Yang, H. He, X. Liu, H. Dai and L. Zhang, Appl. Microbiol. Biotechnol., 2014, 98, 3753–3758, DOI:10.1007/s00253-013-5409-5
.
- X. Wang, Y. Mou, J. Hu, N. Wang, L. Zhao, L. Liu, S. Wang and D. Meng, Chem. Biodiversity, 2014, 11, 133–139, DOI:10.1002/cbdv.201300115
.
- J. Peng, H. Gao, X. Zhang, S. Wang, C. Wu, Q. Gu, P. Guo, T. Zhu and D. Li, J. Nat. Prod., 2014, 77, 2218–2223, DOI:10.1021/np500469b
.
- J. Peng, H. Gao, J. Li, J. Ai, M. Geng, G. Zhang, T. Zhu, Q. Gu and D. Li, J. Org. Chem., 2014, 79, 7895–7904, DOI:10.1021/jo5010179
.
- M. Chen, X.-M. Fu, C.-J. Kong and C.-Y. Wang, Nat. Prod. Res., 2014, 28, 895–900, DOI:10.1080/14786419.2014.891114
.
- F.-P. Miao, X.-R. Liang, X.-H. Liu and N.-Y. Ji, J. Nat. Prod., 2014, 77, 429–432, DOI:10.1021/np401047w
.
- Y. Zhou, A. Debbab, V. Wray, W. Lin, B. Schulz, R. Trepos, C. Pile, C. Hellio, P. Proksch and A. H. Aly, Tetrahedron Lett., 2014, 55, 2789–2792, DOI:10.1016/j.tetlet.2014.02.062
.
- M. Chen, C.-L. Shao, C.-J. Kong, Z.-G. She and C.-Y. Wang, Chem. Nat. Compd., 2014, 50, 617–620, DOI:10.1007/s10600-014-1037-2
.
- T. Fukuda, Y. Kurihara, A. Kanamoto and H. Tomoda, J. Antibiot., 2014, 67, 593–595, DOI:10.1038/ja.2014.46
.
- Z. G. Khalil, X. Huang, R. Raju, A. M. Piggott and R. J. Capon, J. Org. Chem., 2014, 79, 8700–8705, DOI:10.1021/jo501501z
.
- Y. Liu, S. Zhao, W. Ding, P. Wang, X. Yang and J. Xu, Mar. Drugs, 2014, 12, 5124–5131, DOI:10.3390/md12105124
.
- Q. Tang, K. Guo, X.-Y. Li, X.-Y. Zheng, X.-J. Kong, Z.-H. Zheng, Q.-Y. Xu and X. Deng, Mar. Drugs, 2014, 12, 5993–6002, DOI:10.3390/md12125993
.
- X.-W. Chen, C.-W. Li, C.-B. Cui, W. Hua, T.-J. Zhu and Q.-Q. Gu, Mar. Drugs, 2014, 12, 3116–3137, DOI:10.3390/md12063116
.
- S. S. Ebada, T. Fischer, S. Klaßen, A. Hamacher, Y. O. Roth, M. U. Kassack and E. H. Roth, Nat. Prod. Res., 2014, 28, 1241–1245, DOI:10.1080/14786419.2014.895730
.
- F.-Y. Du, P. Zhang, X.-M. Li, C.-S. Li, C.-M. Cui and B.-G. Wang, J. Nat. Prod., 2014, 77, 1164–1169, DOI:10.1021/np4011037
.
- F.-Y. Du, X.-M. Li, P. Zhang, C.-S. Li and B.-G. Wang, Mar. Drugs, 2014, 12, 2816–2826, DOI:10.3390/md12052816
.
- G. Wu, X. Sun, G. Yu, W. Wang, T. Zhu, Q. Gu and D. Li, J. Nat. Prod., 2014, 77, 270–275, DOI:10.1021/np400833x
.
- Q.-A. Liu, C.-L. Shao, Y.-C. Gu, M. Blum, L.-S. Gan, K.-L. Wang, M. Chen and C.-Y. Wang, J. Agric. Food Chem., 2014, 62, 3183–3191, DOI:10.1021/jf500248z
.
- W. B. Han, Y. H. Lu, A. H. Zhang, G. F. Zhang, Y. N. Mei, N. Jiang, X. Lei, Y. C. Song, S. W. Ng and R. X. Tan, Org. Lett., 2014, 16, 5366–5369, DOI:10.1021/ol502572g
.
- D.-X. Xu, P. Sun, T. Kurtán, A. Mándi, H. Tang, B. Liu, W. H. Gerwick, Z.-W. Wang and W. Zhang, J. Nat. Prod., 2014, 77, 1179–1184, DOI:10.1021/np500024r
.
- N. Khamthong, V. Rukachaisirikul, S. Phongpaichit, S. Preedanon and J. Sakayaroj, Phytochem. Lett., 2014, 10, 59, DOI:10.1016/j.phytol.2014.06.014
.
- H. Harms, V. Rempel, S. Kehraus, M. Kaiser, P. Hufendiek, C. E. Müller and G. M. König, J. Nat. Prod., 2014, 77, 673–677, DOI:10.1021/np400850
.
- M. Fredimoses, X. Zhou, X. Lin, X. Tian, W. Ai, J. Wang, S. Liao, J. Liu, B. Yang, X. Yang and Y. Liu, Mar. Drugs, 2014, 12, 3190–3202, DOI:10.3390/md1206319
.
- X. Yang, M.-C. Kang, Y. Li, E.-A. Kim, S.-M. Kang and Y.-J. Jeon, J. Microbiol. Biotechnol., 2014, 24, 1346–1353, DOI:10.4014/jmb.1405.05035
.
- F.-Y. Du, X.-M. Li, J.-Y. Song, C.-S. Li and B.-G. Wang, Helv. Chim. Acta, 2014, 97, 973–978, DOI:10.1002/hlca.201300358
.
- Z. Liu, G. Xia, S. Chen, Y. Liu, H. Li and Z. She, Mar. Drugs, 2014, 12, 3669–3680, DOI:10.3390/md12063669
.
- M. Chen, C.-L. Shao, K.-L. Wang, Y. Xu, Z.-G. She and C.-Y. Wang, Tetrahedron, 2014, 70, 9132–9138, DOI:10.1016/j.tet.2014.08.055
.
- Y. Li, K.-L. Sun, Y. Wang, P. Fu, P.-P. Liu, C. Wang and W.-M. Zhu, Chin. Chem. Lett., 2014, 24, 1049–1052, DOI:10.1016/j.cclet.2013.07.028
.
- N. M. Gomes, T. Dethoup, N. Singburaudom, L. Gales, A. M. S. Silva and A. Kijjoa, Phytochem. Lett., 2012, 5, 717–720, DOI:10.1016/j.phytol.2012.07.010
.
- Z. Wu, D. Liu, P. Proksch, P. Guo and W. Lin, Mar. Drugs, 2014, 12, 3904–3916, DOI:10.3390/md12073904
.
- E. J. Mejia, S. T. Loveridge, G. Stepan, A. Tsai, G. S. Jones, T. Barnes, K. N. White, M. Drašković, K. Tenney, M. Tsiang, R. Geleziunas, T. Cihlar, N. Pagratis, Y. Tian, H. Yu and P. Crews, J. Nat. Prod., 2014, 77, 618–624, DOI:10.1021/np400889x
.
- A. N. Yurchenko, O. F. Smetanina, A. I. Kalinovsky, M. A. Pushilin, V. P. Glazunov, Y. V. Khudyakova, N. N. Kirichuk, S. P. Ermakova, S. A. Dyshlovoy, E. A. Yurchenko and S. S. Afiyatullov, J. Nat. Prod., 2014, 77, 1321–1328, DOI:10.1021/np500014m
.
- J.-J. Dong, J. Bao, X.-Y. Zhang, X.-Y. Xu, X.-H. Nong and S.-H. Qi, Tetrahedron Lett., 2014, 55, 2749–2753, DOI:10.1016/j.tetlet.2014.03.060
.
- Y. Mizushina, H. Suzuki-Fukudome, T. Takeuchi, K. Takemoto, I. Kuriyama, H. Yoshida, S. Kamisuki and F. Sugawara, Bioorg. Med. Chem., 2014, 22, 1070–1076, DOI:10.1016/j.bmc.2013.12.038
.
- M. García-Caballero, L. Cañedo, A. Fernández-Medarde, M. Á. Medina and A. R. Quesada, Mar. Drugs, 2014, 12, 279–299, DOI:10.3390/md12010279
.
- Y. Liu, X.-M. Li, L.-H. Meng and B.-G. Wang, Phytochem. Lett., 2014, 10, 145–148, DOI:10.1016/j.phytol.2014.08.018
.
- C.-Y. An, X.-M. Li, C.-S. Li, G.-M. Xuand and B.-G. Wang, Mar. Drugs, 2014, 12, 746–756, DOI:10.3390/md12020746
.
- J. Wang, Y. Zhao, L. Men, Y. Zhang, Z. Liu, T. Sun, Y. Geng and Z. Yu, Chem. Nat. Compd., 2014, 50, 405–407, DOI:10.1007/s10600-014-0971-3
.
- J. Peng, X. Zhang, L. Du, W. Wang, T. Zhu, Q. Gu and D. Li, J. Nat. Prod., 2014, 77, 424–428, DOI:10.1021/np400977e
.
- X. Wang, H. Wang, T. Liu and Z. Xin, Appl. Microbiol. Biotechnol., 2014, 98, 4875–4885, DOI:10.1007/s00253-014-5572-3
.
- Y.-L. Sun, X.-Y. Zhang, Z.-H. Zheng, X.-Y. Xu and S.-H. Qi, Nat. Prod. Res., 2014, 28, 239–244, DOI:10.1080/14786419.2013.843177
.
- J.-B. He, Y.-N. Ji, D.-B. Hu, S. Zhang, H. Yan, X.-C. Liu, H.-R. Luo and H.-J. Zhu, Tetrahedron Lett., 2014, 55, 2684–2686, DOI:10.1016/j.tetlet.2014.03.031
.
- X.-D. Li, F.-P. Miao, X.-R. Liang and N.-Y. Ji, Magn. Reson. Chem., 2014, 52, 247–250, DOI:10.1002/mrc.4049
.
- O. I. Zhuravleva, M. P. Sobolevskaya, E. V. Leshchenko, N. N. Kirichuk, V. A. Denisenko, P. S. Dmitrenok, S. A. Dyshlovoy, A. M. Zakharenko, N. Y. Kim and S. S. Afiyatullov, J. Nat. Prod., 2014, 77, 1390–1395, DOI:10.1021/np500151b
.
- O. Zhuravleva, M. Sobolevskaya, S. Afiyatullov, N. Kirichuk, V. Denisenko, P. Dmitrenok, E. Yurchenko and S. Dyshlovoy, Mar. Drugs, 2014, 12, 5930–5943, DOI:10.3390/md12125930
.
- J. Bao, J.-F. Luo, X.-C. Qin, X.-Y. Xu, X.-Y. Zhang, Z.-C. Tu and S.-H. Qi, Bioorg. Med. Chem. Lett., 2014, 24, 2433–2436, DOI:10.1016/j.bmcl.2014.04.028
.
- P.-L. Wang, D.-Y. Li, L.-R. Xie, X. Wu, H.-M. Hua and Z.-L. Li, Nat. Prod. Res., 2014, 28, 290–293, DOI:10.1080/14786419.2013.856906
.
- J. Bao, X.-Y. Zhang, J. Dong, X. Xu, X.-H. Nong and S.-H. Qi, Chem. Lett., 2014, 43, 837–839, DOI:10.1246/cl.140138
.
- C.-S. Li, X.-M. Li, C.-Y. An and B.-G. Wang, Helv. Chim. Acta, 2014, 97, 1440–1444, DOI:10.1002/hlca.201400035
.
- M.-W. Xia, C.-B. Cui, C.-W. Li and C.-J. Wu, Mar. Drugs, 2014, 12, 1545–1568, DOI:10.3390/md12031545
.
- S.-M. Fang, C.-J. Wu, C.-W. Li and C.-B. Cui, Mar. Drugs, 2014, 12, 1788–1814, DOI:10.3390/md12041788
.
- C.-J. Wu, C.-W. Li and C.-B. Cui, Mar. Drugs, 2014, 12, 1815–1838, DOI:10.3390/md12041815
.
- F. J. T. Marante, I. H. B. de Laguna, N. V. Torres and R. Mioso, Appl. Biochem. Biotechnol., 2014, 174, 2426–2434, DOI:10.1007/s12010-014-1180-z
.
- M. P. Sobolevskaya, O. I. Zhuravleva, E. V. Leshchenko, S. S. Afiyatullov, Y. V. Khudyakova, N. Y. Kim, N. N. Kirichuk and S. A. Dyshlovoy, Chem. Nat. Compd., 2014, 50, 1122–1124, DOI:10.1007/s10600-014-1179-2
.
- T. H. Quang, N. T. T. Ngan, W. Ko, D.-C. Kim, C.-S. Yoon, J. H. Sohn, J. H. Yim, Y.-C. Kim and H. Oh, Bioorg. Med. Chem. Lett., 2014, 24, 5787–5791, DOI:10.1016/j.bmcl.2014.10.035
.
- W.-J. Lan, W. Liu, W.-L. Liang, Z. Xu, X. Le, J. Xu, C.-K. Lam, D.-P. Yang, H.-J. Li and L.-Y. Wang, Mar. Drugs, 2014, 12, 4188–4199, DOI:10.3390/md12074188
.
- Y. Luan, H. Wei, Z. Zhang, Q. Che, Y. Liu, T. Zhu, A. Mándi, T. Kurtán, Q. Gu and D. Li, J. Nat. Prod., 2014, 77, 1718–1723, DOI:10.1021/np500458a
.
- S. Niu, D. Liu, X. Hu, P. Proksch, Z. Shao and W. Lin, J. Nat. Prod., 2014, 77, 1021–1030, DOI:10.1021/np5000457
.
- Y. Li, C. Wu, D. Liu, P. Proksch, P. Guo and W. Lin, J. Nat. Prod., 2014, 77, 138–147, DOI:10.1021/np400824u
.
- Y. Li, D. Liu, S. Cen, P. Proksch and W. Lin, Tetrahedron, 2014, 70, 7010–7015, DOI:10.1016/j.tet.2014.07.047
.
- B. Wu, V. Oesker, J. Wiese, S. Malien, R. Schmaljohann and J. F. Imhoff, Mar. Drugs, 2014, 12, 1924–1938, DOI:10.3390/md12041924
.
- D. Kuml, T. Dethoup, S. Buttachon, N. Singburaudom, A. M. S. Silva and A. Kijjoa, Nat. Prod. Commun., 2014, 9, 1147–1197 Search PubMed
.
- L. Chen, Q.-Q. Zhang, X. Hu, M.-W. Gong, W.-W. Zhang, Q.-H. Zheng and Q.-Y. Liu, Heterocycles, 2014, 89, 189–196, DOI:10.3987/com-13-12874
.
- T. Yamada, Y. Mizutani, Y. Umebayashi, N. Inno, M. Kawashima, T. Kikuchi and R. Tanaka, Tetrahedron Lett., 2014, 55, 662–664, DOI:10.1016/j.tetlet.2013.11.107
.
- B. Wu, V. Oesker, J. Wiese, R. Schmaljohann and J. F. Imhoff, Mar. Drugs, 2014, 12, 1208–1219, DOI:10.3390/md12031208
.
- S. El-saedi, J. S. Mezogi and A. A. Akasha, PharmaTutor Mag., 2014, 2, 131–144 Search PubMed
, URL: http://www.pharmatutor.org/articles/phytochemical-studies-verticillium-teneru-acremonium.
- X. Huang, X. Sun, S. Lin, Z. Xiao, H. Li, D. Bo and Z. She, Nat. Prod. Res., 2014, 28, 111–114, DOI:10.1080/14786419.2013.850687
.
- X.-H. Nong, X.-Y. Zhang, X.-Y. Xu, Y.-L. Sun and S.-H. Qi, Nat. Prod. Commun., 2014, 9, 467–475 CAS
.
- N. Kotoku, K. Higashimoto, M. Kurioka, M. Arai, A. Fukuda, Y. Sumii, Y. Sowa, T. Sakai and M. Kobayashi, Bioorg. Med. Chem. Lett., 2014, 24, 3389–3391, DOI:10.1016/j.bmcl.2014.05.083
.
- S. U. Lee, Y. Asami, D. Lee, J. H. Jang, J. S. Ahn and H. Oh, J. Nat. Prod., 2011, 74, 1284–1287, DOI:10.1021/np100880b
.
- P. Lorenzo, R. Álvarez and Á. R. de Lera, Eur. J. Org. Chem., 2014, 2014, 2557–2564, DOI:10.1002/ejoc.201400029
.
- F. Y. Du, X. M. Li, C. S. Li, Z. Shang and B. G. Wang, Bioorg. Med. Chem. Lett., 2012, 22, 4650–4653, DOI:10.1016/j.bmcl.2012.05.088
.
- P. Lorenzo, R. Álvarez and Á. R. de Lera, J. Nat. Prod., 2014, 77, 421–423, DOI:10.1021/np400969u
.
- Y.-L. Sun, J. e. Bao, K.-S. Liu, X.-Y. Zhang, F. He, Y.-F. Wang, X.-H. Nong and S.-H. Qi, Planta Med., 2013, 79, 1474–1479, DOI:10.1055/s-0033-1350805
.
- C. Wink, L. Andernach, T. Opatz and S. R. Waldvogel, Eur. J. Org. Chem., 2014, 2014, 7788–7792, DOI:10.1055/s-0033-1350805
.
- A. Sakurai and Y. Okumura, Bull. Chem. Soc. Jpn., 1979, 52, 540–543, DOI:10.1246/bcsj.52.540
.
- P. Ye, L. Shen, W. Jiang, Y. Ye, C.-T. A. Chen, X. Wu, K. Wang and B. Wu, Mar. Drugs, 2014, 12, 3203–3217, DOI:10.3390/md12063203
.
- T. Yamada, M. Iritani, H. Ohishi, K. Tanaka, K. Minoura, M. Doi and A. Numata, Org. Biomol. Chem., 2007, 5, 3979–3986, 10.1039/b713060k
.
- K. Mizuki, K. Iwahashi, N. Murata, M. Ikeda, Y. Nakai, H. Yoneyama, S. Harusawa and Y. Usami, Org. Lett., 2014, 16, 3760–3763, DOI:10.1021/ol501631r
.
- B. Franck, E. M. Gottschalk, V. Ohnsorge and G. Baumann, Angew. Chem., Int. Ed., 1964, 76, 441–442, DOI:10.1002/anie.196404412
.
- L. Wen, Z. Guo, F. Liu, Q. Wan, Z. Yu, Y. Lin and L. Fu, Huaxue Yanjiu Yu Yingyong, 2009, 21, 198–202 CAS
.
- P. S. Steyn, Tetrahedron, 1970, 26, 51–57, DOI:10.1016/0040-4020(70)85006-2
.
- H. Ren, L. Tian, Q. Q. Gu and W. M. Zhu, Arch. Pharmacal Res., 2006, 29, 59–63, DOI:10.1007/BF02977469
.
- T. Qin and J. A. Porco, Angew. Chem., Int. Ed., 2014, 53, 3107–3110, DOI:10.1002/anie.201311260
.
- L. Chen, T. J. Zhu, Y. Q. Ding, I. A. Khan, Q. Q. Gu and D. H. Li, Tetrahedron Lett., 2012, 53, 325–328, DOI:10.1016/j.tetlet.2011.11.038
.
- C. Qi, T. Qin, D. Suzuki and J. A. Porco Jr, J. Am. Chem. Soc., 2014, 136, 3374–3377, DOI:10.1021/ja500854q
.
- F. H. Song, B. A. Ren, K. Yu, C. X. Chen, H. Guo, N. Yang, H. Gao, X. T. Liu, M. Liu, Y. J. Tong, H. Q. Dai, H. Bai, J. D. Wang and L. X. Zhang, Mar. Drugs, 2012, 10, 1297–1306, DOI:10.3390/md10061297
.
- K. Sorra, K. Mukkanti and S. Pusuluri, Synthesis, 2014, 46, 189–194, DOI:10.1055/s-0033-1338558
.
- J. Silber, B. Ohlendorf, A. Labes, C. Naether and J. F. Imhoff, J. Nat. Prod., 2013, 76, 1461–1467, DOI:10.1021/np400262t
.
- S. Das and R. K. Goswami, J. Org. Chem., 2014, 79, 9778–9791, DOI:10.1021/jo5019798
.
- S. Cai, X. Kong, W. Wang, H. Zhou, T. Zhu, D. Li and Q. Gu, Tetrahedron Lett., 2012, 53, 2615–2617, DOI:10.1016/j.tetlet.2012.03.043
.
- E. M. Boyd and J. Sperry, Org. Lett., 2014, 16, 5056–5059, DOI:10.1021/ol5024097
.
- N. Y. Ji, X. H. Liu, F. P. Miao and M. F. Qiao, Org. Lett., 2013, 15, 2327–2329, DOI:10.1021/ol4009624
.
- A. M. Levinson, Org. Lett., 2014, 16, 4904–4907, DOI:10.1021/ol5024163
.
- C. L. Shao, H. X. Wu, C. Y. Wang, Q. A. Liu, Y. Xu, M. Y. Wei, P. Y. Qian, Y. C. Gu, C. J. Zheng, Z. G. She and Y. C. Lin, J. Nat. Prod., 2011, 74, 629–633, DOI:10.1021/np100641b
.
- Y. Gao, J. Liu, L. Wang, M. Xiao and Y. Du, Eur. J. Org. Chem., 2014, 2014, 2092–2098, DOI:10.1002/ejoc.201301613
.
- P. Sun, D. X. Xu, A. Mandl, T. Kurtan, T. J. Li, B. Schulz and W. Zhang, J. Org. Chem., 2013, 78, 7030–7047, DOI:10.1021/jo400861j
.
- A. Venkanna, B. Siva, B. Poornima, K. S. Babu and J. M. Rao, Tetrahedron Lett., 2014, 55, 403–406, DOI:10.1016/j.tetlet.2013.11.044
.
- D. K. Mohapatra, K. Pulluri, E. Bhimireddy, D. P. Reddy and J. S. Yadav, Asian J. Org. Chem., 2014, 3, 1210–1216, DOI:10.1002/ajoc.201402126
.
- J. Liu, F. Li, E. L. Kim, J. L. Li, J. Hong, K. S. Bae, H. Y. Chung, H. S. Kim and J. H. Jung, J. Nat. Prod., 2011, 74, 1826–1829, DOI:10.1021/np200350b
.
- C. Sreelakshmi, A. B. Rao, M. L. Narasu, P. J. Reddy and B. V. S. Reddy, Tetrahedron Lett., 2014, 55, 1303–1305, DOI:10.1016/j.tetlet.2013.12.089
.
- S. S. Gao, X. M. Li, F. Y. Du, C. S. Li, P. Proksch and B. G. Wang, Mar. Drugs, 2011, 9, 59–70, DOI:10.3390/md9010059
.
- J.-C. Zhao, X.-M. Li, J. Gloer and B.-G. Wang, Mar. Drugs, 2014, 12, 3352–3370, DOI:10.3390/md12063352
.
- C. Iwamoto, K. Minoura, T. Oka, T. Ohta, S. Hagishita and A. Numata, Tetrahedron, 1999, 55, 14353–14368, DOI:10.1016/S0040-4020(99)00884-4
.
- K. Fujioka, H. Yokoe, A. Inoue, K. Soga, M. Tsubuki and K. Shishido, J. Org. Chem., 2014, 79, 7512–7519, DOI:10.1021/jo501225y
.
- T. Hamasaki, M. Fukunaga, Y. Kimura and Y. Hatsuda, Agric. Biol. Chem., 1980, 44, 1685–1687, DOI:10.1271/bbb1961.44.1685
.
- S. Wang, X. M. Li, F. Teuscher, D. L. Li, A. Diesel, R. Ebel, P. Proksch and B. G. Wang, J. Nat. Prod., 2006, 69, 1622–1625, DOI:10.1021/np060248n
.
- K.-S. Kim, X. Cui, D.-S. Lee, W. Ko, J. Sohn, J. Yim, R.-B. An, Y.-C. Kim and H. Oh, Int. J. Mol. Sci., 2014, 15, 23749–23765, DOI:10.3390/ijms151223749
.
- S. Takahashi, Y. Itoh, M. Takeuchi, K. Furuya, K. Kodama, A. Naito, T. Haneishi, S. Sato and C. Tamura, J. Antibiot., 1983, 36, 1418–1420, DOI:10.7164/antibiotics.36.1418
.
- O. F. Smetanina, A. N. Yurchenko, S. S. Afiyatullov, A. I. Kalinovsky, M. A. Pushilin, Y. V. Khudyakova, N. N. Slinkina, S. P. Ermakova and E. A. Yurchenko, Phytochem. Lett., 2012, 5, 165–169, DOI:10.1016/j.phytol.2011.12.002
.
- M. M. Anisimov, E. L. Chaikina, O. F. Smetanina and S. S. Afiyatullov, Nat. Prod. Commun., 2014, 9, 835–836 CAS
.
- H. Nagasawa, A. Isogai, A. Suzuki and S. Tamura, Tetrahedron Lett., 1976, 1601–1604, DOI:10.1016/s0040-4039(01)91627-2
.
- D. L. Li, X. M. Li, T. G. Li, H. Y. Dang and B. G. Wang, Helv. Chim. Acta, 2008, 91, 1888–1893, DOI:10.1002/hlca.200890202
.
- X.-P. Sun, Y. Xu, F. Cao, R.-F. Xu, X.-L. Zhang and C.-Y. Wang, Chem. Nat. Compd., 2014, 50, 1153–1155, DOI:10.1007/s10600-014-1189-0
.
- M. F. Elsebai, V. Rempel, G. Schnakenburg, S. Kehraus, C. E. Müller and G. M. König, ACS Med. Chem. Lett., 2011, 2, 866–869, DOI:10.1021/ml200183z
.
- D.-C. Kim, H.-S. Lee, W. Ko, D.-S. Lee, J. Sohn, J. Yim, Y.-C. Kim and H. Oh, Molecules, 2014, 19, 18073–18089, DOI:10.3390/molecules191118073
.
- R. Myokei, A. Sakurai, C.-F. Chang, Y. Kodaira, N. Takahashi and S. Tamura, Agric. Biol. Chem., 1969, 33, 1491–1500, DOI:10.1271/bbb1961.33.1491
.
- K. Motohashi and S. Inaba, Biosci., Biotechnol., Biochem., 2009, 73, 1898–1900, DOI:10.1271/bbb.90228
.
- H. Kato, T. Yoshida, T. Tokue, Y. Nojiri, H. Hirota, T. Ohta, R. M. Williams and S. Tsukamoto, Angew. Chem., Int. Ed., 2007, 46, 2254–2256, DOI:10.1002/anie.200604381
.
- K. Kito, R. Ookura, T. Kusumi, M. Namikoshi and T. Ooi, Heterocycles, 2009, 78, 2101–2106, DOI:10.3987/com-09-11702
.
- D. Weber, S. Gorzalczany, V. Martino, C. Acevedo, O. Sterner and T. Anke, Z. Naturforsch., C: J. Biosci., 2005, 60, 467–477 CAS
, URL: http://lup.lub.lu.se/record/152371.
- K. Trisuwan, V. Rukachaisirikul, Y. Sukpondma, S. Preedanon, S. Phongpaichit, N. Rungjindamai and J. Sakayaroj, J. Nat. Prod., 2008, 71, 1323–1326, DOI:10.1021/np8002595
.
- J. X. Peng, T. Lin, W. Wang, Z. H. Xin, T. J. Zhu, Q. Q. Gu and D. H. Li, J. Nat. Prod., 2013, 76, 1133–1140, DOI:10.1021/np400200k
.
- W. Jiang, Y. Zhong, L. Shen, X. Wu, Y. Ye, C.-T. A. Chen and B. Wu, Curr. Org. Chem., 2014, 18, 925–934, DOI:10.2174/138527281807140515155705
.
- C. Li, J. Wang, C. Luo, W. Ding and D. G. Cox, Nat. Prod. Res., 2014, 28, 616–621, DOI:10.1080/14786419.2014.887074
.
- C. Li, D. G. Cox, S. Huang and W. Ding, Pharmacogn. Mag., 2014, 10, 410–414, DOI:10.4103/0973-1296.141781
.
- S. S. Ebada, T. Fischer, A. Hamacher, F.-Y. Du, Y. O. Roth, M. U. Kassack, B.-G. Wang and E. H. Roth, Nat. Prod. Res., 2014, 28, 776–781, DOI:10.1080/14786419.2014.880911
.
- F. Salmon-Legagneur and M. Le Gall, Bull. Soc. Chim. Fr., 1966, 553–555 CAS
.
- L. Andernach, L. P. Sandjo, J. C. Liermann, I. Buckel, E. Thines and T. Opatz, Eur. J. Org. Chem., 2013, 5946–5951, DOI:10.1002/ejoc.201300530
.
- Z.-Q. Bai, X. Lin, Y. Wang, J. Wang, X. Zhou, B. Yang, J. Liu, X. Yang, Y. Wang and Y. Liu, Fitoterapia, 2014, 95, 194–202, DOI:10.1016/j.fitote.2014.03.021
.
- H. C. Wu, H. M. Ge, L. Y. Zang, Y. C. Bei, Z. Y. Niu, W. Wei, X. J. Feng, S. Ding, S. W. Ng, P. P. Shenv and R. X. Tan, Org. Biomol. Chem., 2014, 12, 6545–6548, 10.1039/c4ob01123f
.
- X.-P. Du and W.-J. Su, Chem. Nat. Compd., 2014, 50, 214–216, DOI:10.1007/s10600-014-0915-y
.
- S. Komai, T. Hosoe, T. Itabashi, K. Nozawa, K. Okada, T. G. M. de Campos, M. Chikamori, T. Yaguchi, K. Fukushima, M. Miyaji and K. Kawai, Mycotoxins, 2004, 54, 15–19, DOI:10.2520/myco.54.15
.
- J. Beau, N. Mahid, W. N. Burda, L. Harrington, L. N. Shaw, T. Mutka, D. E. Kyle and B. Barisic, Mar. Drugs, 2012, 10, 762–774, DOI:10.3390/md10040762
.
- H. Li, J. Jiang, Z. Liu, S. Lin, G. Xia, X. Xia, B. Ding, L. He, Y. Lu and Z. She, J. Nat. Prod., 2014, 77, 800–806, DOI:10.1021/np400880w
.
-
Y. Shen, X. Du, Z. Zheng, Y. Huang, S. Song and W. Su, Faming Zhuanli Shenqing Gongkai Shuomingshu, CN 1733693 A 20060215, 2006
.
- Y. Wang, L. Wang, Y. Zhuang, F. Kong, C. Zhang and W. Zhu, Mar. Drugs, 2014, 12, 2079–2088, DOI:10.3390/md12042079
.
- C. Zheng, Y. Chen, L.-L. Jiang and X.-M. Shi, Phytochem. Lett., 2014, 10, 272–275, DOI:10.1016/j.phytol.2014.10.011
.
- J. Wang, X. Wei, X. Lu, F. Xu, J. Wan, X. Lin, X. Zhou, S. Liao, B. Yang, Z. Tu and Y. Liu, Tetrahedron, 2014, 70, 9695–9701, DOI:10.1016/j.tet.2014.10.056
.
- L. Hammerschmidt, A. Debbab, T. D. Ngoc, V. Wray, C. P. Hemphil, W. Lin, H. Broetz-Oesterhelt, M. U. Kassack, P. Proksch and A. H. Aly, Tetrahedron Lett., 2014, 55, 3463–3468, DOI:10.1016/j.tetlet.2014.04.063
.
- J. Wang, D. G. Cox, W. Ding, G. Huang, Y. Lin and C. Li, Mar. Drugs, 2014, 12, 2840–2850, DOI:10.3390/md12052840
.
- G. Xia, J. Li, H. Li, Y. Long, S. Lin, Y. Lu, L. He, Y. Lin, L. Liu and Z. She, Mar. Drugs, 2014, 12, 2953–2969, DOI:10.3390/md12052953
.
- Z.-X. Hu, Y.-B. Xue, X.-B. Bi, J.-W. Zhang, Z.-W. Luo, X.-N. Li, G.-M. Yao, J.-P. Wang and Y.-H. Zhang, Mar. Drugs, 2014, 12, 5563–5575, DOI:10.3390/md12115563
.
- W. Ai, X. Wei, X. Lin, L. Sheng, Z. Wang, Z. Tu, X. Yang, X. Zhou, J. Li and Y. Liu, Tetrahedron, 2014, 70, 5806–5814, DOI:10.1016/j.tet.2014.06.041
.
- L.-H. Meng, X.-M. Li, Y. Liu and B.-G. Wang, Org. Lett., 2014, 16, 6052–6055, DOI:10.1021/ol503046u
.
- P. Zhang, L.-H. Meng, A. Mándi, T. Kurtán, X.-M. Li, Y. Liu, X. Li, C.-S. Li and B.-G. Wang, Eur. J. Org. Chem., 2014, 2014, 4029–4036, DOI:10.1002/ejoc.201400067
.
- L.-H. Meng, X.-M. Li, C.-T. Lv, C.-G. Huang and B.-G. Wang, J. Nat. Prod., 2014, 77, 1921–1927, DOI:10.1021/np500382k
.
- J. Li, J. Wang, C.-S. Jiang, G. Li and Y.-W. Guo, J. Asian Nat. Prod. Res., 2014, 16, 542–548, DOI:10.1080/10286020.2014.911290
.
- X.-S. Huang, B. Yang, X.-F. Sun, G.-P. Xia, Y.-Y. Liu, L. Ma and Z.-G. She, Helv. Chim. Acta, 2014, 97, 664–668, DOI:10.1002/hlca.201300248
.
- Z.-F. Zhou, T. Kurtán, X.-H. Yang, A. Mándi, M.-Y. Geng, B.-P. Ye, O. Taglialatela-Scafati and Y.-W. Guo, Org. Lett., 2014, 16, 1390–1393, DOI:10.1021/ol5001523
.
- B. Yang, J. Dong, X. Lin, X. Zhou, Y. Zhang and Y. Liu, Tetrahedron, 2014, 70, 3859–3863, DOI:10.1016/j.tet.2014.04.043
.
- J. Li, X. Yang, Y. Lin, J. Yuan, Y. Lu, X. Zhu, J. Li, M. Li, Y. Lin, J. He and L. Liu, Fitoterapia, 2014, 97, 241–246, DOI:10.1016/j.bmc.2013.12.038
.
- Z.-F. Zhou, X.-H. Yang, H.-L. Liu, Y.-C. Gu, B.-P. Ye and Y.-W. Guo, Helv. Chim. Acta, 2014, 97, 1564–1570, DOI:10.1002/hlca.201400062
.
- H. Luo, X.-M. Li, C.-S. Li and B.-G. Wang, Phytochem. Lett., 2014, 9, 22–25, DOI:10.1016/j.phytol.2014.03.012
.
- C. Zheng, G. Huang, X. Tang, D. Wang, X. Gong, Q. Zhang, X. Song and G. Chen, Chin. J. Org. Chem., 2014, 34, 1172–1176, DOI:10.6023/cjoc201401014
.
- J.-F. Sun, X. Lin, X.-F. Zhou, J. Wan, T. Zhang, B. Yang, X.-W. Yang, Z. Tu and Y. Liu, J. Antibiot., 2014, 67, 451–457, DOI:10.1038/ja.2014.24
.
- F. Kong, Y. Wang, P. Liu, T. Dong and W. Zhu, J. Nat. Prod., 2014, 77, 132–137, DOI:10.1021/np400802d
.
- J. X. Yang, S. X. Qiu, Z. She and Y. Lin, Chem. Nat. Compd., 2014, 50, 424–426, DOI:10.1007/s10600-014-0976-y
.
- W. Zhang, L. Xu, L. Yang, Y. Huang, S. Li and Y. Shen, Fitoterapia, 2014, 96, 146–151, DOI:10.1016/j.fitote.2014.05.001
.
- M. Wibowo, V. Prachyawarakorn, T. Aree, S. Wiyakrutta, C. Mahidol, S. Ruchirawat and P. Kittakoop, Eur. J. Org. Chem., 2014, 2014, 3976–3980, DOI:10.1002/ejoc.201402262
.
- K. Pudhom, T. Teerawatananond and S. Chookpaiboon, Mar. Drugs, 2014, 12, 1271–1280, DOI:10.3390/md12031271
.
- K. Pudhom and T. Teerawatananond, J. Nat. Prod., 2014, 77, 1962–1966, DOI:10.1021/np500068y
.
- X.-M. Zhou, C.-J. Zheng, X.-P. Song, C.-R. Han, W.-H. Chen and G.-Y. Chen, J. Antibiot., 2014, 67, 401–403, DOI:10.1038/ja.2014.6
.
- X.-M. Zhou, C.-J. Zheng, G.-Y. Chen, X.-P. Song, C.-R. Han, G.-N. Li, Y.-H. Fu, W.-H. Chen and Z.-G. Niu, J. Nat. Prod., 2014, 77, 2021–2028, DOI:10.1021/np500340y
.
- B. Yang, J. Dong, X. Lin, H. Tao, X. Zhou and Y. Liu, Nat. Prod. Res., 2014, 28, 967–970, DOI:10.1080/14786419.2014.901318
.
- T. Inuzuka, K. Yamamoto, A. Iwasaki, O. Ohno, K. Suenaga, Y. Kawazoe and D. Uemura, Tetrahedron Lett., 2014, 55, 6711–6714, DOI:10.1016/j.tetlet.2014.10.032
.
- A. Iwasaki, S. Sumimoto, O. Ohno, S. Suda and K. Suenaga, Bull. Chem. Soc. Jpn., 2014, 87, 609–613, DOI:10.1246/bcsj.20140008
.
- A. Iwasaki, O. Ohno, S. Sumimoto, S. Suda and K. Suenaga, RSC Adv., 2014, 4, 12840–12843, 10.1039/c4ra00132j
.
- E. Mevers, T. Matainaho, M. A. V. Di Marzo and W. H. Gerwick, Lipids, 2014, 49, 1127–1132, DOI:10.1007/s11745-014-3949-9
.
- Y. Kato and P. J. Scheuer, J. Am. Chem. Soc., 1974, 96, 2245–2246, DOI:10.1021/ja00814a041
.
- G. R. Pettit, J.-P. Xu, D. L. Doubek, J.-C. Chapuis and J. M. Schmidt, J. Nat. Prod., 2004, 67, 1252–1255, DOI:10.1021/np030198b
.
- D. K. Gupta, P. Kaur, S. T. Leong, L. T. Tan, M. R. Prinsep and J. J. H. Chu, Mar. Drugs, 2014, 12, 115–127, DOI:10.3390/md12010115
.
- T.-H. Bui, V. Wray, M. Nimtz, T. Fossen, M. Preisitsch, G. Schröder, K. Wende, S. E. Heiden and S. Mundt, J. Nat. Prod., 2014, 77, 1287–1296, DOI:10.1021/np401020a
.
- O. Ohno, A. Watanabe, M. Morita and K. Suenaga, Chem. Lett., 2014, 43, 287–289, DOI:10.1246/cl.130960
.
- A. Iwasaki, O. Ohno, S. Sumimoto, S. Suda and K. Suenaga, Tetrahedron Lett., 2014, 55, 4126–4128, DOI:10.1016/j.tetlet.2014.05.099
.
- W. Jiang, W. Zhou, H. Uchida, M. Kikumori, K. Irie, R. Watanabe, T. Suzuki, B. Sakamoto, M. Kamio and H. Nagai, Mar. Drugs, 2014, 12, 2748–2759, DOI:10.3390/md12052748
.
- W. Jiang, S. Tan, Y. Hanaki, K. Irie, H. Uchida, R. Watanabe, T. Suzuki, B. Sakamoto, M. Kamio and H. Nagai, Mar. Drugs, 2014, 12, 5788–5800, DOI:10.3390/md12125788
.
- J. Quintana, L. M. Bayona, L. Castellanos, M. Puyana, P. Camargo, F. Aristizábal, C. Edwards, J. N. Tabudravu, M. Jaspars and F. A. Ramos, Bioorg. Med. Chem., 2014, 22, 6789–6795, DOI:10.1016/j.bmc.2014.10.039
.
- E. Mevers, F. P. J. Haeckl, P. D. Boudreau, T. Byrum, P. C. Dorrestein, F. A. Valeriote and W. H. Gerwick, J. Nat. Prod., 2014, 77, 969–975, DOI:10.1021/np401051z
.
- M. J. Balunas, M. F. Grosso, F. A. Villa, N. Engene, K. L. McPhail, K. Tidgewell, L. M. Pineda, L. Gerwick, C. Spadafora, D. E. Kyle and W. H. Gerwick, Org. Lett., 2012, 14, 3878–3881, DOI:10.1021/ol301607q
.
- V. M. T. Carneiro, C. M. Avila, M. J. Balunas, W. H. Gerwick and R. A. Pilli, J. Org. Chem., 2014, 79, 630–642, DOI:10.1021/jo402339y
.
- M. Morita, O. Ohno and K. Suenaga, Chem. Lett., 2012, 41, 165–167, DOI:10.1246/cl.2012.165
.
- Y. Tanabe, E. Sato, N. Nakajima, A. Ohkubo, O. Ohno and K. Suenaga, Org. Lett., 2014, 16, 2858–2861, DOI:10.1021/ol500996n
.
- H. Luesch, W. Y. Yoshida, R. E. Moore and V. J. Paul, Bioorg. Med. Chem., 2002, 10, 1973–1978, DOI:10.1016/S0968-0896(02)00014-7
.
- Y. Masuda, J. Suzuki, Y. Onda, Y. Fujino, M. Yoshida and T. Doi, J. Org. Chem., 2014, 79, 8000–8009, DOI:10.1021/jo501130b
.
- F. D. Horgen, E. B. Kazmierski, H. E. Westenburg, W. Y. Yoshida and P. J. Scheuer, J. Nat. Prod., 2002, 65, 487–491, DOI:10.1021/np010560r
.
- W. Telle, G. Kelter, H.-H. Fiebig, P. G. Jones and T. Lindel, Beilstein J. Org. Chem., 2014, 10, 316–322, DOI:10.3762/bjoc.10.29
.
- P. G. Williams, W. Y. Yoshida, R. E. Moore and V. J. Paul, J. Nat. Prod., 2004, 67, 49–53, DOI:10.1021/np030215x
.
- J. Han, J. Lian, X. Tian, S. Zhou, X. Zhen and S. Liu, Eur. J. Org. Chem., 2014, 2014, 7232–7238, DOI:10.1002/ejoc.201402977
.
- D. Klein, D. Daloze, J. C. Braekman, L. Hoffmann and V. Demoulin, J. Nat. Prod., 1995, 58, 1781–1785, DOI:10.1021/np50125a025
.
- Z. Lu, M. Yang, P. Chen, X. Xiong and A. Li, Angew. Chem., Int. Ed., 2014, 53, 13840–13844, DOI:10.1002/anie.201406626
.
- J. I. Jimenez, T. Vansach, W. Y. Yoshida, B. Sakamoto, P. Poerzgen and F. D. Horgen, J. Nat. Prod., 2009, 72, 1573–1578, DOI:10.1021/np900173d
.
- R. A. Medina, D. E. Goeger, P. Hills, S. L. Mooberry, N. Huang, L. I. Romero, E. Ortega-Barria, W. H. Gerwick and K. L. McPhail, J. Am. Chem. Soc., 2008, 130, 6324, DOI:10.1021/ja801383f
.
- X. Wang, C. Lv, J. Liu, L. Tang, J. Feng, S. Tang, Z. Wang, Y. Liu, Y. Meng, T. Ye and Z. Xu, Synlett, 2014, 25, 1014–1018, DOI:10.1055/s-0033-1340872
.
- W. He, H.-B. Qiu, Y.-J. Chen, J. Xi and Z.-J. Yao, Tetrahedron Lett., 2014, 55, 6109–6112, DOI:10.1016/j.tetlet.2014.09.047
.
- J. C. Kwan, R. Ratnayake, K. A. Abboud, V. J. Paul and H. Luesch, J. Org. Chem., 2010, 75, 8012–8023, DOI:10.1021/jo1013564
.
- J. C. Kwan, Y. Liu, R. Ratnayake, R. Hatano, A. Kuribara, C. Morimoto, K. Ohnuma, V. J. Paul, T. Ye and H. Luesch, ChemBioChem, 2014, 15, 799–804, DOI:10.1002/cbic.201300762
.
- R. G. Linington, B. R. Clark, E. E. Trimble, A. Almanza, L. D. Urena, D. E. Kyle and W. H. Gerwick, J. Nat. Prod., 2009, 72, 14–17, DOI:10.1021/np8003529
.
- B. Miller, A. J. Friedman, H. Choi, J. Hogan, J. A. McCammon, V. Hook and W. H. Gerwick, J. Nat. Prod., 2014, 77, 92–99, DOI:10.1021/np400727r
.
- B. I. Morinaka, A. L. Vagstad, M. J. Helf, M. Gugger, C. Kegler, M. F. Freeman, H. B. Bode and J. Piel, Angew. Chem., Int. Ed., 2014, 53, 85038507, DOI:10.1002/anie.201400478
.
- Q. Zhang, Y. Yu, J. E. Velasquez and W. A. van der Donk, Proc. Natl. Acad. Sci. U. S. A., 2012, 109, 18361–18366, DOI:10.1073/pnas.1210393109
.
- Q. Zhang, X. Yang, H. Wang and W. A. van der Donk, ACS Chem. Biol., 2014, 9, 2686–2694, DOI:10.1021/cb500622c
.
- M. Minamida, K. Kumagai, D. Ulanova, M. Akakabe, Y. Konishi, A. Tominaga, H. Tanaka, M. Tsuda, E. Fukushi, J. Kawabata, A. Masuda and M. Tsuda, Org. Lett., 2014, 16, 4858–4861, DOI:10.1021/ol5023504
.
- B. S. Hwang, H. S. Kim, W. Yih, E. J. Jeong and J.-R. Rho, Org. Lett., 2014, 16, 5362–5365, DOI:10.1021/ol502567g
.
- H. J. Domínguez, J. G. Napolitano, M. T. Fernández-Sánchez, D. Cabrera-García, A. Novelli, M. Norte, J. J. Fernández and A. H. Daranas, Org. Lett., 2014, 16, 4546–4549, DOI:10.1021/ol502102f
.
- G. Nuzzo, A. Cutignano, A. Sardo and A. Fontana, J. Nat. Prod., 2014, 77, 1524–1527, DOI:10.1021/np500275x
.
- M. Akakabe, K. Kumagai, M. Tsuda, Y. Konishi, A. Tominaga, M. Tsuda, E. Fukushi and J. Kawabata, Tetrahedron Lett., 2014, 55, 3491–3494, DOI:10.1016/j.tetlet.2014.04.086
.
- M. Akakabe, K. Kumagai, M. Tsuda, Y. Konishi, A. Tominaga, M. Tsuda, E. Fukushi and J. Kawabata, Tetrahedron, 2014, 70, 2962–2965, DOI:10.1016/j.tet.2014.03.025
.
- T. Inuzuka, K. Yamada and D. Uemura, Tetrahedron Lett., 2014, 55, 6319–6323, DOI:10.1016/j.tetlet.2014.09.094
.
- T. Kubota, T. Iwai, K. Sakai, T. Gonoi and J. Kobayashi, Org. Lett., 2014, 16, 5624–5627, DOI:10.1021/ol502685z
.
- J. Kilcoyne, C. Nulty, T. Jauffrais, P. McCarron, F. Herve, B. Foley, F. Rise, S. Crain, A. L. Wilkins, M. J. Twiner, P. Hess and C. O. Miles, J. Nat. Prod., 2014, 77, 2465–2474, DOI:10.1021/np500555k
.
- R. Suzuki, R. Irie, Y. Harntaweesup, K. Tachibana, P. T. Holland, D. T. Harwood, F. Shi, V. Beuzenberg, Y. Itoh, S. Pascal, P. J. B. Edwards and M. Satake, Org. Lett., 2014, 16, 5850–5853, DOI:10.1021/ol502700h
.
- A. I. Selwood, A. L. Wilkins, R. Munday, H. Gu, K. F. Smith, L. L. Rhodes and F. Rise, Tetrahedron Lett., 2014, 55, 5508–5510, DOI:10.1016/j.tetlet.2014.08.056
.
- M. García-Altares, L. Tartaglione, C. Dell'Aversano, O. Carnicer, P. de la Iglesia, M. Forino, J. Diogène and P. Ciminiello, Anal. Bioanal. Chem., 2014, 407, 1191–1204, DOI:10.1007/s00216-014-8338-y
.
- J. Kobayashi, N. Yamaguchi and M. Ishibashi, Tetrahedron Lett., 1994, 35, 7049–7050, DOI:10.1016/0040-4039(94)88222-3
.
- T. Iwai, T. Kubota and J. Kobayashi, J. Nat. Prod., 2014, 77, 1541–1544, DOI:10.1021/np5003065
.
- R. Kellmann, T. K. Mihali, Y. J. Jeon, R. Pickford, F. Pomati and B. A. Neilan, Appl. Environ. Microbiol., 2008, 74, 4044–4053, DOI:10.1128/aem.00353-08
.
- S. Tsuchiya, Y. Cho, K. Konoki, K. Nagasawa, Y. Oshima and M. Yotsu-Yamashita, Org. Biomol. Chem., 2014, 12, 3016–3020, 10.1039/c4ob00071d
.
- K. L. Poulson-Ellestad, C. M. Jones, J. Roy, M. R. Viant, F. M. Fernandez, J. Kubanek and B. L. Nunn, Proc. Natl. Acad. Sci. U. S. A., 2014, 111, 9009–9014, DOI:10.1073/pnas.1402130111
.
- M. Satake and A. J. Bourdelais, Org. Lett., 2008, 10, 3465–3468, DOI:10.1016/0041-0101(86)90134-0
.
- K. Calabro, J.-M. Guigonis, J.-L. Teyssié, F. Oberhänsli, J.-P. Goudour, M. Warnau, M.-Y. D. Bottein and O. P. Thomas, Toxins, 2014, 6, 1785–1798, DOI:10.3390/toxins6061785
.
- M. Satake and A. J. Bourdelais, Org. Lett., 2008, 10, 3465–3468, DOI:10.1016/0041-0101(86)90134-0
.
- M. Satake, T. Shirai, Y. Takimoto, T. Kuranaga, K. Tachibana, D. G. Baden and J. L. C. Wright, Heterocycles, 2014, 89, 127–142, DOI:10.3987/COM-13-12880
.
- Y. Zhang, S.-F. Zhang, L. Lin and D.-Z. Wang, Mar. Drugs, 2014, 12, 5698–5718, DOI:10.3390/md12115698
.
- D.-Q. Liu, S.-C. Mao, H.-Y. Zhang, X.-Q. Yu, M.-T. Feng, B. Wang, L.-H. Feng and Y.-W. Guo, Fitoterapia, 2013, 91, 15–20, DOI:10.1016/j.fitote.2013.08.014
.
- H. Yang, D.-Q. Liu, T.-J. Liang, J. Li, A.-H. Liu, P. Yang, K. Lin, X.-Q. Yu, Y.-W. Guo, S.-C. Mao and B. Wang, J. Asian Nat. Prod. Res., 2014, 16, 1158–1165, DOI:10.1080/10286020.2014.965162
.
- B. C. Maiti, R. H. Thomson and M. Mahendran, J. Chem. Res., Synop., 1978, 126–127 CAS
.
- L. Cavalcante-Silva, M. Falcão, A. Vieira, M. Viana, J. de Araújo-Júnior, J. Sousa, T. Silva, J. Barbosa-Filho, F. Noël, G. de Miranda, B. Santos and M. Alexandre-Moreira, Molecules, 2014, 19, 14699–14709, DOI:10.3390/molecules190914699
.
- I. C. Canché Chay, R. G. Cansino, C. I. E. Pinzón, R. O. Torres-Ochoa and R. Martínez, Mar. Drugs, 2014, 12, 1757–1772, DOI:10.3390/md12041757
.
- G. L. Renju, G. M. Kurup and V. R. Bandugula, Tumor Biol., 2014, 35, 10747–10758, DOI:10.1007/s13277-014-2339-5
.
- Z.-M. Hu, L.-B. Juan and D.-L. Duan, Chin. Sci. Bull., 2014, 59, 1479–1481, DOI:10.1007/s11434-014-0143-7
.
- N. Dasyam, A. B. Munkacsi, N. H. Fadzilah, D. S. Senanayake, R. F. O'Toole and R. A. Keyzers, J. Nat. Prod., 2014, 77, 1519–1523, DOI:10.1021/np500171z
.
- S. Cheng, M. Zhao, Z. Sun, W. Yuan, S. Zhang, Z. Xiang, Y. Cai, J. Dong, K. Huang and P. Yan, J. Nat. Prod., 2014, 77, 2685–2693, DOI:10.1021/np5006955
.
- A. Othmani, N. Bouzidi, Y. Viano, Z. Alliche, H. Seridi, Y. Blache, M. El Hattab, J.-F. Briand and G. Culioli, J. Appl. Phycol., 2014, 26, 1573–1584, DOI:10.1007/s10811-013-0185-2
.
- C. Ireland and D. J. Faulkner, J. Org. Chem., 1977, 42, 3157–3162, DOI:10.1021/jo00439a010
.
- J. P. Barbosa, R. C. Pereira and J. L. Abrantes, Planta Med., 2004, 70, 856–860, DOI:10.1055/s-2004-827235
.
- A. Pardo-Vargas, I. de Barcelos Oliveira, P. R. S. Stephens, C. C. Cirne-Santos, I. C. N. de P. Paixão, F. A. Ramos, C. Jiménez, J. Rodríguez, J. A. L. C. Resende, V. L. Teixeira and L. Castellanos, Mar. Drugs, 2014, 12, 4247–4259, DOI:10.3390/md12074247
.
- K. H. Jang, B. H. Lee, B. W. Choi, H. S. Lee and J. Shin, J. Nat. Prod., 2005, 68, 716–723, DOI:10.1021/np058003i
.
- J.-I. Lee, B. J. Park, H. Kim and Y. Seo, Bull. Korean Chem. Soc., 2014, 35, 2867–2869, DOI:10.5012/bkcs.2014.35.9.2867
.
- W.-F. He, L.-G. Yao, H.-L. Liu and Y.-W. Guo, J. Asian Nat. Prod. Res., 2014, 16, 685–689, DOI:10.1080/10286020.2014.924511
.
- Y.-J. Jun, M. Lee, T. Shin, N. Yoon, J.-H. Kim and H.-R. Kim, Molecules, 2014, 19, 15638–15652, DOI:10.3390/molecules191015638
.
- S.-H. Eom, D.-S. Lee, Y.-J. Jung, J.-H. Park, J.-I. Choi, M.-J. Yim, J.-M. Jeon, H.-W. Kim, K.-T. Son, J.-Y. Je, M.-S. Lee and Y.-M. Kim, Appl. Microbiol. Biotechnol., 2014, 98, 9795–9804, DOI:10.1007/s00253-014-6041-8
.
- H. A. Jung, H. J. Jung, H. Y. Jeong, H. J. Kwon, M. Y. Ali and J. S. Choi, Fitoterapia, 2014, 92, 260–269, DOI:10.1016/j.fitote.2013.12.003
.
- F. Karadeniz, K.-H. Kang, J. W. Park, S.-J. Park and S.-K. Kim, Biosci., Biotechnol., Biochem., 2014, 78, 1151–1158, DOI:10.1080/09168451.2014.923282
.
- J.-S. Choi, K. Lee, B.-B. Lee, Y.-C. Kim, Y. D. Kim, Y.-K. Hong, K. K. Cho and I. S. Choi, Bot. Sci., 2014, 92, 425–431 CrossRef
.
- Y.-I. Yang, S.-H. Jung, K.-T. Lee and J.-H. Choi, Int. Immunopharmacol., 2014, 23, 460–468, DOI:10.1016/j.intimp.2014.09.019
.
- H.-J. Choi, J.-H. Park, B. H. Lee, H. Y. Chee, K. B. Lee and S.-M. Oh, Appl. Biochem. Biotechnol., 2014, 173, 957–967, DOI:10.1007/s12010-014-0910-6
.
- J. H. Kwak, Y. He, B. Yoon, S. Koo, Z. Yang, E. J. Kang, B. H. Lee, S.-Y. Han, Y. C. Yoo, K. B. Lee and J. S. Kim, Chem. Commun., 2014, 50, 13045–13048, 10.1039/c4cc04270k
.
- V.-T. Nguyen, Z.-J. Qian, B. Lee, S.-J. Heo, K.-N. Kim, Y.-J. Jeon, W. S. Park, I.-W. Choi, C. H. Jang, S.-C. Ko, S.-J. Park, Y.-T. Kim, G.-H. Kim, D.-S. Lee, M.-J. Yim, J.-Y. Je and W.-K. Jung, Algae, 2014, 29, 355–366, DOI:10.4490/algae.2014.29.4.355
.
- Y. Zhang, H. Fang, Q. Xie, J. Sun, R. Liu, Z. Hong, R. Yi and H. Wu, Molecules, 2014, 19, 2100–2113, DOI:10.3390/molecules19022100
.
- M.-C. Kang, K.-N. Kim, H. H. C. Lakmal, E.-A. Kim, W. A. J. P. Wijesinghe, X. Yang, S.-J. Heo and Y.-J. Jeon, Environ. Toxicol. Pharmacol., 2014, 38, 607–615, DOI:10.1016/j.etap.2014.08.001
.
- S.-H. Lee, S.-M. Kang, S.-C. Ko, S.-H. Moon, B.-T. Jeon, D. H. Lee and Y.-J. Jeon, Food Funct., 2014, 5, 2602–2608, 10.1039/c4fo00420e
.
- K. C. Kim, M. J. Piao, J. Zheng, C. W. Yao, J. W. Cha, M. H. S. R. Kumara, X. Han, H. K. Kang, N. H. Lee and J. W. Hyun, Biomol. Ther., 2014, 22, 301–307, DOI:10.4062/biomolther.2014.044
.
- G. Lopes, G. Daletos, P. Proksch, P. B. Andrade and P. Valentão, Mar. Drugs, 2014, 12, 1406–1418, DOI:10.3390/md12031406
.
- A. H. Banskota, R. Stefanova, S. Sperker, S. Lall, J. S. Craigie and J. T. Hafting, J. Appl. Phycol., 2014, 26, 1565–1571, DOI:10.1007/s10811-013-0174-5
.
- A. H. Banskota, R. Stefanova, S. Sperker, S. P. Lall, J. S. Craigie, J. T. Hafting and A. T. Critchley, Phytochemistry, 2014, 101, 101–108, DOI:10.1016/j.phytochem.2014.02.004
.
- T. Umezawa, Y. Oguri, H. Matsuura, S. Yamazaki, M. Suzuki, E. Yoshimura, T. Furuta, Y. Nogata, Y. Serisawa, K. Matsuyama-Serisawa, T. Abe, F. Matsuda, M. Suzuki and T. Okino, Angew. Chem., Int. Ed., 2014, 53, 3909–3912, DOI:10.1002/anie.201311175
.
- A. Gutiérrez-Cepeda, A. H. Daranas, J. J. Fernández, M. Norte and M. L. Souto, Mar. Drugs, 2014, 12, 4031–4044, DOI:10.3390/md12074031
.
- K. Kokkotou, E. Ioannou, M. Nomikou, F. Pitterl, A. Vonaparti, E. Siapi, M. Zervou and V. Roussis, Phytochemistry, 2014, 108, 208–219, DOI:10.1016/j.phytochem.2014.10.007
.
- C. A. Motti, P. Thomas-Hall, K. A. Hagiwara, C. J. Simmons, R. Willis and A. D. Wright, J. Nat. Prod., 2014, 77, 1193–1200, DOI:10.1021/np500059h
.
- X.-Q. Yu, W.-F. He, D.-Q. Liu, M.-T. Feng, Y. Fang, B. Wang, L.-H. Feng, Y.-W. Guo and S.-C. Mao, Phytochemistry, 2014, 103, 162–170, DOI:10.1016/j.phytochem.2014.03.021
.
- F. L. da Silva Machado, T. L. B. Ventura, L. M. de Souza Gestinari, V. Cassano, J. Antônio, L. C. Resende, C. s. R. Kaiser, E. B. Lasunskaia, M. F. Muzitano and A. R. Soares, Molecules, 2014, 19, 3181–3192, DOI:10.3390/molecules19033181
.
- R. F. Angawi, W. M. Alarif, R. I. Hamza, F. A. Badria and S.-E. N. Ayyad, Helv. Chim. Acta, 2014, 97, 1388–1395, DOI:10.1002/hlca.201300464
.
- M. Kladi, D. Ntountaniotis, M. Zervou, C. Vagias, E. Ioannou and V. Roussis, Tetrahedron Lett., 2014, 55, 2835–2837, DOI:10.1016/j.tetlet.2014.03.083
.
- H.-Y. Fang, S.-F. Chiou, C. Uvarani, Z.-H. Wen, C.-H. Hsu, Y.-C. Wu, W.-L. Wang, C.-C. Liaw and J.-H. Sheu, Bull. Chem. Soc. Jpn., 2014, 87, 1278–1280, DOI:10.1246/bcsj.20140165
.
- X. Xu, L. Yin, J. Gao, L. Gao and F. Song, Chem. Biodiversity, 2014, 11, 807–811, DOI:10.1002/cbdv.201300239
.
- S. Greff, M. Zubia, G. Genta-Jouve, L. Massi, T. Perez and O. P. Thomas, J. Nat. Prod., 2014, 77, 1150–1155, DOI:10.1021/np401094h
.
- S. Takahashi, M. Yasuda, T. Nakamura, K. Hatano, K. Matsuoka and H. Koshino, J. Org. Chem., 2014, 79, 9373–9380, DOI:10.1021/jo501228v
.
- L. R. de Carvalho, M. T. Fujii, N. F. Roque and J. H. G. Lago, Phytochemistry, 2006, 67, 1331–1335, DOI:10.1016/j.phytochem.2006.04.020
.
- A. G. González, J. Derias, J. D. Martin and C. Pérez, Tetrahedron Lett., 1974, 15, 1249–1250, DOI:10.1016/s0040-4039(01)82457-6
.
- A. E. Leung, R. Rubbiani, G. Gasser and K. L. Tuck, Org. Biomol. Chem., 2014, 12, 8239–8246, 10.1039/c4ob01662a
.
- N. Mihopoulos, C. Vagias, E. Mikros, M. Scoullos and V. Roussis, Tetrahedron Lett., 2001, 42, 3749–3752, DOI:10.1016/s0040-4039(01)00538-x
.
- C. V. Vogel, H. Pietraszkiewicz, O. M. Sabry, W. H. Gerwick, F. A. Valeriote and C. D. Vanderwal, Angew. Chem., Int. Ed., 2014, 53, 12205–12209, DOI:10.1002/anie.201407726
.
- J. H. Cardellina, S. B. Horsley, J. Clardy, S. R. Leftow and J. Meinwald, Can. J. Chem., 1982, 60, 2675–2677, DOI:10.1139/v82-384
.
- G. Kim, T.-i. Sohn, D. Kim and R. S. Paton, Angew. Chem., Int. Ed., 2014, 53, 272–276, DOI:10.1002/anie.201308077
.
- M. Kuniyoshi, P. G. Wahome, T. Miono, T. Hashimoto, M. Yokoyama, K. L. Shrestha and T. Higa, J. Nat. Prod., 2005, 68, 1314–1317, DOI:10.1021/np058004a
.
- C. Recsei, B. Chan and C. S. P. McErlean, J. Org. Chem., 2014, 79, 880–887, DOI:10.1021/jo402790x
.
- R. de Nys, A. D. Wright, G. M. König, O. Sticher and P. M. Alino, J. Nat. Prod., 1993, 56, 877–883, DOI:10.1021/np50096a011
.
- W. A. J. P. Wijesinghe, M.-C. Kang, W.-W. Lee, H.-S. Lee, T. Kamada, C. S. Vairappan and Y.-J. Jeon, Algae, 2014, 29, 333–341, DOI:10.4490/algae.2014.29.4.333
.
- W. A. J. P. Wijesinghe, E.-A. Kim, M.-C. Kang, W.-W. Lee, H.-S. Lee, C. S. Vairappan and Y.-J. Jeon, Environ. Toxicol. Pharmacol., 2014, 37, 110–117, DOI:10.1016/j.etap.2013.11.006
.
- A. Abdel-Lateff, W. M. Alarif, H. Z. Asfour, S.-E. N. Ayyad, A. Khedr, F. A. Badria and S. S. Al-lihaibi, Environ. Toxicol. Pharmacol., 2014, 37, 928–935, DOI:10.1016/j.etap.2014.03.005
.
- G. A. Mohamed, A. E. E. Abd-Elrazek, H. A. Hassanean, D. T. A. Youssef and R. van Soest, Phytochem. Lett., 2014, 9, 51–58, DOI:10.1016/j.phytol.2014.04.008
.
- C.-K. Kim, I.-H. Song, H. Y. Park, Y.-J. Lee, H.-S. Lee, C. J. Sim, D.-C. Oh, K.-B. Oh and J. Shin, J. Nat. Prod., 2014, 77, 1396–1403, DOI:10.1021/np500156n
.
- T. Kubota, H. Suzuki, A. Takahashi-Nakaguchi, J. Fromont, T. Gonoi and J. Kobayashi, RSC Adv., 2014, 4, 11073–11079, 10.1039/c3ra47796g
.
- C.-W. Chiu, H.-J. Su, M.-C. Lu, W.-H. Wang, J.-H. Sheu and J.-H. Su, Bull. Chem. Soc. Jpn., 2014, 87, 1231–1234, DOI:10.1246/bcsj.20140188
.
- S.-E. N. Ayyad, R. A. Alarif, E. A. Saqer and F. A. Badria, Z. Naturforsch., C: J. Biosci., 2014, 69, 117–123, DOI:10.5560/znc.2013-0088
.
- K. Takada, S. Okada and S. Matsunaga, Fish. Sci., 2014, 80, 1057–1064, DOI:10.1007/s12562-014-0776-0
.
- Y.-S. Juan, C.-C. Lee, C.-W. Tsao, M.-C. Lu, M. El-Shazly, H.-C. Shih, Y.-C. Chen, Y.-C. Wu and J.-H. Su, Int. J. Mol. Sci., 2014, 15, 16511–16521, DOI:10.3390/ijms150916511
.
- H. Kim, K.-J. Kim, J.-T. Yeon, S. H. Kim, D. H. Won, H. Choi, S.-J. Nam, Y.-J. Son and H. Kang, Mar. Drugs, 2014, 12, 2054–2065, DOI:10.3390/md12042054
.
- L.-F. Liang, T. Wang, Y.-S. Cai, W.-F. He, P. Sun, Y.-F. Li, Q. Huang, O. Taglialatela-Scafati, H.-Y. Wang and Y.-W. Guo, Eur. J. Med. Chem., 2014, 79, 290–297, DOI:10.1016/j.ejmech.2014.04.003
.
- Y. Hitora, K. Takada, S. Okada and S. Matsunaga, Tetrahedron, 2011, 67, 4530–4534, DOI:10.1016/j.tet.2011.04.085
.
- K. Mori, K. Akasaka and S. Matsunaga, Tetrahedron, 2014, 70, 392–401, DOI:10.1016/j.tet.2013.11.045
.
-
I. Carletti, G. Massiot and C. Debitus, WO 2011/051380 A1, 2011
.
- E. De Gussem, W. Herrebout, S. Specklin, C. Meyer, J. Cossy and P. Bultinck, Chem.–Eur. J., 2014, 20, 17385–17394, DOI:10.1002/chem.201404822
.
- G. Chianese, M. Persico, F. Yang, H.-W. Lin, Y.-W. Guo, N. Basilico, S. Parapini, D. Taramelli, O. Taglialatela-Scafati and C. Fattorusso, Bioorg. Med. Chem., 2014, 22, 4572–4580, DOI:10.1016/j.bmc.2014.07.034
.
- A. Trianto, N. J. de Voodg and J. Tanaka, J. Asian Nat. Prod. Res., 2014, 16, 163–168, DOI:10.1080/10286020.2013.844128
.
- M. Carbone, L. Núñez-Pons, M. L. Ciavatta, F. Castelluccio, C. Avila and M. Gavagnin, Nat. Prod. Commun., 2014, 9, 469–539 CAS
.
- D. P. Clark, J. Carroll, S. Naylor and P. Crews, J. Org. Chem., 1998, 63, 8757–8764, DOI:10.1021/jo980758p
.
- H. Tajima, T. Wakimoto, K. Takada, Y. Ise and I. Abe, J. Nat. Prod., 2014, 77, 154–158, DOI:10.1021/np400668k
.
- L. Coello, F. Reyes, M. J. Martín, C. Cuevas and R. Fernández, J. Nat. Prod., 2014, 77, 298–303, DOI:10.1021/np400888e
.
- M. Pelay-Gimeno, Y. Garcia-Ramos, M. J. Martin, J. Spengler, J. M. Molina-Guijarro, S. Munt, A. M. Francesch, C. Cuevas, J. Tulla-Puche and F. Albericio, Nat. Commun., 2013, 4, 2352, DOI:10.1038/ncomms3352
.
- M. J. Martín, R. Rodríguez-Acebes, Y. García-Ramos, V. Martínez, C. Murcia, I. Digón, I. Marco, M. Pelay-Gimeno, R. Fernández, F. Reyes, A. M. Francesch, S. Munt, J. Tulla-Puche, F. Albericio and C. Cuevas, J. Am. Chem. Soc., 2014, 136, 6754–6762, DOI:10.1021/ja502744a
.
- T. D. Tran, N. B. Pham, G. A. Fechner, J. N. A. Hooper and R. J. Quinn, Mar. Drugs, 2014, 12, 3399–3415, DOI:10.3390/md12063399
.
- K. C. Tan, T. Wakimoto and I. Abe, Org. Lett., 2014, 16, 3256–3259, DOI:10.1021/ol501271v
.
- X. Wang, B. I. Morinaka and T. F. Molinski, J. Nat. Prod., 2014, 77, 625–630, DOI:10.1021/np400891s
.
- K.-X. Zhan, W.-H. Jiao, F. Yang, J. Li, S.-P. Wang, Y.-S. Li, B.-N. Han and H.-W. Lin, J. Nat. Prod., 2014, 77, 2678–2684, DOI:10.1021/np5006778
.
- J. Song, J.-e. Jeon, T. H. Won, C. J. Sim, D.-C. Oh, K.-B. Oh and J. Shin, Mar. Drugs, 2014, 12, 2760–2770, DOI:10.3390/md12052760
.
- D. T. A. Youssef, L. A. Shaala, G. A. Mohamed, J. M. Badr, F. H. Bamanie and S. R. M. Ibrahim, Mar. Drugs, 2014, 12, 1911–1923, DOI:10.3390/md12041911
.
- H. Li, J. J. Bowling, M. Sua, J. Hong, B.-J. Lee, M. T. Hamann and J. H. Jung, Biochim. Biophys. Acta, Gen. Subj., 2014, 1840, 977–984, DOI:10.1016/j.bbagen.2013.11.001
.
- C.-D. Pham, R. Hartmann, P. Böhler, B. Stork, S. Wesselborg, W. Lin, D. Lai and P. Proksch, Org. Lett., 2014, 16, 266–269, DOI:10.1021/ol403241v
.
- D. Uemura, K. Takahashi, T. Yamamoto, C. Katayama, J. Tanaka, Y. Okumura and Y. Hirata, J. Am. Chem. Soc., 1985, 107, 4796–4798, DOI:10.1016/S0040-4039(00)98121-8
.
- Y. Hirata and D. Uemura, Pure Appl. Chem., 1986, 58, 701–710, DOI:10.1351/pac198658050701
.
- A. Ueda, A. Yamamoto, D. Kato and Y. Kishi, J. Am. Chem. Soc., 2014, 136, 5171–5176, DOI:10.1021/ja5013307
.
- Y. Chen, Y. Peng, C. Gao and R. Huang, Nat. Prod. Res., 2014, 28, 1010–1014, DOI:10.1080/14786419.2014.903397
.
- A. S. Dewi, T. A. Hadi, N. D. Fajarningsih, J. T. Blanchfield, P. V. Bernhardt and M. J. Garson, Aust. J. Chem., 2014, 67, 1205–1210, DOI:10.1071/ch14107
.
- A. Furusato, H. Kato, T. Nehira, K. Eguchi, T. Kawabata, Y. Fujiwara, F. Losung, R. E. P. Mangindaan, N. J. de Voogd, M. Takeya, H. Yokosawa and S. Tsukamoto, Org. Lett., 2014, 16, 3888–3891, DOI:10.1021/ol5015569
.
- A. H. El-Desoky, H. Kato, K. Eguchi, T. Kawabata, Y. Fujiwara, F. Losung, R. E. P. Mangindaan, N. J. de Voogd, M. Takeya, H. Yokosawa and S. Tsukamoto, J. Nat. Prod., 2014, 77, 1536–1540, DOI:10.1021/np500290a
.
- J.-Z. Sun, C.-S. Jiang, X.-Q. Chen, K.-S. Chen, X.-C. Zhen, R. W. M. van Soest and Y.-W. Guo, Tetrahedron, 2014, 70, 3166–3171, DOI:10.1016/j.tet.2014.03.051
.
- S. Matsunaga, R. Kishi, K. Otsuka, M. J. Fujita, M. Oikawa and R. Sakai, Org. Lett., 2014, 16, 3090–3093, DOI:10.1021/ol5011888
.
- W.-F. He, D.-Q. Xue, L.-G. Yao, J.-Y. Li, J. Li and Y.-W. Guo, Mar. Drugs, 2014, 12, 3982–3993, DOI:10.3390/md12073982
.
- N. Tanaka, R. Momose, A. Takahashi-Nakaguchi, T. Gonoi, J. Fromont and J. Kobayashi, Tetrahedron, 2014, 70, 832–837, DOI:10.1016/j.tet.2013.12.032
.
- E. Sakai, H. Kato, H. Rotinsulu, F. Losung, R. E. P. Mangindaan, N. J. de Voogd, H. Yokosawa and S. Tsukamoto, J. Nat. Med., 2014, 68, 215–219, DOI:10.1007/s11418-013-0778-8
.
- S. Khokhar, Y. Feng, M. R. Campitelli, M. G. Ekins, J. N. A. Hooper, K. D. Beattie, M. C. Sadowski, C. C. Nelson and R. A. Davis, Bioorg. Med. Chem. Lett., 2014, 24, 3329–3332, DOI:10.1016/j.bmcl.2014.05.104
.
- J. Dai, J. I. Jimenez, M. Kelly and P. G. Williams, J. Org. Chem., 2010, 75, 2399–2402, DOI:10.1021/jo902566n
.
- A. Skiredj, M. A. Beniddir, D. Joseph, K. Leblanc, G. Bernadat, L. Evanno and E. Poupon, Angew. Chem., Int. Ed., 2014, 53, 6419–6424, DOI:10.1002/anie.201403454
.
- M. Arai, C. Han, Y. Yamano, A. Setiawan and M. Kobayashi, J. Nat. Med., 2014, 68, 372–376, DOI:10.1007/s11418-013-0811-y
.
- H.-B. Yu, F. Yang, F. Sun, J. Li, W.-H. Jiao, J.-H. Gan, W.-Z. Hu and H.-W. Lin, Mar. Drugs, 2014, 12, 6003–6013, DOI:10.3390/md12126003
.
- H.-B. Yu, F. Yang, F. Sun, G.-Y. Ma, J.-H. Gan, W.-Z. Hu, B.-N. Han, W.-H. Jiao and H.-W. Lin, J. Nat. Prod., 2014, 77, 2124–2129, DOI:10.1021/np500583z
.
- T. D. Tran, N. B. Pham and R. J. Quinn, Eur. J. Org. Chem., 2014, 2014, 4805–4816, DOI:10.1002/ejoc.201402372
.
- A. Ahond, M. B. Zurita, M. Colin, C. Fizames, P. Laboute, F. Lavelle, D. Laurent, C. Poupat, J. Pusset and M. Pusset, C. R. Acad. Sci., Ser. II: Mec., Phys., Chim., Sci. Terre Univers, 1988, 307, 145–148 CAS
.
- S.-Y. Fung, V. Sofiyev, J. Schneiderman, A. F. Hirschfeld, R. E. Victor, K. Woods, J. S. Piotrowski, R. Deshpande, S. C. Li, N. J. de Voogd, C. L. Myers, C. Boone, R. J. Andersen and S. E. Turvey, ACS Chem. Biol., 2014, 9, 247–257, DOI:10.1021/cb400740c
.
- T. N. Makarieva, E. K. Ogurtsova, V. A. Denisenko, P. S. Dmitrenok, K. M. Tabakmakher, A. G. Guzii, E. A. Pislyagin, A. A. Es'kov, V. B. Kozhemyako, D. L. Aminin, Y.-M. Wang and V. A. Stonik, Org. Lett., 2014, 16, 4292–4295, DOI:10.1021/ol502013f
.
- T. Grkovic, J. Blees, M. Bayer, N. Colburn, C. Thomas, C. Henrich, M. Peach, J. McMahon, T. Schmid and K. Gustafson, Mar. Drugs, 2014, 12, 4593–4601, DOI:10.3390/md12084593
.
- E. Gros, A. Al-Mourabit, M.-T. Martin, J. Sorres, J. Vacelet, M. Frederich, M. Aknin, Y. Kashman and A. Gauvin-Bialecki, J. Nat. Prod., 2014, 77, 818–823, DOI:10.1021/np4009283
.
- F. Plisson, P. Prasad, X. Xiao, A. M. Piggott, X.-c. Huang, Z. Khalil and R. J. Capon, Org. Biomol. Chem., 2014, 12, 1579–1584, 10.1039/c4ob00091a
.
- T. Kusama, N. Tanaka, A. Takahashi-Nakaguchi, T. Gonoi, J. Fromont and J. Kobayashi, Chem. Pharm. Bull., 2014, 62, 499–503, DOI:10.1248/cpb.c14-00077
.
- T. Kusama, N. Tanaka, K. Sakai, T. Gonoi, J. Fromont, Y. Kashiwada and J. Kobayashi, Org. Lett., 2014, 16, 3916–3918, DOI:10.1021/ol501664b
.
- T. Kusama, N. Tanaka, K. Sakai, T. Gonoi, J. Fromont, Y. Kashiwada and J. Kobayashi, Org. Lett., 2014, 16, 5176–5179, DOI:10.1021/ol502528m
.
- S. Urban, P. D. Leone, A. R. Carroll, G. A. Fechner, J. Smith, J. N. A. Hooper and R. J. Quinn, J. Org. Chem., 1999, 64, 731–735, DOI:10.1021/jo981034g
.
- R. A. Rodriguez, D. B. Steed, Y. Kawamata, S. Su, P. A. Smith, T. C. Steed, F. E. Romesberg and P. S. Baran, J. Am. Chem. Soc., 2014, 136, 15403–15413, DOI:10.1021/ja508632y
.
- R. P. Walker, D. J. Faulkner, D. van Engen and J. Clardy, J. Am. Chem. Soc., 1981, 103, 6772–6773, DOI:10.1021/ja00412a052
.
- M. Assmann and M. Köck, Z. Naturforsch., C: J. Biosci., 2002, 57, 157–160 CAS
, URL: http://www.znaturforsch.com/ac/v57c/s57c0157.pdf.
- P. A. Keifer, R. E. Schwartz, M. E. S. Koker, R. G. Hughes, D. Rittschof and K. L. Rinehart, J. Org. Chem., 1991, 56, 2965–2975, DOI:10.1021/jo00009a008
.
- J. Kobayashi, M. Tsuda, T. Murayama, H. Nakamura, Y. Ohizumi, M. Ishibashi, M. Iwamura, T. Ohta and S. Nozoe, Tetrahedron, 1990, 46, 5579–5586, DOI:10.1016/s0040-4020(01)87756-5
.
- S. Nishimura, S. Matsunaga, M. Shibazaki, K. Suzuki and K. Furihata, Org. Lett., 2003, 5, 2255–2257, DOI:10.1021/ol034564u
.
- A. Grube, S. Immel, P. S. Baran and M. Köck, Angew. Chem., Int. Ed., 2007, 46, 6721–6724, DOI:10.1002/anie.200701935
.
- R. B. Kinnel, H. Gehrken, R. Swali, G. Skoropowski and P. J. Scheuer, J. Org. Chem., 1998, 63, 3281–3286, DOI:10.1021/jo971987z
.
- Z. Ma, X. Wang, X. Wang, R. A. Rodriguez, C. E. Moore, S. Gao, X. Tan, Y. Ma, A. L. Rheingold, P. S. Baran and C. Chen, Science, 2014, 346, 219–224, DOI:10.1126/science.1255677
.
- L.-W. Tian, Y. Feng, Y. Shimizu, T. A. Pfeifer, C. Wellington, J. N. A. Hooper and R. J. Quinn, Bioorg. Med. Chem. Lett., 2014, 24, 3537–3540, DOI:10.1016/j.bmcl.2014.05.054
.
- L.-W. Tian, Y. Feng, Y. Shimizu, T. Pfeifer, C. Wellington, J. N. A. Hooper and R. J. Quinn, J. Nat. Prod., 2014, 77, 1210–1214, DOI:10.1021/np500119e
.
- M. M. K. Kumar, J. D. Naik, K. Satyavathi, H. Ramana, P. R. Varma, K. P. Nagasree, D. Smitha and D. V. Rao, Nat. Prod. Res., 2014, 28, 888–894, DOI:10.1080/14786419.2014.891112
.
- B. Wang, Y. Lin, Y. Chen and R. Huang, Nat. Prod. Commun., 2014, 9, 471–473 CAS
.
- M. Farrugia, N. Trotter, S. Vijayasarathy, A. A. Salim, Z. G. Khalil, E. Lacey and R. J. Capon, Tetrahedron Lett., 2014, 55, 5902–5904, DOI:10.1016/j.tetlet.2014.08.116
.
- D. R. Abou-Hussein, J. M. Badr and D. T. A. Youssef, Nat. Prod. Res., 2014, 28, 1134–1141, DOI:10.1080/14786419.2014.915828
.
- Q. Göthel and M. Köck, Beilstein J. Org. Chem., 2014, 10, 613–621, DOI:10.3762/bjoc.10.52
.
- N. K. Utkin and V. A. Denisenko, Nat. Prod. Commun., 2014, 9, 757–765 Search PubMed
.
- W.-H. Jiao, J. Li, Q. Liu, T.-T. Xu, G.-H. Shi, H.-B. Yu, F. Yang, B.-N. Han, M. Li and H.-W. Lin, Molecules, 2014, 19, 18025–18032, DOI:10.3390/molecules191118025
.
- W.-H. Jiao, T.-T. Xu, H.-B. Yu, F.-R. Mu, J. Li, Y.-S. Li, F. Yang, B.-N. Hana and H.-W. Lin, RSC Adv., 2014, 4, 9236–9246, 10.1039/c3ra47265e
.
- W.-H. Jiao, T.-T. Xu, H.-B. Yu, G.-D. Chen, X.-J. Huang, F. Yang, Y.-S. Li, B.-N. Han, X.-Y. Liu and H.-W. Lin, J. Nat. Prod., 2014, 77, 346–350, DOI:10.1021/np4009392
.
- G. Daletos, N. J. de Voogd, W. E. G. Müller, V. Wray, W. H. Lin, D. Feger, M. Kubbutat, A. H. Aly and P. Proksch, J. Nat. Prod., 2014, 77, 218–226, DOI:10.1021/np400633m
.
- M. Yamakuma, H. Kato, K. Matsuo, A. H. El-Desoky, T. Kawabata, F. Losung, R. E. P. Mangindaan, N. J. de Voogd, H. Yokosawa and S. Tsukamoto, Heterocycles, 2014, 89, 2605–2610, DOI:10.3987/com-14-13087
.
- R. M. Centko, A. Steinø, F. I. Rosell, B. O. Patrick, N. de Voogd, A. G. Mauk and R. J. Andersen, Org. Lett., 2014, 16, 6480–6483, DOI:10.1021/ol503337f
.
- A. Bisio, E. Fedele, A. Pittaluga, G. Olivero, M. Grilli, J. Chen, G. Mele, N. Malafronte, N. De Tommasi, F. Leddae, R. Manconi, R. Pronzato and M. Marchi, Nat. Prod. Commun., 2014, 9, 1581–1585 Search PubMed
.
- W. Cheng, D. Liu, F. Zhang, Q. Zhang, P. Pedpradab, P. Proksch, H. Liang and W. Lin, Tetrahedron, 2014, 70, 3576–3583, DOI:10.1016/j.tet.2014.04.008
.
- D. Hahn, J. Chin, H. Kim, I. Yang, D. H. Won, M. Ekins, H. Choi, S.-J. Nam and H. Kang, Tetrahedron Lett., 2014, 55, 4716–4719, DOI:10.1016/j.tetlet.2014.07.019
.
- W. Liu, K.-J. Liang, C.-Y. Chiang, M.-C. Lu and J.-H. Su, Chem. Pharm. Bull., 2014, 62, 392–394, DOI:10.1248/cpb.c13-00851
.
- P. V. Kiem, C. V. Minh, N. X. Nhiem, N. T. Cuc, N. V. Quang, H. L. T. Anh, B. H. Tai, P. H. Yen, N. T. Hoai, K. Y. Ho, N. Kim, S. J. Park and S. H. Kim, Magn. Reson. Chem., 2014, 52, 51–56, DOI:10.1002/mrc.4030
.
- J.-R. Rho, J. Korean Magn. Reson. Soc., 2014, 18, 82–88, DOI:10.6564/JKMRS.2014.18.2.082
.
- F. Yang, Y. Zou, R.-P. Wang, M. Hamann, H.-J. Zhang, W.-H. Jiao, B.-N. Han, S.-J. Song and H.-W. Lin, Mar. Drugs, 2014, 12, 4399–4416, DOI:10.3390/md12084399
.
- S. M. Parrish, W. Y. Yoshida, T. P. Kondratyuk, E.-J. Park, J. M. Pezzuto, M. Kelly and P. G. Williams, J. Nat. Prod., 2014, 77, 1644–1649, DOI:10.1021/np500256w
.
- J. M. Wojnar, K. O. Dowle and P. T. Northcote, J. Nat. Prod., 2014, 77, 2288–2295, DOI:10.1021/np500549g
.
- S.-J. Piao, W.-H. Jiao, F. Yang, Y.-H. Yi, Y.-T. Di, B.-N. Han and H.-W. Lin, Mar. Drugs, 2014, 12, 4096–4109, DOI:10.3390/md12074096
.
- C. Festa, C. Cassiano, M. V. D'Auria, C. Debitus, M. C. Monti and S. De Marino, Org. Biomol. Chem., 2014, 12, 8646–8655, 10.1039/c4ob01510j
.
- Y.-J. Lee, J.-W. Lee, D.-G. Lee, H.-S. Lee, J. Kang and J. Yun, Int. J. Mol. Sci., 2014, 15, 20045–20053, DOI:10.3390/ijms151120045
.
- L. Yin, H. Li, X. Chen and Y. Qiu, Rec. Nat. Prod., 2014, 8, 417–421 Search PubMed
, URL: http://www.acgpubs.org/RNP/2014/Volume8/Issue%201/58-RNP-1309-433.pdf.
- F. Yang, J.-H. Gan, X.-Y. Liu and H.-W. Lin, Nat. Prod. Commun., 2014, 9, 763–767 CAS
.
- J.-K. Woo, C.-K. Kim, S.-H. Kim, H. Kim, D.-C. Oh, K.-B. Oh and J. Shin, Org. Lett., 2014, 16, 2826–2829, DOI:10.1021/ol500868s
.
- Y. Lee, W. Wang, H. Kim, A. G. Giri, D. H. Won, D. Hahn, K. R. Baek, J. Lee, I. Yang, H. Choi, S.-J. Nam and H. Kang, Bioorg. Med. Chem. Lett., 2014, 24, 4095–4098, DOI:10.1016/j.bmcl.2014.07.066
.
- C. Viegelmann, J. Parker, T. Ooi, C. Clements, G. Abbott, L. Young, J. Kennedy, A. D. W. Dobson and R. Edrada-Ebel, Mar. Drugs, 2014, 12, 2937–2952, DOI:10.3390/md12052937
.
- V. Sepe, C. D'Amore, R. Ummarino, B. Renga, M. V. D'Auria, E. Novellino, A. Sinisi, O. Taglialatela-Scafati, Y. Nakao, V. Limongelli, A. Zampella and S. Fiorucci, Eur. J. Med. Chem., 2014, 73, 126–134, DOI:10.1016/j.ejmech.2013.12.005
.
- S. N. Sunassee, T. Ransom, C. J. Henrich, J. A. Beutler, D. G. Covell, J. B. McMahon and K. R. Gustafson, J. Nat. Prod., 2014, 77, 2475–2480, DOI:10.1021/np500556t
.
- I. Yang, H. Choi, D. H. Won, S.-J. Nam and H. Kang, Bull. Korean Chem. Soc., 2014, 35, 3360–3362, DOI:10.5012/bkcs.2014.35.11.3360
.
- G. Chianese, V. Sepe, V. Limongelli, B. Renga, C. D'Amore, A. Zampella, O. Taglialatela-Scafati and S. Fiorucci, Steroids, 2014, 83, 80–85, DOI:10.1016/j.steroids.2014.02.003
.
- J. Gong, P. Sun, N. Jiang, R. Riccio, G. Lauro, G. Bifulco, T.-J. Li, W. H. Gerwick and W. Zhang, Org. Lett., 2014, 16, 2224–2227, DOI:10.1021/ol5007345
.
- K. Machida, T. Abe, D. Arai, M. Okamoto, I. Shimizu, N. J. de Voogd, N. Fusetani and Y. Nakao, Org. Lett., 2014, 16, 1539–1541, DOI:10.1021/ol5000023
.
- D.-J. Jin, S.-A. Tang, G.-S. Xing, W.-J. Zhao, C. Zhao, H.-Q. Duan and W.-H. Lin, J. Asian Nat. Prod. Res., 2014, 16, 427–433, DOI:10.1080/10286020.2014.911288
.
- S.-E. N. Ayyad, R. F. Angawi, E. Saqer, A. Abdel-Lateff and F. A. Badria, Pharmacogn. Mag., 2014, 10, 334–341, DOI:10.4103/0973-1296.133292
.
- A. Zampella, C. Giannini, C. Debitus, C. Roussakis and M. V. D'Auria, J. Nat. Prod., 1999, 62, 332–334, DOI:10.1021/np9803225
.
- J. J. La Clair, S. T. Loveridge, K. Tenney, M. O'Neil-Johnson, E. Chapman and P. Crews, PLoS One, 2014, 9, e100474, DOI:10.1371/journal.pone.0100474
.
- G. D. Sala, T. Hochmuth, R. Teta, V. Costantino and A. Mangoni, Mar. Drugs, 2014, 12, 5425–5440, DOI:10.3390/md12115425
.
- S. Sakemi, T. Ichiba, S. Kohmoto, G. Saucy and T. Higa, J. Am. Chem. Soc., 1988, 110, 4851–4853, DOI:10.1021/ja00222a068
.
- T. Hamada, S. Matsunaga, G. Yano and N. Fusetani, J. Am. Chem. Soc., 2005, 127, 110–118, DOI:10.1021/ja045749e
.
- J. Kobayashi, M. Sato, M. Ishibashi, H. Shigemori, T. Nakamura and Y. Ohizumi, J. Chem. Soc., Perkin Trans. 1, 1991, 10, 2609–2611, 10.1039/p19910002609
.
- N. Fusetani, S. Matsunaga, H. Matsumoto and Y. Takebayashi, J. Am. Chem. Soc., 1990, 112, 7053–7054, DOI:10.1021/ja00175a045
.
- D. G. Corley, R. Herb, R. E. Moore, P. J. Scheuer and V. J. Paul, J. Org. Chem., 1988, 53, 3644–3646, DOI:10.1021/jo00250a053
.
- L. M. West, P. T. Northcote and C. N. Battershill, J. Org. Chem., 2000, 65, 445–449, DOI:10.1021/jo991296y
.
- A. E. Prota, K. Bargsten, P. T. Northcote, M. Marsh, K.-H. Altmann, J. H. Miller, J. F. Diaz and M. O. Steinmetz, Angew. Chem., Int. Ed., 2014, 53, 1621–1625, DOI:10.1002/anie.201307749
.
- H. Nakamura, J. Kobayashi, Y. Ohizumi and Y. Hirata, Tetrahedron Lett., 1982, 23, 5555–5558, DOI:10.1016/s0040-4039(00)85893-1
.
- F. Funk, K. Krüger, C. Henninger, W. Wätjen, P. Proksch, J. Thomale and G. Fritz, Anti-Cancer Drugs, 2014, 25, 917–929, DOI:10.1097/cad.0000000000000119
.
- M.-J. Kim, S. W. Woo, M.-S. Kim, J.-E. Park and J.-K. Hwang, J. Asian Nat. Prod. Res., 2014, 16, 1139–1147, DOI:10.1080/10286020.2014.983092
.
- R. Capon, E. L. Ghisalberti, P. R. Jefferies, B. W. Skelton and A. H. White, J. Chem. Soc., Perkin Trans. 1, 1981, 2464–2467, 10.1039/p19810002464
.
- K. A. Salam, A. Furuta, N. Noda, S. Tsuneda, Y. Sekiguchi, A. Yamashita, K. Moriishi, M. Nakakoshi, H. Tani, S. R. Roy, J. Tanaka, M. Tsubuki and N. Akimitsu, Molecules, 2014, 19, 4006–4020, DOI:10.3390/molecules19044006
.
- M. Xu, R. A. Davis, Y. J. Feng, M. L. Sykes, T. Shelper, V. M. Avery, D. Camp and R. J. Quinn, J. Nat. Prod., 2012, 75, 1001–1005, DOI:10.1021/np300147d
.
- M. S. Buchanan, A. R. Carroll, G. A. Fechner, A. Boyle, M. M. Simpson, R. Addepalli, V. M. Avery, J. N. A. Hooper, N. Su, H. Chen and R. J. Quinn, Bioorg. Med. Chem. Lett., 2007, 17, 6860–6863, DOI:10.1016/j.bmcl.2007.10.021
.
- R. A. Davis, D. Vullo, C. T. Supuran and S.-A. Poulsen, BioMed Res. Int., 2014, 2014, 374079, DOI:10.1155/2014/374079
.
- R. Kazlauskas, P. T. Murphy, R. J. Quinn and R. J. Wells, Tetrahedron Lett., 1976, 2631–2634, DOI:10.1016/s0040-4039(00)91753-2
.
- C. Cassiano, R. Esposito, A. Tosco, A. Zampella, M. V. D'Auria, R. Riccio, A. Casapullo and M. C. Monti, Chem. Commun., 2014, 50, 406–408, 10.1039/C3CC45454A
.
- S. Urban and R. J. Capon, Lipids, 1997, 32, 675–677, DOI:10.1007/s11745-997-0086-0
.
- A. M. Langseter, Y. Stenstrøm and L. Skattebøl, Molecules, 2014, 19, 3804–3812, DOI:10.3390/molecules19033804
.
- K. Watanabe, Y. Tsuda, Y. Yamane, K. Takahashi, K. Iguchi, H. Naoki and T. Fujita, Tennen Yuki Kagobutsu Toronkai Koen Yoshishu, 2000, 42, 367–372 Search PubMed
.
- N. G. Shallu, G. L. Kad and J. Singh, Nat. Prod. Res., 2014, 28, 424–430, DOI:10.1080/14786419.2013.867855
.
- S. Sirirath, J. Tanaka, I. I. Ohtani, T. Ichiba, R. Rachmat, K. Ueda, T. Usui, H. Osada and T. Higa, J. Nat. Prod., 2002, 65, 1820–1823, DOI:10.1021/np0200865
.
- K. M. Reddy, J. Shashidhar and S. Ghosh, Org. Biomol. Chem., 2014, 12, 4002–4012, 10.1039/c4ob00250d
.
- K. W. L. Yong, J. D. de Voss, J. N. A. Hooper and M. J. Garson, J. Nat. Prod., 2011, 74, 194–207, DOI:10.1021/np100620x
.
- H. Sugimura, S. Sato, K. Tokudome and T. Yamada, Org. Lett., 2014, 16, 3384–3387, DOI:10.1021/ol501446w
.
- Y. Chen, K. B. Killday, P. J. McCarthy, R. Schmoler, K. Chilson, C. Selitrennikoff, S. A. Pomponi and A. E. Wright, J. Nat. Prod., 2001, 64, 262–264, DOI:10.1021/np000368
.
- C. Jimenez-Romero, I. Ortiz, J. Vicente, B. Vera, A. D. Rodriguez, S. Nam and R. Jove, J. Nat. Prod., 2010, 73, 1694–1700, DOI:10.1021/np100461t
.
- X.-Y. Tian, J.-W. Han, Q. Zhao and H. N. C. Wong, Org. Biomol. Chem., 2014, 12, 3686–3700, 10.1039/c4ob00448e
.
- C. Festa, S. De Marino, M. V. D'Auria, E. Deharo, G. Gonzalez, C. Deyssard, S. Petek, G. Bifulco and A. Zampella, Tetrahedron, 2012, 68, 10157–10163, DOI:10.1016/j.tet.2012.09.106
.
- C. M. Rasik and M. K. Brown, Angew. Chem., Int. Ed., 2014, 53, 14522–14526, DOI:10.1002/anie.201408055
.
- A. Bishara, A. Rudi, M. Aknin, D. Neumann, N. Ben-Califa and Y. Kashman, Org. Lett., 2008, 10, 4307–4309, DOI:10.1021/ol801750y
.
- J. N. deGruyter and W. A. Maio, Org. Lett., 2014, 16, 5196–5199, DOI:10.1021/ol5025585
.
- Y. J. Feng, A. R. Carroll, D. M. Pass, J. K. Archbold, V. M. Avery and R. J. Quinn, J. Nat. Prod., 2008, 71, 8–11, DOI:10.1021/np070094r
.
- G. Santhakumar and R. J. Payne, Org. Lett., 2014, 16, 4500–4503, DOI:10.1021/ol502045u
.
- M. V. D'Auria, V. Sepe, R. D'Orsi, F. Bellotta, C. Debitus and A. Zampella, Tetrahedron, 2007, 63, 131–140, DOI:10.1016/j.tet.2006.10.032
.
- M. Kikuchi and H. Konno, Org. Lett., 2014, 16, 4324–4327, DOI:10.1021/ol5020619
.
- X. Luo, F. Li, J. Hong, C.-O. Lee, C. J. Sim, K. S. Im and J. H. Jung, J. Nat. Prod., 2006, 69, 567–571, DOI:10.1021/np0503552
.
- R. Towada and S. Kuwahara, Tetrahedron, 2014, 70, 3774–3781, DOI:10.1016/j.tet.2014.04.040
.
- Y. Naka, T. Maki and S. Matsunaga, Tennen Yuki Kagobutsu Toronkai Koen Yoshishu, 2000, 42, 19–24 Search PubMed
.
- Y. Gao, Q. Shan, J. Liu, L. Wang and Y. Du, Org. Biomol. Chem., 2014, 12, 2071–2079, 10.1039/c3ob42364f
.
- H. Sorek, A. Rudi, M. Aknin, E. M. Gaydou and Y. Kashman, J. Nat. Prod., 2010, 73, 456–458, DOI:10.1021/np900500c
.
- M. Amat, G. Guignard, N. Llor and J. Bosch, J. Org. Chem., 2014, 79, 2792–2802, DOI:10.1021/jo5002627
.
- T. Kubota, T. Nishi, E. Fukushi, J. Kawabata, J. Fromont and J. Kobayashi, Tetrahedron Lett., 2007, 48, 4983–4985, DOI:10.1016/j.tetlet.2007.05.121
.
- S. G. Davies, A. M. Fletcher, P. M. Roberts, R. S. Shah, A. L. Thompson and J. E. Thomson, Org. Lett., 2014, 16, 1354–1357, DOI:10.1021/ol500096r
.
- R. A. Davis, S. Duffy, S. Fletcher, V. M. Avery and R. J. Quinn, J. Org. Chem., 2013, 78, 9608–9613, DOI:10.1021/jo400988y
.
- R. H. Pouwer, S. M. Deydier, P. V. Le, B. D. Schwartz, N. C. Franken, R. A. Davis, S. A. Charman, M. D. Edstein, T. S. Skinner-Adams, K. T. Andrews, I. D. Jenkins and R. J. Quinn, ACS Med. Chem. Lett., 2014, 5, 178–182, DOI:10.1021/ml400447v
.
- M. Kuramoto, N. Miyake, Y. Ishimaru, N. Ono and H. Uno, Org. Lett., 2008, 10, 5465–5468, DOI:10.1021/ol802263j
.
- M. Iwata, K. Kanoh, T. Imaoka and K. Nagasawa, Chem. Commun., 2014, 50, 6991–6994, 10.1039/c4cc00137k
.
- F. A. Khan, S. Ahmad, N. Kodipelli, G. Shivange and R. Anindya, Org. Biomol. Chem., 2014, 12, 3847–3865, 10.1039/c3ob42537a
.
- C. Pieri, D. Borselli, C. Di Giorgio, M. De Méo, J.-M. Bolla, N. Vidal, S. Combes and J. M. Brunel, J. Med. Chem., 2014, 57, 4263–4272, DOI:10.1021/jm500194e
.
- N. Fusetani, Y. Masuda, Y. Nakao and S. Matsunaga, Tetrahedron, 2001, 57, 7507–7511, DOI:10.1016/S0040-4020(01)00735-9
.
- I. Mancini, G. Guella, P. Laboute, C. Debitus and F. Pietra, J. Chem. Soc., Perkin Trans. 1, 1993, 3121–3125, 10.1039/p19930003121
.
- H. Kubo, K. Matsui, T. Saitoh and S. Nishiyama, Tetrahedron, 2014, 70, 6392–6397, DOI:10.1016/j.tet.2014.07.049
.
- P. A. Searle and T. F. Molinski, J. Org. Chem., 1995, 60, 4296–4298, DOI:10.1021/jo00118a059
.
- H. Ding, W. Li, Z. Ruan, R. Yang, Z. Mao, Q. Xiao and J. Wu, Beilstein J. Org. Chem., 2014, 10, 1681–1685, DOI:10.1002/ejoc.201403091
.
- Z. V. Peitsinis, D. A. Melidou, J. G. Stefanakis, H. Evgenidou and A. E. Koumbis, Eur. J. Org. Chem., 2014, 2014, 8160–8166, DOI:10.3762/bjoc.10.176
.
- R. Kazlauskas, P. T. Murphy, R. G. Warren, R. J. Wells and J. F. Blount, Aust. J. Chem., 1978, 31, 2685–2697, DOI:10.1071/ch9782685
.
- A. Fernández, E. Alvarez, R. Alvarez-Manzaneda, R. Chahboun and E. Alvarez-Manzaneda, Chem. Commun., 2014, 50, 13100–13102, 10.1039/c4cc05116e
.
- S. Urban and R. J. Capon, Aust. J. Chem., 1996, 49, 611–615, DOI:10.1071/ch9960611
.
- M. Göhl and K. Seifert, Eur. J. Org. Chem., 2014, 2014, 6975–6982, DOI:10.1002/ejoc.201402873
.
- R. M. Rosser and D. J. Faulkner, J. Org. Chem., 1984, 49, 5157–5160, DOI:10.1021/jo00200a029
.
- M. Gans and F. Bracher, Tetrahedron, 2014, 70, 1084–1090, DOI:10.1016/j.tet.2013.11.065
.
- R. A. Keyzers, J. Daoust, M. T. Davies-Coleman, R. Van Soest, A. Balgi, E. Donohue, M. Roberge and R. J. Andersen, Org. Lett., 2008, 10, 2959–2962, DOI:10.1021/ol800937u
.
- S.-S. Wang, Y. Shi and W.-S. Tian, Org. Lett., 2014, 16, 2177–2179, DOI:10.1021/ol500727c
.
- S. J. Robinson, E. K. Hoobler, M. Riener, S. T. Loveridge, K. Tenney, F. A. Valeriote, T. R. Holman and P. Crews, J. Nat. Prod., 2009, 72, 1857–1863, DOI:10.1021/np900465e
.
- E. Boulifa, A. Fernández, E. Alvarez, R. Alvarez-Manzaneda, A. I. Mansour, R. Chahboun and E. Alvarez-Manzaneda, J. Org. Chem., 2014, 79, 10689–10695, DOI:10.1021/jo502048y
.
- M. Tsoukatou, H. Siapi, C. Vagias and V. Roussis, J. Nat. Prod., 2003, 66, 444–446, DOI:10.1021/np020471u
.
- T. Tonoi, K. Mameda, M. Fujishiro and Y. Yoshinaga, Beilstein J. Org. Chem., 2014, 10, 2421–2427, DOI:10.3762/bjoc.10.252
.
- D. L. Dixson, D. Abrego and M. E. Hay, Science, 2014, 345, 892–897, DOI:10.1126/science.1255057
.
- C. Gao, L. Lin, B. Long, Y. Chen, B. He, H. Sun and R. Huang, Nat. Prod. Res., 2014, 28, 473–476, DOI:10.1080/14786419.2013.879134
.
- Z.-H. Sun, Y.-H. Cai, C.-Q. Fan, G.-H. Tang, H.-B. Luo and S. Yin, Mar. Drugs, 2014, 12, 672–681, DOI:10.3390/md12020672
.
- C. Audoin, V. Cocandeau, O. Thomas, A. Bruschini, S. Holderith and G. Genta-Jouve, Metabolites, 2014, 4, 421–432, DOI:10.3390/metabo4020421
.
- F. Cen-Pacheco, M. Norte, J. J. Fernández and A. H. Daranas, Org. Lett., 2014, 16, 2880–2883, DOI:10.1021/ol500860v
.
- F. Cen-Pacheco, M. Martín, J. Fernández and A. H. Daranas, Mar. Drugs, 2014, 12, 5188–5196, DOI:10.3390/md12105188
.
- Y.-B. Cheng, C.-C. Lan, W.-C. Liu, W.-C. Lai, Y.-C. Tsai, M. Y. Chiang, Y.-C. Wu and F.-R. Chang, Tetrahedron Lett., 2014, 55, 5369–5372, DOI:10.1016/j.tetlet.2014.07.101
.
- P. Ciminiello, C. Dell'Aversano, E. D. Iacovo, M. Forino, L. Tartaglione, M. Pelin, S. Sosa, A. Tubaro, O. Chaloin, M. Poli and G. Bignami, J. Nat. Prod., 2014, 77, 351–357, DOI:10.1021/np4009514
.
- P. Ciminiello, Chem. Res. Toxicol., 2009, 22, 1851–1859, DOI:10.1021/tx900259v
.
- P. Ciminiello, C. Dell'Aversano, E. D. Iacovo, M. Forino, A. Randazzo and L. Tartaglione, J. Org. Chem., 2014, 79, 72–79, DOI:10.1021/jo4022953
.
- J. Zhang, Y. Liang, X.-J. Liao, Z. Deng and S.-H. Xu, Nat. Prod. Res., 2014, 28, 150–155, DOI:10.1080/14786419.2013.857668
.
- B. Yang, X. Wei, J. Huang, X. Lin, J. Liu, S. Liao, J. Wang, X. Zhou, L. Wang and Y. Liu, Mar. Drugs, 2014, 12, 5316–5327, DOI:10.3390/md12105316
.
- J. Zhang, Y. Liang, L.-C. Li and S.-H. Xu, Helv. Chim. Acta, 2014, 97, 128–136, DOI:10.1002/hlca.201300296
.
- P. Georgantea, E. Ioannou, C. Vagias and V. Roussis, Phytochem. Lett., 2014, 8, 86–91, DOI:10.1016/j.phytol.2014.02.006
.
- H.-M. Chung, W.-H. Wang, T.-L. Hwang, J.-J. Chen, L.-S. Fang, Z.-H. Wen, Y.-B. Wang, Y.-C. Wu and P.-J. Sung, Int. J. Mol. Sci., 2014, 15, 15679–15688, DOI:10.3390/ijms150915679
.
- H.-M. Chung, W.-H. Wang, T.-L. Hwang, L.-S. Fang, Z.-H. Wen, J.-J. Chen, Y.-C. Wu and P.-J. Sung, Mar. Drugs, 2014, 12, 5856–5863, DOI:10.3390/md12125856
.
- H.-M. Chung, W.-H. Wang, T.-L. Hwang, J.-J. Li, L.-S. Fang, Y.-C. Wu and P.-J. Sung, Molecules, 2014, 19, 12320–12327, DOI:10.3390/molecules190812320
.
- Y.-J. Xio, J.-H. Su, Y.-J. Tseng, B.-W. Chen, W. Liu and J.-H. Sheu, Mar. Drugs, 2014, 12, 4495–4503, DOI:10.3390/md12084495
.
- M. Chen, L. Han, Y. Wang, X.-L. Zhang and C.-Y. Wang, Nat. Prod. Res., 2014, 28, 1147–1151, DOI:10.1080/14786419.2014.918122
.
- S.-Y. Cheng, N.-L. Shih, K.-Y. Hou, M.-J. Ger, C.-N. Yang, S.-K. Wang and C.-Y. Duh, Bioorg. Med. Chem. Lett., 2014, 24, 473–475, DOI:10.1016/j.bmcl.2013.12.037
.
- J. L. von Salm, N. G. Wilson, B. A. Vesely, D. E. Kyle, J. Cuce and B. J. Baker, Org. Lett., 2014, 16, 2630–2633, DOI:10.1021/ol500792x
.
- E. S. Elkhayat, S. R. M. Ibrahim, M. A. Fouad and G. A. Mohamed, Tetrahedron, 2014, 70, 3822–3825, DOI:10.1016/j.tet.2014.03.056
.
- S. Rajaram, D. Ramesh, U. Ramulu, M. Anjum, P. Kumar, U. S. N. Murthy, M. Altaf Hussain, G. Narahari Sastry and Y. Venkateswarlu, Indian J. Chem., Sect. B: Org. Chem. Incl. Med. Chem., 2014, 53, 1086–1090 Search PubMed
, URL: http://nopr.niscair.res.in/handle/123456789/29262.
- L. Xue, P.-L. Li, Z. Liang, X.-L. Tang and G.-Q. Li, Biochem. Syst. Ecol., 2014, 57, 48–51, DOI:10.1016/j.bse.2014.06.010
.
- J.-J. Zheng, C.-L. Shao, M. Chen, L.-S. Gan, Y.-C. Fang, X.-H. Wang and C.-Y. Wang, Mar. Drugs, 2014, 12, 1569–1579, DOI:10.3390/md12031569
.
- S. Gaisser, L. Kellenberger, A. L. Kaja, A. J. Weston, R. E. Lill, G. Wirtz, S. G. Kendrew, L. Low, R. M. Sheridan, B. Wilkinson, I. S. Galloway, K. Stutzman-Engwall, H. A. I. McArthur, J. Staunton and P. F. Leadlay, Org. Biomol. Chem., 2003, 1, 2840–2847, 10.1039/b304022d
.
- C. Gao, Y. Wang, Y. Chen, B. He, R. Zhang, M. Xu and R. Huang, Chem. Biodiversity, 2014, 11, 812–818, DOI:10.1002/cbdv.201300265
.
- S.-Y. Cheng, S.-K. Wang and C.-Y. Duh, Mar. Drugs, 2014, 12, 6028–6037, DOI:10.3390/md12126028
.
- S.-Y. Cheng, N.-L. Shih, C.-T. Chuang, S.-F. Chiou, C.-N. Yang, S.-K. Wang and C.-Y. Duh, Bioorg. Med. Chem. Lett., 2014, 24, 1562–1564, DOI:10.1016/j.bmcl.2014.01.073
.
- N. P. Thao, B. T. T. Luyen, N. T. T. Ngan, S. B. Song, N. X. Cuong, N. H. Nam, P. V. Kiem, Y. H. Kim and C. V. Minh, Bioorg. Med. Chem. Lett., 2014, 24, 228–232, DOI:10.1016/j.bmcl.2013.11.033
.
- J. Figueroa, B. Vera and A. D. Rodríguez, Helv. Chim. Acta, 2014, 97, 712–721, DOI:10.1002/hlca.201300282
.
- W.-Y. Lin, B.-W. Chen, C.-Y. Huang, Z.-H. Wen, P.-J. Sung, J.-H. Su, C.-F. Dai and J.-H. She, Mar. Drugs, 2014, 12, 840–850, DOI:10.3390/md12020840
.
- S. S. Al-Lihaibi, W. M. Alarif, A. Abdel-Lateff, S.-E. N. Ayyad, A. B. Abdel-Naim, F. F. El-Senduny and F. A. Badria, Eur. J. Med. Chem., 2014, 81, 314–322, DOI:10.1016/j.ejmech.2014.05.016
.
- A. Elkhateeb, A. A. El-Beih, A. M. Gamal-Eldeen, M. A. Alhammady, S. Ohta, P. W. Paré and M.-E. F. Hegazy, Mar. Drugs, 2014, 12, 1977–1986, DOI:10.3390/md12041977
.
- Z.-B. Cheng, Q. Liao, Y. Chen, C.-Q. Fan, Z.-Y. Huang, X.-J. Xu and S. Yin, Magn. Reson. Chem., 2014, 52, 515–520, DOI:10.1002/mrc.4108
.
- N. A. Eltahawy, A. K. Ibrahim, M. M. Radwan, M. A. El-Sohly, H. A. Hassanean and S. A. Ahmed, Tetrahedron Lett., 2014, 55, 3984–3988, DOI:10.1016/j.tetlet.2014.05.013
.
- L.-F. Lei, M.-F. Chen, T. Wang, X.-X. He, B.-X. Liu, Y. Deng, X.-J. Chen, Y.-T. Li, S.-Y. Guan, J.-H. Yao, W. Li, W.-C. Ye, D.-M. Zhang and C.-X. Zhang, Tetrahedron, 2014, 70, 6851–6858, DOI:10.1016/j.tet.2014.07.042
.
- Y.-J. Tseng, Y.-C. Yang, S.-K. Wang and C.-Y. Duh, Mar. Drugs, 2014, 12, 3371–3380, DOI:10.3390/md12063371
.
- K.-E. Lillsunde, C. Festa, H. Adel, S. de Marino, V. Lombardi, S. Tilvi, D. Nawrot, A. Zampella, L. D'Souza, M. D'Auria and P. Tammela, Mar. Drugs, 2014, 12, 4045–4068, DOI:10.3390/md12074045
.
- K.-H. Lin, Y.-J. Tseng, B.-W. Chen, T.-L. Hwang, H.-Y. Chen, C.-F. Dai and J.-H. Sheu, Org. Lett., 2014, 16, 1314–1317, DOI:10.1021/ol403723b
.
- K.-H. Chen, C.-F. Dai, T.-L. Hwang, C.-Y. Chen, J.-J. Li, J.-J. Chen, Y.-C. Wu, J.-H. Sheu, W.-H. Wang and P.-J. Sung, Mar. Drugs, 2014, 12, 385–393, DOI:10.3390/md12010385
.
- A. Bahl, S. M. Jachak, K. Palaniveloo, T. Ramachandram, C. S. Vairappan and H. K. Chopra, Nat. Prod. Commun., 2014, 9, 1139–1180 CAS
.
- M.-P. La, J. Li, C. Li, H. Tang, B.-S. Liu, P. Sun, C.-L. Zhuang, T.-J. Li and W. Zhang, Mar. Drugs, 2014, 12, 6178–6189, DOI:10.3390/md12126178
.
- Y.-D. Su, C.-H. Cheng, W.-F. Chen, Y.-C. Chang, Y.-H. Chen, T.-L. Hwang, Z.-H. Wen, W.-H. Wang, L.-S. Fang, J.-J. Chen, Y.-C. Wu, J.-H. Sheu and P.-J. Sung, Tetrahedron Lett., 2014, 55, 6065–6067, DOI:10.1016/j.tetlet.2014.09.032
.
- W. Zhou, J. Li, H.-C. E, B.-S. Liu, H. Tang, W. H. Gerwick, H.-M. Hua and W. Zhang, Mar. Drugs, 2014, 12, 589–600, DOI:10.3390/md12020589
.
- H. Lei, J.-F. Sun, Z. Han, X.-F. Zhou, B. Yang and Y. Liu, RSC Adv., 2014, 4, 5261–5271, 10.1039/c3ra46163g
.
- Y.-M. Zhou, C.-L. Shao, H. Huang, X.-L. Zhang and C.-Y. Wang, Nat. Prod. Res., 2014, 28, 7–11, DOI:10.1080/14786419.2013.827191
.
- C.-C. Liaw, Y.-B. Cheng, Y.-S. Lin, Y.-H. Kuo, T.-L. Hwang and Y.-C. Shen, Mar. Drugs, 2014, 12, 4677–4692, DOI:10.3390/md12084677
.
- C.-H. Gao, B.-J. He, Y.-N. Chen, K. Ke, L. Lin, B. Long and R.-M. Huang, Z. Naturforsch., B: J. Chem. Sci., 2014, 69, 116–120, DOI:10.5560/znb.2014-3213
.
- Y.-M. Zhou, M. Chen, X.-M. Fu, Y.-C. Fang and C.-Y. Wang, Nat. Prod. Res., 2014, 28, 1176–1181, DOI:10.1080/14786419.2014.923423
.
- T.-H. Chen, C.-H. Cheng, Y.-H. Chen, M.-C. Lu, L.-S. Fang, W.-F. Chen, Z.-H. Wen, W.-H. Wang, Y.-C. Wu and P.-J. Sung, Nat. Prod. Commun., 2014, 9, 613–617 CAS
.
- F.-Y. Chang, F.-J. Hsu, C.-J. Tai, W.-C. Wei, N.-S. Yang and J.-H. Sheu, Mar. Drugs, 2014, 12, 3060–3071, DOI:10.3390/md12053060
.
- T.-H. Chen, W.-F. Chen, Z.-H. Wen, M.-C. Lu, W.-H. Wang, J.-J. Li, Y.-C. Wu and P.-J. Sung, Mar. Drugs, 2014, 12, 2144–2155, DOI:10.3390/md12042144
.
- Y.-N. Lee, C.-J. Tai, T.-L. Hwang and J.-H. Sheu, Mar. Drugs, 2014, 12, 1148–1156, DOI:10.3390/md12021148
.
- C.-J. Tai, U. Chokkalingam, Y. Cheng, S.-P. Shih, M.-C. Lu, J.-H. Su, T.-L. Hwang and J.-H. Sheu, Int. J. Mol. Sci., 2014, 15, 21865–21874, DOI:10.3390/ijms151221865
.
- T.-Z. Huang, B.-W. Chen, C.-Y. Huang, T.-L. Hwang, C.-F. Dai and J.-H. Sheu, Mar. Drugs, 2014, 12, 2446–2457, DOI:10.3390/md12052446
.
- M. C. Lin, B. W. Chen, C. Y. Huang, C. F. Dai, T. L. Hwang and J. H. Sheu, J. Nat. Prod., 2013, 76, 1661–1667, DOI:10.1021/np400372v
.
- P. K. Roy, M. C. Roy, J. Taira and K. Ueda, Tetrahedron Lett., 2014, 55, 1421–1423, DOI:10.1016/j.tetlet.2014.01.035
.
- E. H. Andrianasolo, L. Haramaty, E. White, R. Lutz and P. Falkowski, Mar. Drugs, 2014, 12, 1102–1115, DOI:10.3390/md12021102
.
- Y.-C. Lin, S.-S. Wang, C.-H. Chen, Y.-H. Kuo and Y.-C. Shen, Mar. Drugs, 2014, 12, 3477–3486, DOI:10.3390/md12063477
.
- L.-F. Liang, T. Kurtán, A. Mándi, L.-X. Gao, J. Li, W. Zhang and Y.-W. Guo, Eur. J. Org. Chem., 2014, 2014, 1841–1847, DOI:10.1002/ejoc.201301683
.
- M. Zhang, K. Long, K. Ma, X. Huang and H. Wu, J. Nat. Prod., 1995, 58, 414–418, DOI:10.1021/np50117a010
.
- Z.-B. Cheng, Y.-L. Deng, C.-Q. Fan, Q.-H. Han, S.-L. Lin, G.-H. Tang, H.-B. Luo and S. Yin, J. Nat. Prod., 2014, 77, 1928–1936, DOI:10.1021/np500394d
.
- C.-Y. Kuo, Y.-S. Juan, M.-C. Lu, M. Chiang, C.-F. Dai, Y.-C. Wu and P.-J. Sung, Int. J. Mol. Sci., 2014, 15, 10136–10149, DOI:10.3390/ijms150610136
.
- M. Liu, C.-L. Shao, M. Chen, J. Qi, Y. Wang, Y.-C. Fang and C.-Y. Wang, Chem. Biodiversity, 2014, 11, 1109–1120, DOI:10.1002/cbdv.201400021
.
- W.-T. Chen, H.-L. Liu, L.-G. Yao and Y.-W. Guo, Steroids, 2014, 92, 56–61, DOI:10.1016/j.steroids.2014.08.027
.
- F. Cao, C.-L. Shao, Y. Wang, K.-X. Xu, X. Qi and C.-Y. Wang, Helv. Chim. Acta, 2014, 97, 900–908, DOI:10.1002/hlca.201300397
.
- F. Cao, C.-L. Shao, M. Chen, M.-Q. Zhang, K.-X. Xu, H. Meng and C.-Y. Wang, J. Nat. Prod., 2014, 77, 1488–1493, DOI:10.1021/np500252q
.
- N. P. Thao, B. T. T. Luyen, Y. N. Sun, S. B. Song, N. V. Thanh, N. X. Cuong, N. H. Nam, P. V. Kiem, Y. H. Kim and C. V. Minh, Bioorg. Med. Chem. Lett., 2014, 24, 2834–2838, DOI:10.1016/j.bmcl.2014.04.103
.
- R. Xu, M.-H. Jin, Y. Jiao, G.-S. Xing, W.-J. Zhao, C. Zhao, H.-Q. Duan and S.-A. Tang, J. Asian Nat. Prod. Res., 2014, 16, 351–357, DOI:10.1080/10286020.2013.879469
.
- M. Moritz, L. Marostica, É. Bianco, M. Almeida, J. Carraro, G. Cabrera, J. Palermo, C. Simões and E. Schenkel, Mar. Drugs, 2014, 12, 5864–5880, DOI:10.3390/md12125864
.
- J. Zhang, Y. Liang, K.-L. Wang, X.-J. Liao, Z. Deng and S.-H. Xu, Steroids, 2014, 79, 1–6, DOI:10.1016/j.steroids.2013.10.007
.
- S. Yu, X. Ye, L. Chen, X.-Y. Lian and Z. Zhang, Steroids, 2014, 88, 19–25, DOI:10.1016/j.steroids.2014.06.013
.
- V. Junior, F. Zara, S. Marangoni, D. de Toyama, A. J. de Souza, S. C. de Oliveira and M. Toyama, J. Venomous Anim. Toxins Incl. Trop. Dis., 2014, 20, 10, DOI:10.1186/1678-9199-20-10
.
- A. A. Rodríguez, E. Salceda, A. G. Garateix, A. J. Zaharenko, S. Peigneur, O. López, T. Pons, M. Richardson, M. Díaz, Y. Hernández, L. Ständker, J. Tytgat and E. Soto, Peptides, 2014, 53, 3–12, DOI:10.1016/j.peptides.2013.06.003
.
- M. G. Barzilai, R. Kahn, N. Regev, D. Gordon, Y. Moran and M. Gurevitz, Biochem. J., 2014, 463, 271–277, DOI:10.1042/BJ20140576
.
- N. Nesher, E. Zlotkin and B. Hochner, Biochem. J., 2014, 461, 51–59, DOI:10.1042/BJ20131454
.
- V. Antonini, V. Pérez-Barzaga, S. Bampi, D. Pentón, D. Martínez, M. D. Serra and M. Tejuca, PLoS One, 2014, 9, e110824, DOI:10.1371/journal.pone.0110824
.
- L. León, E. A. Lissi, G. Celedón, G. Gonzalez, F. Pazos, C. Alvarez and M. E. Lanio, Protein J., 2014, 33, 493–501, DOI:10.1007/s10930-014-9582-x
.
- L. L. Pedrera, M. L. Fanani, U. Ros, M. E. Lanio, B. Maggio and C. Álvarez, Biochim. Biophys. Acta, Biomembr., 2014, 1838, 1752–1759, DOI:10.1016/j.bbamem.2014.03.011
.
- W. E. Houssen and M. Jaspars, J. Nat. Prod., 2005, 68, 453–455, DOI:10.1021/np049666n
.
- S. S. More, A. Raghunadh, R. Shankar, N. B. Patel, D. S. Bhalerao and U. K. S. Kumar, Helv. Chim. Acta, 2014, 97, 403–413, DOI:10.1002/hlca.201300318
.
- T. Iwagawa, M. Miyazaki, H. Okamura, M. Nakatani, M. Doe and K. Takemura, Tetrahedron Lett., 2003, 44, 2533–2535, DOI:10.1016/S0040-4039(03)00331-9
.
- A. Skiredj, M. A. Beniddir, D. Joseph, K. Leblanc, G. Bernadat, L. Evanno and E. Poupon, Org. Lett., 2014, 16, 4980–4983, DOI:10.1021/ol502177m
.
- F. J. Schmitz, E. D. Lorance and L. S. Ciereszko, J. Org. Chem., 1969, 34, 1989–1990, DOI:10.1021/jo01258a110
.
- S. J. Gharpure, L. N. Nanda and M. K. Shukla, Org. Lett., 2014, 16, 6424–6427, DOI:10.1021/ol503246k
.
- A. D. Rodríguez, C. Ramírez and I. I. Rodríguez, Tetrahedron Lett., 1999, 43, 7627–7631, DOI:10.1016/S0040-4039(99)01559-2
.
- A. D. Rodriguez, C. Ramirez, V. Medina and Y. P. Shi, Tetrahedron Lett., 2000, 41, 5177–5180, DOI:10.1016/S0040-4039(00)00767-X
.
- I. I. Rodríguez, A. D. Rodríguez, Y. Wang and S. G. Franzblau, Tetrahedron Lett., 2006, 47, 3229–3232, DOI:10.1016/j.tetlet.2006.03.048
.
- I. T. Chen, I. Baitinger, L. Schreyer and D. Trauner, Org. Lett., 2014, 16, 166–169, DOI:10.1021/ol403156r
.
- D. R. Williams and A. A. Shah, J. Am. Chem. Soc., 2014, 8829–8836, DOI:10.1021/ja5043462
.
- A. D. Rodriguez and J. G. Shi, J. Org. Chem., 1998, 63, 420–421, DOI:10.1021/jo971884g
.
- J. Marrero, A. D. Rodriguez and C. L. Barnes, Org. Lett., 2005, 7, 1877–1880, DOI:10.1021/ol0505961
.
- D. Stichnoth, P. Kölle, T. J. Kimbrough, E. Riedle, R. de Vivie-Riedle and D. Trauner, Nat. Commun., 2014, 5, 5597, DOI:10.1038/ncomms6597
.
- N. Toelle, H. Weinstabl, T. Gaich and J. Mulzer, Angew. Chem., Int. Ed., 2014, 53, 3859–3862, DOI:10.1002/anie.201400617
.
- M. Alam and P. Sharama, J. Org. Chem., 1989, 54, 1896–1900, DOI:10.1021/jo00269a027
.
- D. Friedrich and L. A. Paquette, J. Nat. Prod., 2002, 65, 126–130, DOI:10.1021/np0103822
.
- J. S. Clark, L. Delion and L. J. Farrugia, Org. Lett., 2014, 16, 4300–4303, DOI:10.1021/ol5020152
.
- G. Yue, Y. Zhang, L. Fang, C.-c. Li, T. Luo and Z. Yang, Angew. Chem., Int. Ed., 2014, 53, 1837–1840, DOI:10.1002/anie.201309449
.
- T. Higa, J. Tanaka, Y. Tsukitani and H. Kikuchi, Chem. Lett., 1981, 1647–1650, DOI:10.1246/cl.1981.1647
.
- R. Somaiah, K. Ravindar, R. Cencic, J. Pelletier and P. Deslongchamps, J. Med. Chem., 2014, 57, 2511–2523, DOI:10.1021/jm401799j
.
- N. Thao, J. No, B. Luyen, G. Yang, S. Byun, J. Goo, K. Kim, N. Cuong, N. Nam, C. V. Minh, T. Schmidt, J. Kang and Y. Kim, Molecules, 2014, 19, 7869–7880, DOI:10.3390/molecules1906786
.
- N. P. Thao, N. H. Nam, N. X. Cuong, B. T. T. Luyen, B. H. Tai, J. E. Kim, S. B. Song, P. V. Kiem, C. V. Minh and Y. H. Kim, Arch. Pharmacal Res., 2014, 37, 706–712, DOI:10.1007/s12272-013-0230-3
.
- R. G. Kerr, S. Brophy and D. J. Derksen, Bioorg. Med. Chem. Lett., 2014, 24, 4804–4806, DOI:10.1016/j.bmcl.2014.09.008
.
- A. Pardo-Vargas, F. A. Ramos, C. C. Cirne-Santos, P. R. Stephens, I. C. P. Paixão, V. L. Teixeira and L. Castellanos, Bioorg. Med. Chem. Lett., 2014, 24, 4381–4383, DOI:10.1016/j.bmcl.2014.08.019
.
- V. Sepe, F. D. Leva, C. D'Amore, C. Festa, S. De Marino, B. Renga, M. D'Auria, E. Novellino, V. Limongelli, L. D'Souza, M. Majik, A. Zampella and S. Fiorucci, Mar. Drugs, 2014, 12, 3091–3115, DOI:10.3390/md12063091
.
- R. Kazlauskas, P. T. Murphy and R. J. Wells, Aust. J. Chem., 1978, 31, 1817–1824, DOI:10.1071/ch9781817
.
- N.-F. Chen, S.-Y. Huang, C.-H. Lu, C.-L. Chen, C.-W. Feng, C.-H. Chen, H.-C. Hung, Y.-Y. Lin, P.-J. Sung, C.-S. Sung, S.-N. Yang, H.-M. Wang, Y.-C. Chang, J.-H. Sheu, W.-F. Chen and Z.-H. Wen, Mar. Drugs, 2014, 12, 3792–3817, DOI:10.3390/md12073792
.
- J.-J. Lin, J.-H. Su, C.-C. Tsai, Y.-J. Chen, M.-H. Liao and Y.-J. Wu, Mar. Drugs, 2014, 12, 4783–4798, DOI:10.3390/md12094783
.
- C.-C. Su, J. Chen, Z.-H. Din, J.-H. Su, Z.-Y. Yang, Y.-J. Chen, R. Wang and Y.-J. Wu, Mar. Drugs, 2014, 12, 5295–5315, DOI:10.3390/md12105295
.
- P. T. Szymanski, S. A. Ahmed, M. M. Radwan, S. I. Khalifa and H. Fahmy, Nat. Prod. Commun., 2014, 9, 151–154 CAS
.
- P. T. Szymanski, S. A. Ahmed, M. M. Radwan, S. I. Khalifa and H. Fahmy, Nat. Prod. Commun., 2014, 9, 1143–1146 CAS
.
- L. P. Patiño, C. Muniain, M. E. Knott, L. Puricelli and J. A. Palermo, J. Nat. Prod., 2014, 77, 1170–1178, DOI:10.1021/np500012y
.
- X.-R. Tian, H.-F. Tang, J.-T. Feng, Y.-S. Li, H.-W. Lin, X.-P. Fan and X. Zhang, Mar. Drugs, 2014, 12, 1987–2003, DOI:10.3390/md12041987
.
- X.-R. Tian, H.-F. Tang, Y.-S. Li, H.-W. Lin, X.-P. Fan, J.-T. Feng and X. Zhang, Phytochem. Lett., 2014, 9, 1–6, DOI:10.1016/j.phytol.2014.03.010
.
- Y. Kamano, H. P. Zhang, Y. Ichihara, H. Kizu, K. Komiyama and G. R. Pettit, Tetrahedron Lett., 1995, 36, 2783–2784, DOI:10.1016/0040-4039(95)00395-S
.
- P. D. Fernandes, R. S. Zardo, G. S. M. Figueiredo, B. V. Silva and A. C. Pinto, Life Sci., 2014, 116, 16–24, DOI:10.1016/j.lfs.2014.08.019
.
- J. Kilcoyne, P. McCarron, M. J. Twiner, C. Nulty, S. Crain, M. A. Quilliam, F. Rise, A. L. Wilkins and C. O. Miles, Chem. Res. Toxicol., 2014, 27, 587–600, DOI:10.1021/tx400434b
.
- M. Carbone, M. L. Ciavatta, G. De Rinaldis, F. Castelluccio, E. Mollo and M. Gavagnin, Tetrahedron, 2014, 70, 3770–3773, DOI:10.1016/j.tet.2014.04.046
.
- M. Gavagnin, A. Spinella, A. Crispino, R. D. A. Epifanio, A. Marin and G. Cimino, Gazz. Chim. Ital., 1993, 123, 205–208 CAS
.
- V. J. Paul, P. Ciminiello and W. Fenical, Phytochemistry, 1988, 27, 1011–1014, DOI:10.1016/0031-9422(88)80262-0
.
- M. L. Ciavatta, G. Nuzzo, K. Takada, V. Mathieu, R. Kiss, G. Villani and M. Gavagnin, J. Nat. Prod., 2014, 77, 1678–1684, DOI:10.1021/np500298h
.
- W.-F. He, Y. Li, M.-T. Feng, M. Gavagnin, E. Mollo, S.-C. Mao and Y.-W. Guo, Fitoterapia, 2014, 96, 109–114, DOI:10.1016/j.fitote.2014.04.011
.
- F. R. Pereira, M. F. C. Santos, D. E. Williams, R. J. Andersen, V. Padula, A. G. Ferreira and R. G. S. Berlinck, J. Braz. Chem. Soc., 2014, 25, 788–794, DOI:10.5935/0103-5053.20140037
.
- S. Khor, S. A. Wood, L. Salvitti, D. I. Taylor, J. Adamson, P. McNabb and S. C. Cary, Mar. Drugs, 2014, 12, 1–16, DOI:10.3390/md12010001
.
- M.-E. F. Hegazy, A. Y. Moustfa, A. El-H, H. Mohamed, M. A. Alhammady, S. E. I. Elbehairi, S. Ohta and P. W. Paré, Tetrahedron Lett., 2014, 55, 1711–1714, DOI:10.1016/j.tetlet.2014.01.096
.
- C. Jiménez-Romero, A. M. S. Mayer and A. D. Rodríguez, Bioorg. Med. Chem. Lett., 2014, 24, 344–348, DOI:10.1016/j.bmcl.2013.11.008
.
- B. Esmaeelian, C. A. Abbott, R. K. Le Leu and K. Benkendorff, Mar. Drugs, 2014, 12, 17–35, DOI:10.3390/md12010017
.
- D. S. Dalisay, E. W. Rogers, A. S. Edison and T. F. Molinski, J. Nat. Prod., 2009, 72, 732–738, DOI:10.1021/np8007649
.
- H. Wahyudi, W. Tantisantisom and S. R. McAlpine, Tetrahedron Lett., 2014, 55, 2389–2393, DOI:10.1016/j.tetlet.2014.02.106
.
- A. L. Pietkiewicz, H. Wahyudi, J. R. McConnell and S. R. McAlpine, Tetrahedron Lett., 2014, 55, 6979–6982, DOI:10.1016/j.tetlet.2014.10.089
.
- G. R. Pettit, S. B. Singh, F. Hogan, P. Lloyd-Williams, D. L. Herald, D. D. Burkett and P. J. Clewlow, J. Am. Chem. Soc., 1989, 111, 5463–5465, DOI:10.1021/ja00196a061
.
- A. Maderna, M. Doroski, C. Subramanyam, A. Porte, C. A. Leverett, B. C. Vetelino, Z. Chen, H. Risley, K. Parris, J. Pandit, A. H. Varghese, S. Shanker, C. Song, S. C. K. Sukuru, K. A. Farley, M. M. Wagenaar, M. J. Shapiro, S. Musto, M.-H. Lam, F. Loganzo and C. J. O'Donnell, J. Med. Chem., 2014, 57, 10527–10543, DOI:10.1021/jm501649k
.
- Y. Sun, Y. Lin, X. Cao, L. Xiang and J. Qi, Int. J. Mol. Sci., 2014, 15, 21660–21673, DOI:10.3390/ijms151221660
.
- E. Hernando, V. Soto-Cerrato, S. Cortés-Arroyo, R. Pérez-Tomás and R. Quesada, Org. Biomol. Chem., 2014, 12, 1771–1778, 10.1039/C3OB42341G
.
- R. J. Andersen, D. J. Faulkner and H. Cun-heng, J. Am. Chem. Soc., 1985, 107, 5492–5495, DOI:10.1021/ja00305a027
.
- C. Ballot, A. Martoriati, M. Jendoubi, S. Buche, P. Formstecher, L. Mortier, J. Kluza and P. Marchetti, Mar. Drugs, 2014, 12, 779–798, DOI:10.3390/md12020779
.
- H. Liu, H. Zhang, H. Zheng, S. Wang, Z. Guo and G. Zhang, J. Agric. Food Chem., 2014, 62, 12384–12391, DOI:10.1021/jf504648f
.
- B. Nguyen, J.-P. Le Caer, R. Aráoz, R. Thai, H. Lamthanh, E. Benoit and J. Molgó, Toxicon, 2014, 91, 155–163, DOI:10.1016/j.toxicon.2014.10.006
.
- D.-D. Yuana, Y.-H. Hana, C.-G. Wanga and C.-W. Chi, Toxicon, 2007, 49, 1135–1149, DOI:10.1016/j.toxicon.2007.02.011
.
- A. D. Santos, J. M. McIntosh, D. R. Hillyard, L. J. Cruz and B. M. Olivera, J. Biol. Chem., 2004, 279, 17596–17606, DOI:10.1074/jbc.M309654200
.
- B. Nguyen, J.-P. Caer, G. Mourier, R. Thai, H. Lamthanh, D. Servent, E. Benoit and J. Molgó, Mar. Drugs, 2014, 12, 3449–3465, DOI:10.3390/md12063449
.
- E. K. M. Lebbe, S. Peigneur, M. Maiti, B. G. Mille, P. Devi, S. Ravichandran, E. Lescrinier, E. Waelkens, L. D'Souza, P. Herdewijn and J. Tytgat, Toxicon, 2014, 91, 145–154, DOI:10.1016/j.toxicon.2014.08.074
.
- R. Sonti, K. N. S. Rao, S. Chidanand, K. H. Gowd, S. Raghothama and P. Balaram, Chem.–Eur. J., 2014, 20, 5075–5086, DOI:10.1002/chem.201303687
.
- S. Peigneur, M. Paolini-Bertrand, H. Gaertner, D. Biass, A. Violette, R. Stocklin, P. Favreau, J. Tytgat and O. Hartley, J. Biol. Chem., 2014, 289, 35341–35350, DOI:10.1074/jbc.M114.610436
.
- S. Chhabra, A. Belgi, P. Bartels, B. J. van Lierop, S. D. Robinson, S. N. Kompella, A. Hung, B. P. Callaghan, D. J. Adams, A. J. Robinson and R. S. Norton, J. Med. Chem., 2014, 57, 9933–9944, DOI:10.1021/jm501126u
.
- Y. Wu, X. Wu, J. Yu, X. Zhu, D. Zhangsun and S. Luo, Molecules, 2014, 19, 966–979, DOI:10.3390/molecules19010966
.
- S. V. Sambasivarao, J. Roberts, V. S. Bharadwaj, J. G. Slingsby, C. Rohleder, C. Mallory, J. R. Groome, O. M. McDougal and C. M. Maupin, ChemBioChem, 2014, 15, 413–424, DOI:10.1002/cbic.201300577
.
- S. Wang, T. Du, Z. Liu, S. Wang, Y. Wu, J. Ding, L. Jiang and Q. Dai, Biochem. Biophys. Res. Commun., 2014, 454, 151–156, DOI:10.1016/j.bbrc.2014.10.055
.
- M. Li, S. Chang, L. Yang, J. Shi, K. McFarland, X. Yang, A. Moller, C. Wang, X. Zou, C. Chi and J. Cui, J. Biol. Chem., 2014, 289, 4735–4742, DOI:10.1074/jbc.M113.535898
.
- S. R. M. Ibrahim, G. A. Mohamed, L. A. Shaala, D. T. A. Youssef and A. A. Gab-Alla, Nat. Prod. Res., 2014, 28, 1591–1597, DOI:10.1080/14786419.2014.927874
.
- T. H. Won, M. You, S.-H. Lee, B. J. Rho, D.-C. Oh, K.-B. Oh and J. Shin, Mar. Drugs, 2014, 12, 3754–3769, DOI:10.3390/md12063754
.
- G. A. Mohamed, S. R. M. Ibrahim, J. M. Badr and D. T. A. Youssef, Tetrahedron, 2014, 70, 35–40, DOI:10.3390/toxins6020693
.
- L. A. Shaala, D. T. A. Youssef, S. R. M. Ibrahim, G. A. Mohamed, J. B. Badr, A. L. Risinger and S. L. Mooberry, Mar. Drugs, 2014, 12, 5021–5034, DOI:10.3390/md12095021
.
- M. Tadesse, J. Svenson, K. Sepčić, L. Trembleau, M. Engqvist, J. H. Andersen, M. Jaspars, K. Stensvåg and T. Haug, J. Nat. Prod., 2014, 77, 364–369, DOI:10.1021/np401002s
.
- D. Smitha, M. M. K. Kumar, H. Ramana and D. V. Rao, Nat. Prod. Res., 2014, 28, 12–17, DOI:10.1080/14786419.2013.827194
.
- C. Imperatore, F. D'Aniello, A. Aiello, S. Fiorucci, C. D'Amore, V. Sepe and M. Menna, Mar. Drugs, 2014, 12, 2066–2078, DOI:10.3390/md12042066
.
- M. T. Jamison, C. N. Boddy and T. F. Molinski, J. Org. Chem., 2014, 79, 9992–9997, DOI:10.1021/jo501486p
.
- W. Hassan, R. Edrada, R. Ebel, V. Wray and P. Proksch, Mar. Drugs, 2004, 2, 88–100, DOI:10.3390/md203088
.
- C. Murcia, L. Coello, R. Fernández, M. J. Martín, F. Reyes, A. Francesch, S. Munt and C. Cuevas, Mar. Drugs, 2014, 12, 1116–1130, DOI:10.3390/md12021116
.
- J. C. Kwan, M. D. B. Tianero, M. S. Donia, T. P. Wyche, T. S. Bugni and E. W. Schmidt, PLoS One, 2014, 9, e95850, DOI:10.1371/journal.pone.0095850
.
- W. E. Houssen, A. F. Bent, A. R. McEwan, N. Pieiller, J. Tabudravu, J. Koehnke, G. Mann, R. I. Adaba, L. Thomas, U. W. Hawas, H. Liu, U. Schwarz-Linek, M. C. M. Smith, J. H. Naismith and M. Jaspars, Angew. Chem., Int. Ed., 2014, 53, 14171–14174, DOI:10.1002/anie.201408082
.
- B. C. M. Potts, D. J. Faulkner, J. A. Chan, G. C. Simolike, P. Offen, M. E. Hemling and T. A. Francis, J. Am. Chem. Soc., 1991, 113, 6321–6322, DOI:10.1021/ja00016a087
.
- H. Fuwa, T. Muto, K. Sekine and M. Sasaki, Chem.–Eur. J., 2014, 20, 1848–1860, DOI:10.1002/chem.201303713
.
- F.-M. Zhang and Y.-Q. Tu, Tetrahedron Lett., 2014, 55, 3784–3787, DOI:10.1016/j.tetlet.2014.05.065
.
- H. H. Issa, J. Tanaka, R. Rachmat, A. Setiawan, A. Trianto and T. Higa, Mar. Drugs, 2005, 3, 78–83, DOI:10.3390/md303078
.
- J. In, S. Lee, Y. Kwon and S. Kim, Chem.–Eur. J., 2014, 20, 17433–17442, DOI:10.1002/chem.201404316
.
- N. Gonzalez, J. Rodriguez and C. Jimenez, J. Org. Chem., 1999, 64, 5705–5707, DOI:10.1021/jo9903914
.
- J. Ren and R. Tong, J. Org. Chem., 2014, 79, 6987–6995, DOI:10.1021/jo501142q
.
- J. Sikorska, A. M. Hau, C. Anklin, S. Parker-Nance, M. T. Davies-Coleman, J. E. Ishmael and K. L. McPhail, J. Org. Chem., 2012, 77, 6066–6075, DOI:10.1021/jo3008622
.
- H. Lei, J. Yan, J. Yu, Y. Liu, Z. Wang, Z. Xu and T. Ye, Angew. Chem., Int. Ed., 2014, 53, 6533–6537, DOI:10.1002/anie.201403542
.
- L. Garrido, J. Org. Chem., 2003, 68, 293–299, DOI:10.1021/jo020487p
.
- M. Matveenko, G. Liang and E. M. W. Lauterwasser, J. Am. Chem. Soc., 2012, 134, 9291–9295, DOI:10.1021/ja301326k
.
- Y. Momoi, K.-i. Okuyama, H. Toya, K. Sugimoto, K. Okano and H. Tokuyama, Angew. Chem., Int. Ed., 2014, 53, 13215–13219, DOI:10.1002/anie.201407686
.
- A. N. Pearce, D. R. Appleton, R. C. Babcock and B. R. Copp, Tetrahedron Lett., 2003, 44, 3897–3899, DOI:10.1016/S0040-4039(03)00831-1
.
- A. E. R. Jolibois, W. Lewis and C. J. Moody, Org. Lett., 2014, 16, 1064–1067, DOI:10.1021/ol403598k
.
- M. Tadesse, M. B. Strom, J. Svenson, M. Jaspars, B. F. Milne, V. Torfoss, J. H. Andersen, E. Hansen, K. Stensvag and T. Haug, Org. Lett., 2010, 12, 4752–4755, DOI:10.1021/ol101707u
.
- N. V. Shymanska, I. H. An and J. G. Pierce, Angew. Chem., Int. Ed., 2014, 53, 5401–5404, DOI:10.1002/anie.201402310
.
- R. Trepos, G. Cervin, C. Hellio, H. Pavia, W. Stensen, K. Stensvåg, J.-S. Svendsen, T. Haug and J. Svenson, J. Nat. Prod., 2014, 77, 2105–2113, DOI:10.1021/np5005032
.
- D. M. Tapiolas, B. F. Bowden, E. Abou-Mansour, R. H. Willis, J. R. Doyle, A. N. Muirhead, C. Liptrot, L. E. Llewellyn and C. W. Wolff, J. Nat. Prod., 2009, 72, 1115–1120, DOI:10.1021/np900099j
.
- M. Liberio, M. Sadowski, C. Nelson and R. Davis, Mar. Drugs, 2014, 12, 5222–5239, DOI:10.3390/md12105222
.
- K. Nozawa, M. Tsuda, H. Ishiyama, T. Sasaki, T. Tsuruo and R. Kobayashi, Bioorg. Med. Chem., 2006, 14, 1063–1067, DOI:10.1016/j.bmc.2005.09.033
.
- S. Kazami, M. Takaine, H. Itoh, T. Kubota, J. Kobayashi and T. Usui, Biol. Pharm. Bull., 2014, 37, 1944–1947, DOI:10.1248/bpb.b14-00548
.
- H. Atmaca and S. Uzunoglu, Eur. Cytokine Network, 2014, 25, 1–7, DOI:10.1684/ecn.2014.0347
.
- D.-S. Lee, X. Cui, W. Ko, K.-S. Kim, I. C. Kim, J. H. Yim, R.-B. An, Y.-C. Kim and H. Oh, Arch. Pharmacal Res., 2014, 37, 983–991, DOI:10.1007/s12272-013-0269-18
.
- N. P. Mishchenko, E. A. Vasileva and S. A. Fedoreyev, Tetrahedron Lett., 2014, 55, 5967–5969, DOI:10.1016/j.tetlet.2014.09.018
.
- A. A. Kicha, A. I. Kalinovskii, N. V. Ivanchina, T. V. Malyarenko, R. S. Popov, F. K. Long and N. A. Hung, Chem. Nat. Compd., 2014, 50, 1032–1036, DOI:10.1007/s10600-014-1153-z6
.
- N. P. Thao, B. T. T. Luyen, T. L. Vien, B. H. Tai, D. L. Dat, N. X. Cuong, N. H. Nam, P. V. Kiem, C. V. Minh and Y. H. Kim, Nat. Prod. Commun., 2014, 9, 615–623 Search PubMed
.
- A. S. Silchenko, A. I. Kalinovsky, S. A. Avilov, P. V. Andryjaschenko, P. S. Dmitrenok, E. A. Yurchenko, I. Y. Dolmatov, A. M. Savchenko and V. I. Kalinin, Nat. Prod. Commun., 2014, 9, 1421–1429 CAS
.
- R. S. Popov, S. A. Avilov, A. S. Silchenko, A. I. Kalinovsky, P. S. Dmitrenok, B. B. Grebnev, N. V. Ivanchina and V. I. Kalinin, Biochem. Syst. Ecol., 2014, 57, 191–197, DOI:10.1016/j.bse.2014.08.009
.
- V. P. Careaga, C. Bueno, C. Muniain, L. Alché and M. S. Maier, Nat. Prod. Res., 2014, 28, 213–220, DOI:10.1080/14786419.2012.751596
.
- X.-H. Wang, Z.-R. Zou, Y.-H. Yi, H. Han, L. Li and M.-X. Pan, Mar. Drugs, 2014, 12, 2004–2018, DOI:10.3390/md12042004
.
- T. V. Malyarenko, A. A. Kicha, N. V. Ivanchina, A. I. Kalinovsky, R. S. Popov, O. S. Vishchuk and V. A. Stonik, Steroids, 2014, 87, 119–127, DOI:10.1016/j.steroids.2014.05.027
.
- M. Elbandy, J. R. Rho and R. Afifi, Eur. Food Res. Technol., 2014, 238, 937–955, DOI:10.1007/s00217-014-2171-6
.
- Y. Bahrami, W. Zhang, T. Chataway and C. Franco, Mar. Drugs, 2014, 12, 4439–4473, DOI:10.3390/md12084439
.
- M. Demeyer, J. De Winter, G. Caulier, I. Eeckhaut, P. Flammang and P. Gerbaux, Comp. Biochem. Physiol., Part B: Biochem. Mol. Biol., 2014, 168, 1–11, DOI:10.1016/j.cbpb.2013.10.004
.
- J. M. Sciani, M. C. Sampaio, B. C. Zychar, L. R. de
C. Gonçalves, R. Giorgi, T. de O. Nogueira, R. L. de Melo, C. de F. P. Teixeira and D. C. Pimenta, Peptides, 2014, 53, 13–21, DOI:10.1016/j.peptides.2013.07.031
.
- N. Ueki, K. Someya, Y. Matsuo, K. Wakamatsu and H. Mukai, Pept. Sci., 2007, 88, 190–198, DOI:10.1002/bip.20687
.
- R. E. Moore, H. Singh and P. J. Scheuer, Tetrahedron Lett., 1968, 4581–4583, DOI:10.1016/S0040-4039(01)99190-7
.
- N. D. Pokhilo, G. I. Mel'man, V. P. Glazunov and V. F. Anufriev, Chem. Nat. Compd., 2014, 50, 417–419, DOI:10.1007/s10600-014-0974-0
.
- R. E. Moore, H. Singh and P. J. Scheuer, J. Org. Chem., 1966, 31, 3645–3650, DOI:10.1021/jo01349a040
.
- E. Peña-Cabrera and L. S. Liebeskind, J. Org. Chem., 2002, 67, 1689–1691, DOI:10.1021/jo016034m
.
- S. Jeong, H. Kim, I.-S. Song, S. Noh, J. Marquez, K. Ko, B. Rhee, N. Kim, N. Mishchenko, S. Fedoreyev, V. Stonik and J. Han, Mar. Drugs, 2014, 12, 4602–4615, DOI:10.3390/md12084602
.
- S. H. Jeong, H. K. Kim, I.-S. Song, S. J. Lee, K. S. Ko, B. D. Rhee, N. Kim, N. P. Mishchenko, S. A. Fedoryev, V. A. Stonik and J. Han, Mar. Drugs, 2014, 12, 2922–2936, DOI:10.3390/md12052922
.
- S. Lee, J. Pronto, B.-E. Sarankhuu, K. Ko, B. Rhee, N. Kim, N. Mishchenko, S. Fedoreyev, V. Stonik and J. Han, Mar. Drugs, 2014, 12, 3560–3573, DOI:10.3390/md12063560
.
- N. P. Mischenko, S. A. Fedoreyev, N. D. Pokhilo, V. P. Anufriev, V. A. Denisenko and V. P. Glazunov, J. Nat. Prod., 2005, 68, 1390–1393, DOI:10.1021/np049585r
.
- V. P. Glazunov, D. V. Berdyshev and V. L. Novikov, Russ. Chem. Bull., 2014, 63, 1993–1999, DOI:10.1007/s11172-014-0690-8
.
- I. R. Smith and M. D. Sutherland, Aust. J. Chem., 1971, 24, 1487–1499, DOI:10.1071/CH9711487
.
- L.-C. Chen, Y.-Y. Lin, Y.-H. Jean, Y. Lu, W.-F. Chen, S.-N. Yang, H.-M. Wang, I.-Y. Jang, I.-M. Chen, J.-H. Su, P.-J. Sung, J.-H. Sheu and Z.-H. Wen, Molecules, 2014, 19, 14667–14686, DOI:10.3390/molecules190914667
.
- E. Turner, R. Klevit, L. J. Hager and B. M. Shapiro, Biochemistry, 1987, 26, 4028–4036, DOI:10.1021/bi00387a043
.
- A. Mirzahosseini, S. Hosztafi, G. Tóth and B. Noszál, ARKIVOC, 2014, 2014, 1–9, DOI:10.3998/ark.5550190.p008.872
.
- H. Song, A. S. Her, F. Raso, Z. Zhen, Y. Huo and P. Liu, Org. Lett., 2014, 16, 2122–2125, DOI:10.1021/ol5005438
.
- A. Mirzahosseini, G. Orgován, S. Hosztafi and B. Noszál, Anal. Bioanal. Chem., 2014, 406, 2377–2387, DOI:10.1007/s00216-014-7631-0
.
- G. Russo, M. Russo, I. Castellano, A. Napolitano and A. Palumbo, Mar. Drugs, 2014, 12, 4069–4085, DOI:10.3390/md12074069
.
- D. M. Pereira, G. Correia-da-Silva, P. Valentão, N. Teixeira and P. B. Andrade, PLoS One, 2014, 9, e88341, DOI:10.1371/journal.pone.0088341
.
- D. M. Pereira, G. Correia-da-Silva, P. Valentão, N. Teixeira and P. B. Andrade, Mar. Drugs, 2014, 12, 54–68, DOI:10.3390/md12010054
.
- C. E. Jones, C. B. Otara, N. D. Younan, J. H. Viles and M. R. Elphick, Biochim. Biophys. Acta, Biomembr., 2014, 1844, 1842–1850, DOI:10.1016/j.bbapap.2014.08.001
.
- M. Girard, J. Belanger, J. W. ApSimon, F. X. Garneau, C. Harvey and J. R. Brisson, Can. J. Chem., 1990, 68, 11–18, DOI:10.1139/v90-003
.
- J. Al Shemaili, E. Mensah-Brown, K. Parekh, S. A. Thomas, S. Attoub, B. Hellman, F. Nyberg, A. Adem, P. Collin and T. E. Adrian, Eur. J. Cancer, 2014, 50, 1391–1398, DOI:10.1016/j.ejca.2014.01.002
.
- T. H. Quang, D.-S. Lee, S. J. Han, I. C. Kim, J. H. Yim, Y.-C. Kim and H. Oh, Bull. Korean Chem. Soc., 2014, 35, 2335–2341, DOI:10.5012/bkcs.2014.35.8.2335
.
- J. Wang, H. Han, X. Chen, Y. Yi and H. Sun, Mar. Drugs, 2014, 12, 4274–4290, DOI:10.3390/md12084274
.
- Y. Wang, J. Wang, R. C. Yanagita, C. Liu, X. Hu, P. Dong, C. Xue and Y. Xue, Biosci., Biotechnol., Biochem., 2014, 78, 139–146, DOI:10.1080/09168451.2014.877830
.
- A. S. Silchenko, A. I. Kalinovsky, S. A. Avilov, P. V. Andryjaschenko, P. S. Dmitrenok, E. A. Martyyas, V. I. Kalinin, P. Jayasandhya, G. C. Rajan and K. P. Padmakumar, Nat. Prod. Commun., 2013, 8, 301–310 CAS
.
- E. A. Pislyagin, D. L. Aminin, A. S. Silchenko, S. A. Avilov, P. V. Andryjashchenko, V. I. Kalinin and K. Padmakumar, Nat. Prod. Commun., 2014, 9, 771–772 CAS
.
- I. H. Hwang, R. Kulkarni, M. H. Yang, S. J. Choo, W. Zhou, S. M. Lee, T. S. Jang, G.-S. Jeong, H. W. Chang and M. Na, Arch. Pharmacal Res., 2014, 37, 1252–1263, DOI:10.1007/s12272-014-0374-9
.
- X.-X. Yi, Y. Chen, W.-P. Xie, M.-B. Xu, Y.-N. Chen, C.-H. Gao and R.-M. Huang, Mar. Drugs, 2014, 12, 2515–2525, DOI:10.3390/md12052515
.
- C.-H. Gao, X.-X. Yi, W.-P. Xie, Y.-N. Chen, M.-B. Xu, Z.-W. Su, L. Yu and R.-M. Huang, Mar. Drugs, 2014, 12, 4353–4360, DOI:10.3390/md12084353
.
- H. Wang, M.-Y. Li, F. Z. Katele, T. Satyanandamurty, J. Wu and G. Bringmann, Beilstein J. Org. Chem., 2014, 10, 276–281, DOI:10.3762/bjoc.10.23
.
- X. Luo, Y. Huang, S. Zhang, H. Yin, C. Li and Q. Li, Biochem. Syst. Ecol., 2014, 56, 191–195, DOI:10.1016/j.bse.2014.05.018
.
- Z.-F. Zhou, O. Taglialatela-Scafati, H.-L. Liu, Y.-C. Gu, L.-Y. Kong and Y.-W. Guo, Fitoterapia, 2014, 97, 192–197, DOI:10.1016/j.fitote.2014.06.009
.
- C. Sarigaputi, D. Sommit, T. Teerawatananond and K. Pudhom, J. Nat. Prod., 2014, 77, 2037–2043, DOI:10.1021/np5003687
.
- Z.-F. Zhou, H.-L. Liu, W. Zhang, T. Kurtán, A. Mándi, A. Bényei, J. Li, O. Taglialatela-Scafati and Y.-W. Guo, Tetrahedron, 2014, 70, 6444–6449, DOI:10.1016/j.tet.2014.07.027
.
- Y.-B. Wu, X. Qing, C.-H. Huo, H.-M. Yan, Q.-W. Shi, F. Sauriol, Y.-C. Gu and H. Kiyota, Tetrahedron, 2014, 70, 4557–4562, DOI:10.1016/j.tet.2014.04.062
.
- M.-Y. Li, Q. Xiao, T. Satyanandamurty and J. Wu, Chem. Biodiversity, 2014, 11, 262–275, DOI:10.1002/cbdv.201300057
.
- W. Chen, L. Shen, M. Li, Q. Xiao, T. Satyanandamurty and J. Wu, Fitoterapia, 2014, 94, 108–113, DOI:10.1016/j.fitote.2014.02.001
.
- Y. Wu, Y. Bai, X. Guo, J. Qi, M. Dong, F. Sauriol, Q. Shi, Y. Gu and C. Huo, Chem. Nat. Compd., 2014, 50, 314–316, DOI:10.1007/s10600-014-0940-x
.
- C. Ito, S. Katsuno, Y. Kondo, H. T.-W. Tan and H. Furukawa, Chem. Pharm. Bull., 2000, 48, 339–343, DOI:10.1248/cpb.48.339
.
- R. Jain, O. Monthakantirat, P. Tengamnuay and W. De-Eknamkul, Molecules, 2014, 19, 6809–6821, DOI:10.3390/molecules19056809
.
- G.-C. Gao, X.-M. Luo, X.-Y. Wei, S.-H. Qi, H. Yin, Z.-H. Xiao and S. Zhang, Helv. Chim. Acta, 2010, 93, 339–344, DOI:10.1002/hlca.200900193
.
- J.-X. Liu, M.-Q. Luo, M. Xia, Q. Wu, S.-M. Long, Y. Hu, G.-C. Gao, X.-L. Yao, M. He, H. Su, X.-M. Luo and S.-Z. Yao, Mar. Drugs, 2014, 12, 2790–2801, DOI:10.3390/md12052790
.
- M. S. J. Carliera, J. Guitton, F. Bévalot, L. Fanton and Y. Gaillard, J. Chromatogr. B: Anal. Technol. Biomed. Life Sci., 2014, 962, 1–8, DOI:10.1016/j.jchromb.2014.05.014
.
- Y. Deng, Y. Liao, J. Li, L. Yang, H. Zhong, Q. Zhou and Z. Qing, Nat. Prod. Commun., 2014, 9, 1265–1268 CAS
.
- A. S. Devi, J. Rajkunar and T. K. Beenish, Asian J. Chem., 2014, 26, 458–460, DOI:10.14233/ajchem.2014.15442
.
- J. Boukouvalas and M.-A. Jean, Tetrahedron Lett., 2014, 55, 4248–4250, DOI:10.1016/j.tetlet.2014.05.054
.
- J.-P. Yin, C.-L. Tang, L.-X. Gao, W.-P. Ma, J.-Y. Li, Y. Li, J. Li and F.-J. Nan, Org. Biomol. Chem., 2014, 12, 3441–3445, 10.1039/c4ob00214h
.
- X. L. Chen, H. L. Liu, J. Li, G. R. Xin and Y. W. Guo, Org. Lett., 2011, 13, 5032–5035, DOI:10.1021/ol201809q
.
- U. W. Hawas, Chem. Nat. Compd., 2014, 50, 629–632, DOI:10.1007/s10600-014-1040-74
.
- M. M. D. Mohammed, A.-H. A. Hamdy, N. M. El-Fiky, W. S. A. Mettwally, A. A. El-Beih and N. Kobayashi, Nat. Prod. Res., 2014, 28, 377–382, DOI:10.1080/14786419.2013.869694
.
- J. Rodríguez, R. M. Nieto, M. Blanco, F. A. Valeriote, C. Jiménez and P. Crews, Org. Lett., 2014, 16, 464–467, DOI:10.1021/ol403350e
.
- T. V. Ovchinnikova, G. M. Aleshina, S. V. Balandin, A. D. Krasnosdembskaya, M. L. Markelov, E. I. Frolova, Y. F. Leonova, A. A. Tagaev, E. G. Krasnodembsky and V. N. Kokryakov, FEBS Lett., 2004, 577, 209–214, DOI:10.1016/j.febslet.2004.10.012
.
- A. L. Maltseva, O. N. Kotenko, V. N. Kokryakov, V. V. Starunov and A. D. Krasnodembskaya, Frontiers in Physiology, 2014, 5, 497, DOI:10.3389/fphys.2014.00497
.
- M. Nakamura, T. Suzuki, N. Ishizaka, J. Sato and S. Inouye, Tetrahedron, 2014, 70, 2161–2168, DOI:10.1016/j.tet.2014.01.075
.
- Y. Kudo, J. Finn, K. Fukushima, S. Sakugawa, Y. Cho, K. Konoki and M. Yotsu-Yamashita, J. Nat. Prod., 2014, 77, 1000–1004, DOI:10.1021/np401097n
.
- S. Subramanian, N. W. Ross and S. L. MacKinnon, Mar. Biotechnol., 2009, 11, 748–757, DOI:10.1007/s10126-009-9189-y
.
- M. Cantisani, E. Finamore, E. Mignogna, A. Falanga, G. F. Nicoletti, C. Pedone, G. Morelli, M. Leone, M. Galdiero and S. Galdiero, Antimicrob. Agents Chemother., 2014, 58, 5280–5290, DOI:10.1128/AAC.02395-149
.
- H.-N. Huang, C.-Y. Pan, Y.-L. Chan, J.-Y. Chen and C.-J. Wu, Antimicrob. Agents Chemother., 2014, 58, 1538–1545, DOI:10.1128/aac.02427-133
.
- M. S. Taleshi, R. K. Seidler-Egdal, K. B. Jensen, T. Schwerdtle and K. A. Francesconi, Organometallics, 2014, 33, 1397–1403, DOI:10.1021/om40110926
.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5np00156k |
| This journal is © The Royal Society of Chemistry 2016 |