Enantioselective palladium-catalyzed arylation of N-tosylarylimines with arylboronic acids using a chiral 2,2′-bipyridine ligand†
Xiang
Gao
,
Bo
Wu
,
Zhong
Yan
and
Yong-Gui
Zhou
*
State Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian 116023, P. R. China. E-mail: ygzhou@dicp.ac.cn
First published on 24th November 2015
Abstract
With the aid of an axially chiral 2,2′-bipyridine ligand, we have successfully developed a palladium-catalyzed method for the enantioselective arylation of N-tosylarylimines, furnishing the chiral diarylmethamines with high yields and enantioselectivities under very mild conditions. An exogenous base was avoided and imine hydrolysis was inhibited in this transformation.
Transition-metal-catalyzed asymmetric addition of organometallic reagents to imines represents one of the most straightforward approaches for the construction of chiral diarylmethamines, which are synthetically important chiral building blocks for pharmaceutically active compounds.1 Thanks to the seminal work by Hayashi,2 Carreira3 and Lin,4 the chiral dienes flourished as steering ligands in rhodium-catalyzed asymmetric reactions, especially the arylation of imines with excellent yields and enantioselectivities.5 Pioneered by these studies, ene-based ligands were extensively studied, P-olefin, N-olefin and S-olefin ligands were developed and applied in rhodium-catalyzed asymmetric addition of arylboronic acids to enones or imines with excellent stereocontrol.6 Therefore, rhodium complexes coordinated with ene-based ligands have emerged as the most versatile catalysts for the asymmetric arylation of imines by arylboronic acids.
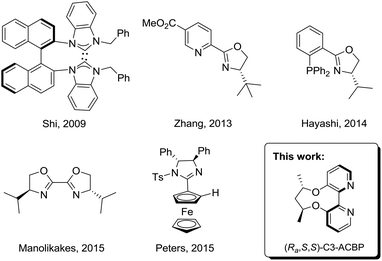
In contrast to rhodium, other transition-metals have received relatively less attention. Several reports have described the copper- or zirconium-catalyzed asymmetric addition of allylboronates or dialkylzinc reagents to ketimines.7 Recently, palladium-catalyzed addition of arylboronic acids to imines has become a subject of keen interest due to the high efficacy and robustness of palladium catalysts.8 A wide array of palladium catalysts coordinated with N-heterocyclic carbene,9 pyridine–oxazoline,10 phosphinooxazoline,11 bisoxazoline,12 and imidazoline13 ligands have been reported in the asymmetric arylation of imines (Scheme 1).14 These elegant studies by Zhang, Hayashi and Peters authenticate that the catalytic proficiency of palladium catalysts has been comparable to that of the best rhodium catalysts in terms of enantioselectivity and activity. Despite this valuable progress made in this area, highly-efficient ligands occupied in this asymmetric transformation are limited and developing new ligands is still desirable. Herein, we report an example of using palladium complexes coordinated with axially chiral 2,2′-bipyridine ligands for the enantioselective arylation of N-tosylarylimines with arylboronic acids, affording the chiral diarylmethamines with up to 94% ee.
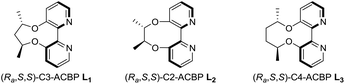
In 2007, Lu and co-workers disclosed a palladium-catalyzed arylation of N-tosylarylimines promoted by an achiral 2,2′-bipyridine ligand.15a They found that the 2,2′-bipyridine ligand was crucial in the reaction by enabling the arylpalladium species to become more nucleophilic, thus making the addition reaction possible. However, with no easily accessible chiral bipyridine ligands in hand, they turned to use chiral pyridine–oxazoline ligands to study the asymmetric version and moderate ee values were obtained.15b Recently, we have reported the design and synthesis of a series of axially chiral 2,2′-bipyridine ligands Cn-ACBP, they exhibited excellent stereocontrol in palladium-catalyzed asymmetric carbene migratory insertion reactions.16 Therefore, we envisioned that our chiral bipyridine ligands could effectively promote the palladium-catalyzed asymmetric arylation of N-tosylarylimines with arylboronic acids.
We began our investigation on the addition of phenylboronic acid to (E)-N-(4-chlorobenzylidene)-4-methylbenzene-sulfonamide 1a catalyzed by Pd(L1)(OCOCF3)2 in trifluoroethanol (TFE) at 60 °C. Gratifyingly, the reaction proceeded smoothly without an exogenous base and furnished 3aa in 99% yield and 82% ee (Table 1, entry 1). Notably, imine hydrolysis couldn't be detected even when the reaction was performed in air. Lowering the reaction temperature resulted in a slight improvement of enantioselectivities (Table 1, entries 2–4). To our satisfactory, the reaction even proceeded well at 0 °C, affording 3aa in 99% yield and 89% ee within 12 h (Table 1, entry 5). Next, a solvent screening revealed that dichloromethane and 1,2-dichloroethane led to a drastic decrease in both reactivity and enantioselectivity (Table 1, entries 6 and 7). Surprisingly, no product was detected when employing MeOH as the solvent (Table 1, entry 8). While reactivity retained, there was a significant drop in enantioselectivity when utilizing C2-ACBP L2 as the ligand (Table 1, entry 9), and the low enantioselectivity might be ascribable to the small dihedral angle.17 C4-ACBP L3 was also examined and a slightly lower ee value was obtained (Table 1, entry 10). Therefore, the optimal reaction conditions were established: using Pd(L1)(OCOCF3)2 as the catalyst and TFE as the solvent to perform the reaction at 0 °C.
| Entry | L | Solvent | T (°C) | Yieldb (%) | eec (%) |
|---|---|---|---|---|---|
| a Reaction conditions: Pd(L)(OCOCF3)2 (5.0 mol%), 1a (0.20 mmol), 2a (0.30 mmol), solvent (3 mL), 5–96 h. b Isolated yield. c Determined by HPLC. d The catalyst was prepared in situ. N/R: No reactivity, DCM: dichloromethane, DCE: 1,2-dichloroethane. | |||||
| 1 | L1 | TFE | 60 | 99 | 82 |
| 2 | L1 | TFE | 40 | 99 | 83 |
| 3 | L1 | TFE | 25 | 99 | 86 |
| 4 | L1 | TFE | 10 | 99 | 87 |
| 5 | L1 | TFE | 0 | 99 | 89 |
| 6 | L1 | DCM | 0 | 41 | 89 |
| 7 | L1 | DCE | 0 | 59 | 88 |
| 8 | L1 | MeOH | 0 | N/R | — |
| 9d | L2 | TFE | 0 | 99 | 4 |
| 10d | L3 | TFE | 0 | 99 | 88 |
|
|||||
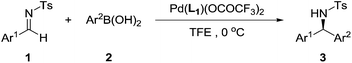
Having identified the optimal reaction conditions, we turned to investigate the substrate scope, and the results are summarized in Table 2. Excellent yields could be obtained regardless of electronic properties of N-tosylarylimines (Table 2, entries 1–12). Though a slight decrease in reactivity was observed when electron-deficient N-tosylarylimines were used (Table 2, entries 2 and 3), enantioselectivities were consistently maintained (89–90% ee). It is noteworthy that electron-rich N-tosylarylimines gave relatively higher ee values than the electron-deficient ones (Table 2, entries 4–12), excellent enantioselectivities were generally obtained (90–93% ee). ortho-Substituents on the N-tosylarylimines dramatically decreased the activity, but excellent yields and enantioselectivities could still be achieved after a prolonged time (entries 6 and 7).
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
| Entry | Ar | t (h) | Yieldb (%) | eec (%) |
|---|---|---|---|---|
| a Reaction conditions: Pd(L1)(OCOCF3)2 (5.0 mol%), 1 (0.20 mmol), 2a (0.30 mmol), TFE (3.0 mL), 0 °C. b Isolated yield. c Determined by HPLC. d Determined by HPLC of the product after being protected by benzoyl chloride. | ||||
| 1 | 4-ClC6H4 (1a) | 12 | 99 (3aa) | 89 (S) |
| 2 | 4-BrC6H4 (1b) | 35 | 96 (3ba) | 89 (S) |
| 3 | 2-BrC6H4 (1c) | 24 | 90 (3ca) | 90 (S) |
| 4 | 4-MeC6H4 (1d) | 22 | 91 (3da) | 90 (S) |
| 5 | 4-MeOC6H4 (1e) | 35 | 99 (3ea) | 91 (S) |
| 6d | 2-HOC6H4 (1f) | 35 | 76 (3fa) | 91 (−) |
| 7 | 2-MeOC6H4 (1g) | 24 | 95 (3ga) | 91 (S) |
| 8 | 3,4-(MeO)2C6H3 (1h) | 42 | 94 (3ha) | 92 (−) |
| 9 | 3,5-(MeO)2C6H3 (1i) | 22 | 90 (3ia) | 90 (−) |
| 10 | 3,4,5-(MeO)3C6H2 (1j) | 36 | 99 (3ja) | 92 (−) |
| 11 | 2-Br-4,5-(MeO)2C6H2 (1k) | 21 | 99 (3ka) | 93 (−) |
| 12 | 1-Naphthyl (1l) | 24 | 99 (3la) | 90 (S) |
In addition, a variety of arylboronic acids was examined for addition to different N-tosylarylimines (Table 3). Steric properties of the substrates obviously affected the reaction activity (Table 3, entries 1–6), the reaction could complete within 24 h when using p-tolylboronic acid (Table 3, entry 1), however, when o-tolylboronic acid was used, it would take 10 days before the total consumption of 1 (Table 3, entry 3). It is amazing to see that the catalyst remained active in such a long reaction time and no imine hydrolysis or aryl–aryl coupling was detected during this period. The desired product was obtained with 97% yield and 94% ee. It was also found that the electronic properties of the arylboronic acids had little influence on enantioselectivities (Table 3, entries 1–10), arylboronic acids bearing electron-withdrawing group Br and F all gave excellent yields and enantioselectivities (Table 3, entries 7 and 8). Extending the reaction time could give complete conversion for all cases, furnishing the corresponding adducts in excellent enantioselectivities (90–94% ee). Additionally, we also employed pyridine-3-boronic acid and furan-2-boronic acid as reaction partners, but unfortunately, neither of the two heteroaryl boronic acids fitted in this protocol and no product was detected, which might attribute to deactivation of the catalyst caused by the coordination of heteroatom with palladium.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
| Entry | 1 | Ar2 | t (h) | Yieldb (%) | eec (%) |
|---|---|---|---|---|---|
| a Reaction conditions: Pd(L1)(OCOCF3)2 (5.0 mol%), 1 (0.20 mmol), 2 (0.30 mmol), TFE (3.0 mL), 0 °C. b Isolated yields based on imines. c Determined by chiral HPLC. | |||||
| 1 | 1k | 4-MeC6H4 (2b) | 24 | 98 (3kb) | 93 (+) |
| 2 | 1k | 3-MeC6H4 (2c) | 36 | 99 (3kc) | 93 (+) |
| 3 | 1k | 2-MeC6H4 (2d) | 240 | 97 (3kd) | 94 (+) |
| 4 | 1k | 4-MeOC6H4 (2e) | 24 | 98 (3ke) | 92 (+) |
| 5 | 1k | 3-MeOC6H4 (2f) | 36 | 99 (3kf) | 93 (+) |
| 6 | 1k | 2-MeOC6H4 (2g) | 24 | 99 (3kg) | 94 (+) |
| 7 | 1k | 4-FC6H4 (2h) | 120 | 99 (3kh) | 94 (+) |
| 8 | 1k | 4-BrC6H4 (2i) | 144 | 95 (3ki) | 93 (+) |
| 9 | 1k | 4-BnOC6H4 (2j) | 24 | 99 (3kj) | 92 (+) |
| 10 | 1k | 1-Naphthyl (2k) | 168 | 99 (3kk) | 92 (+) |
| 11 | 1d | 4-MeC6H4 (2b) | 24 | 87 (3db) | 90 (S) |
| 12 | 1d | 2-MeOC6H4 (2g) | 36 | 77 (3dg) | 90 (R) |
| 13 | 1h | 4-MeC6H4 (2b) | 24 | 98 (3hb) | 90 (−) |
| 14 | 1h | 2-MeOC6H4 (2g) | 48 | 80 (3hg) | 90 (+) |
| 15 | 1j | 4-MeC6H4 (2b) | 24 | 99(3jb) | 92 (+) |
| 16 | 1j | 2-MeOC6H4 (2g) | 48 | 99 (3jg) | 90 (+) |
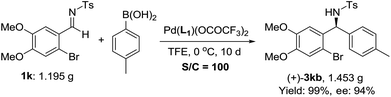
To further demonstrate the practicality, 1 mol% catalyst was used in the addition of 4-methylphenylboronic acid to imine 1k on a gram scale, and the desired product was obtained in 94% ee and 99% yield (Scheme 2). The corresponding product 3kb could be conveniently converted into chiral 3-substituted isoindolinone derivatives, a useful framework frequently found in bioactive molecules.18
Conclusions
In conclusion, we have successfully established a new catalytic protocol by employing Pd-bipyridine complexes as catalysts for asymmetric arylation of N-tosylarylimines, the corresponding chiral diarylmethamines could be obtained with up to 94% ee. This represents the first example of palladium-catalyzed arylation of N-tosylarylimines promoted by a chiral 2,2′-bipyridine ligand. Highlights of this methodology involve an exogenous-base-free transmetalation process, inhibition of imine hydrolysis, and mild reaction conditions. Studies to extend the scope of this methodology and probe the reaction mechanism are currently underway in our laboratory.Acknowledgements
We are grateful for the financial support from the National Natural Science Foundation of China (21372220 & 21532006).Notes and references
- For reviews, see: (a) R. Bloch, Chem. Rev., 1998, 98, 1407 CrossRef CAS PubMed; (b) K. Yamada and K. Tomioka, Chem. Rev., 2008, 108, 2874 CrossRef CAS PubMed; (c) G. K. Friestad and A. K. Mathies, Tetrahedron, 2007, 63, 2541 CrossRef CAS; (d) S. Kobayashi, Y. Mori, J. S. Fossey and M. M. Salter, Chem. Rev., 2011, 111, 2626 CrossRef CAS PubMed.
- (a) T. Hayashi and M. Ishigedani, J. Am. Chem. Soc., 2000, 122, 976 CrossRef CAS; (b) T. Hayashi, K. Ueyama, N. Tokunaga and K. Yoshida, J. Am. Chem. Soc., 2003, 125, 11508 CrossRef CAS PubMed; (c) T. Hayashi, M. Kawai and N. Tokunaga, Angew. Chem., Int. Ed., 2004, 43, 6125 CrossRef CAS PubMed; (d) N. Tokunaga, Y. Otomaru, K. Okamoto, K. Ueyama, R. Shintani and T. Hayashi, J. Am. Chem. Soc., 2004, 126, 13584 CrossRef CAS PubMed.
- (a) C. Fisher, C. Defieber, T. Suzuki and E. M. Carreira, J. Am. Chem. Soc., 2004, 126, 1628 CrossRef PubMed; (b) C. Defieber, J.-F. Paquin, S. Serna and E. M. Carreira, Org. Lett., 2004, 6, 3873 CrossRef CAS PubMed.
- (a) Z.-Q. Wang, C.-G. Feng, M.-H. Xu and G.-Q. Lin, J. Am. Chem. Soc., 2007, 129, 5336 CrossRef CAS PubMed; (b) C.-G. Feng, Z.-Q. Wang, P. Tian, M.-H. Xu and G.-Q. Lin, Chem. – Asian J., 2008, 3, 1511 CrossRef CAS PubMed; (c) C.-G. Feng, Z.-Q. Wang, C. Shao, M.-H. Xu and G.-Q. Lin, Org. Lett., 2008, 10, 4101 CrossRef CAS PubMed.
- For reviews on chiral olefins as steering ligands in asymmetric catalysis: (a) F. Glorius, Angew. Chem., Int. Ed., 2004, 43, 3364 CrossRef CAS PubMed; (b) C. Defieber, H. Grützmacher and E. M. Carreira, Angew. Chem., Int. Ed., 2008, 47, 4482 CrossRef CAS PubMed; (c) J. B. Johnson and T. Rovis, Angew. Chem., Int. Ed., 2008, 47, 840 CrossRef CAS PubMed; (d) R. Shintani and T. Hayashi, Aldrichimica Acta, 2009, 42, 31 CAS; (e) P. Tian, H.-Q. Dong and G.-Q. Lin, ACS Catal., 2012, 2, 95 CrossRef CAS; (f) Y. Li and M.-H. Xu, Chem. Commun., 2014, 50, 3771 RSC. For selected examples, see: (g) K. M.-H. Lim and T. Hayashi, J. Am. Chem. Soc., 2015, 137, 3201 CrossRef CAS PubMed; (h) Y. Huang and T. Hayashi, J. Am. Chem. Soc., 2015, 137, 7556 CrossRef CAS PubMed; (i) C. M. So, S. Kume and T. Hayashi, J. Am. Chem. Soc., 2013, 135, 10990 CrossRef CAS PubMed; (j) Y.-J. Chen, Y.-H. Chen, C.-G. Feng and G.-Q. Lin, Org. Lett., 2014, 16, 3400 CrossRef CAS PubMed; (k) Z. Cui, H.-J. Yu, R.-F. Yang, W.-Y. Gao, C.-G. Feng and G.-Q. Lin, J. Am. Chem. Soc., 2011, 133, 12394 CrossRef CAS PubMed.
- For selected examples on chiral olefin ligands, see: (a) H. Wang, T. Jiang and M.-H. Xu, J. Am. Chem. Soc., 2013, 129, 5336 CrossRef PubMed; (b) T. Jiang, Z. Wang and M.-H. Xu, Org. Lett., 2015, 17, 528 CrossRef CAS PubMed; (c) M. Ogasawara, Y.-Y. Tseng, S. Arae, T. Morita, T. Nakaya, W.-Y. Wu, T. Takahashi and K. Kamikawa, J. Am. Chem. Soc., 2014, 136, 9377 CrossRef CAS PubMed; (d) R. Shintani, W.-L. Duan, T. Nagano, A. Okada and T. Hayashi, Angew. Chem., Int. Ed., 2005, 44, 4611 CrossRef CAS PubMed; (e) M. Roggen and E. M. Carreira, J. Am. Chem. Soc., 2010, 132, 11917 CrossRef CAS PubMed; (f) M. A. Schafroth, D. Sarlah, S. Krautwald and E. M. Carreira, J. Am. Chem. Soc., 2012, 134, 20276 CrossRef CAS PubMed; (g) S.-S. Jin, H. Wang and M.-H. Xu, Chem. Commun., 2011, 47, 7230 RSC; (h) S.-S. Jin, H. Wang, T.-S. Zhu and M.-H. Xu, Org. Biomol. Chem., 2012, 10, 1764 RSC.
- (a) R. Wada, T. Shibuguchi, S. Makino, K. Oisaki, M. Kanai and M. Shibasaki, J. Am. Chem. Soc., 2006, 128, 7687 CrossRef CAS PubMed; (b) C. Lauzon and A. Charette, Org. Lett., 2006, 8, 2743 CrossRef CAS PubMed; (c) P. Fu, M. L. Snapper and A. H. Hoveyda, J. Am. Chem. Soc., 2008, 130, 5530 CrossRef CAS PubMed.
- For a recent review, see: Y.-W. Sun, P.-L. Zhu, Q. Xu and M. Shi, RSC Adv., 2013, 3, 3153 RSC.
- G.-N. Ma, T. Zhang and M. Shi, Org. Lett., 2009, 11, 875 CrossRef CAS PubMed.
- G. Yang and W. Zhang, Angew. Chem., Int. Ed., 2013, 52, 7540 CrossRef CAS PubMed.
- C. Jiang, Y. Lu and T. Hayashi, Angew. Chem., Int. Ed., 2014, 53, 9936 CrossRef CAS PubMed.
- T. Beisel and G. Manolikakes, Org. Lett., 2015, 17, 3162 CrossRef CAS PubMed.
- C. Schrapel and R. Peters, Angew. Chem., Int. Ed., 2015, 54, 10289 CrossRef CAS PubMed.
- For other recent examples on palladium-catalyzed arylation of imines, see: (a) J. Chen, X. Lu, W. Lou, Y. Ye, H. Jiang and W. Zeng, J. Org. Chem., 2012, 77, 8541 CrossRef CAS PubMed; (b) Y. Álvarez-Casao, D. Monge, E. Álvarez, R. Fernández and J. M. Lassaletta, Org. Lett., 2015, 17, 5104 CrossRef PubMed.
- (a) H. Dai and X. Lu, Org. Lett., 2007, 9, 3077 CrossRef CAS PubMed; (b) H. Dai and X. Lu, Tetrahedron Lett., 2009, 50, 3478 CrossRef CAS.
- X. Gao, B. Wu, W.-X. Huang, M.-W. Chen and Y.-G. Zhou, Angew. Chem., Int. Ed., 2015, 54, 11956 CrossRef CAS PubMed . L2 was synthesized by Milani in 1996, see: B. Milani, E. Alessio, G. Mestroni, E. Zangrando, L. Randaccio and G. Consiglio, J. Chem. Soc., Dalton Trans., 1996, 1021 RSC.
- J. Durand, E. Zangrando, C. Carfagna and B. Milani, Dalton Trans., 2008, 2171 RSC.
- (a) M. Fujioka, T. Morimoto, T. Tsumagari, H. Tanimoto, Y. Nishiyama and K. Kakiuchi, J. Org. Chem., 2012, 77, 2911 CrossRef CAS PubMed; (b) T. L. Stuk, B. K. Assink, R. C. Bates, D. T. Erdman, V. Fedij, S. M. Jennings, J. A. Lassig, R. J. Smith and T. L. Smith, Org. Process Res. Dev., 2003, 7, 851 CrossRef CAS; (c) J. R. Atack, Expert Opin. Invest. Drugs, 2005, 14, 601 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ob02330k |
| This journal is © The Royal Society of Chemistry 2016 |