Open Access Article
Open Access ArticleThe total synthesis and functional evaluation of fourteen stereoisomers of yaku'amide B. The importance of stereochemistry for hydrophobicity and cytotoxicity†
Hiroyuki
Mutoh
,
Yusuke
Sesoko
,
Takefumi
Kuranaga‡
,
Hiroaki
Itoh
and
Masayuki
Inoue
*
Graduate School of Pharmaceutical Sciences, The University of Tokyo, 7-3-1 Hongo, Bunkyo-ku, Tokyo 113-0033, Japan. E-mail: inoue@mol.f.u-tokyo.ac.jp; Fax: +81 3-5841-0568
First published on 15th April 2016
Abstract
Yaku'amide B is a highly unsaturated linear tridecapeptide and an extremely potent cytotoxin. Herein, we describe the synthesis of fourteen new stereoisomers of yaku'amide B using a unified assembly strategy. The hydrophobicities and cytotoxicities of these analogues were analyzed, along with those of four previously prepared isomers. Although all of the analogues share a common planar structure, their log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D values varied significantly (3.39–5.32), presumably reflecting their distinct three-dimensional shapes. Subnanomolar-level cytotoxicity was observed for the natural yaku'amide B and its epimer of the N-terminal acyl group, whereas the other sixteen isomers exhibited 13- to 1200-fold weaker activities than that of the natural isomer. These data indicated the importance of the overall stereostructure of the 13-mer sequence of yaku'amide B for exerting its potent toxicity.
D values varied significantly (3.39–5.32), presumably reflecting their distinct three-dimensional shapes. Subnanomolar-level cytotoxicity was observed for the natural yaku'amide B and its epimer of the N-terminal acyl group, whereas the other sixteen isomers exhibited 13- to 1200-fold weaker activities than that of the natural isomer. These data indicated the importance of the overall stereostructure of the 13-mer sequence of yaku'amide B for exerting its potent toxicity.
Introduction
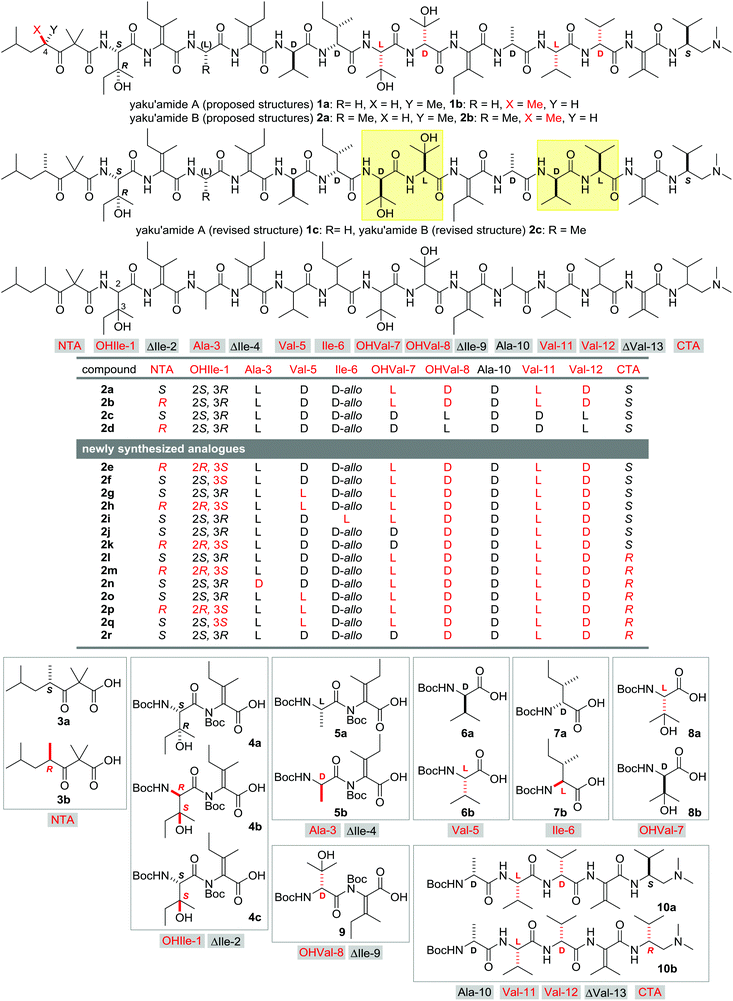
Yaku'amides A (1) and B (2) were isolated from a deep sea sponge Ceratopsion sp. (Fig. 1) and were reported to exhibit exceptionally potent cytotoxicity towards P388 murine leukemia cells.1 Cytotoxicity assays of 1 using a panel of 39 human cancer cell lines (JFCR39)2 unveiled its distinct growth-inhibitory profile in comparison with 38 clinically available anticancer drugs, suggesting its potentially novel mode of action. The linear tridecapeptide sequences of 1 and 2 only differ in their third residue (Gly-3 for 1 and Ala-3 for 2), and both compounds contain four tetrasubstituted α,β-dehydroamino acids3 (ΔIle-2, ΔIle-4, ΔIle-9, and ΔVal-13) and seven other non-proteinogenic amino acids. Additionally, an N-terminal acyl (NTA) group and a C-terminal amine (CTA) cap the N- and C-termini, respectively. These unusual structural features and potent biological activities of 1 and 2 have spurred interest in the chemical and biological communities.4Since only minute amounts of 1 and 2 have been obtained from natural sources (1: 1.3 mg; 2: 0.3 mg), their structural determination is incomplete and detailed biological studies have been hampered. To address these issues, we launched synthetic studies of 1 and 2 as the initial phase for further investigations of their structures and bioactivities. Consequently, the C4-epimers of the NTA moiety of the proposed structures 1a/b and 2a/b were chemically constructed by developing a new protocol for construction of the α,β-unsaturated amino acid residues.5,6 Namely, Cu-catalyzed C(sp2)–N bond formation between the primary amides and the alkenyl iodides was adopted to construct four building blocks with the tetrasubstituted enamide moieties (4a, 5a, 9, and 10a).7 Then, the enamide building blocks and the protected amino acids were condensed stepwise to furnish the possible proposed structures 1a/b and 2a/b. However, both the NTA-epimers 1a/b and 2a/b exhibited different chromatographic behaviors from naturally occurring 1 and 2, respectively. This finding prompted investigations directed toward determining the true structures of 1 and 2. Structural information was gathered through degradative studies of 0.05 mg of the available 2 and synthetic preparation of all the possible structures of the degraded partial structures. These studies allowed us to establish the structures of the natural 1 and 2 to be 1c and 2c. Both the revised structures possess the inverse configurations at the Cα-positions of the four amino acid residues (OHVal-7, -8, Val-11, and -12) of the proposed structures, and the S-configuration at C4 of NTA. Compounds 1c and 2c were then prepared by total syntheses to confirm their structural authenticity.
During the process of structural revision by a combination of degradation and synthesis, the total syntheses of the multiple potential isomers of the natural 2 were independently performed without achieving the revised structure 2c. Specifically, the fourteen analogues 2e–r having different amino acid stereochemistries from those of the proposed structures 2a/b were synthesized. Upon analysis by ultrahigh-performance liquid chromatography (UHPLC), all of the analogues 2e–r provided retention times distinct from that of the natural 2, indicating that they did not correspond to the true structure. Although the syntheses of 2e–r did not contribute to the structural elucidation of 2c, this focused library of stereoisomers provided us with a valuable opportunity for investigating the structure–activity relationship (SAR) of yaku'amide B (2c).8 Compounds 2e–r share the same planar structure and thus their functional evaluations would clarify the stereochemical requirement of the amino-acid residues for the unique physicochemical and potent biological properties of 2c. Furthermore, the SAR study would offer useful insight into the unknown mode of action of 2c. Herein, we report the syntheses and functional analyses of the fourteen analogues 2e–r of yaku'amide B (2c). These studies showed that the configurational changes of the main chain of 2c significantly affected the hydrophobicities and greatly diminished the cytotoxicities. Only 2c and its C4-epimer of NTA 2d displayed subnanomolar-level cytotoxicity towards P388 cells, demonstrating the biological importance of the main chain stereochemistry.
Results and discussion
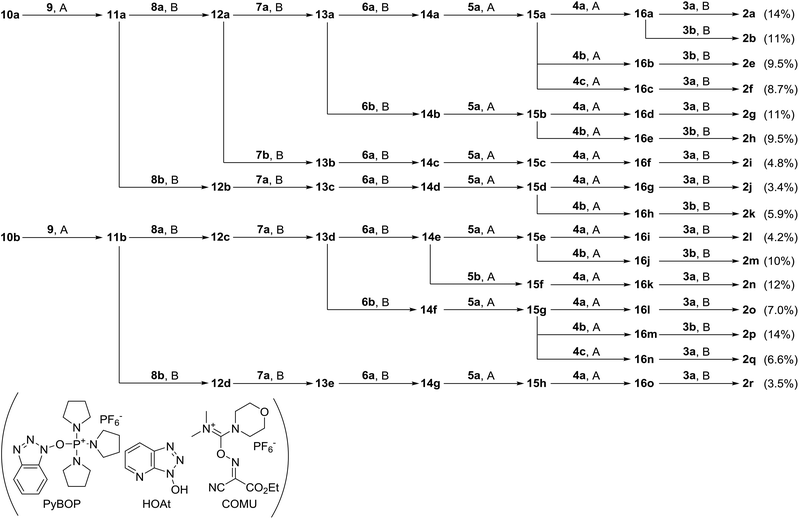
The fourteen new analogues of yaku'amide B, 2e–r, have different configurations from the natural 2c at NTA, OHIle-1, Ala-3, Val-5, Ile-6, OHVal-7, OHVal-8, Val-11, Val-12, and/or CTA (Fig. 1), while the double-bond geometries of ΔIle-2, -4, and -9 and the D-stereochemistry of Ala-10 are retained. As 2e–r were designed based on the proposed structures 2a/b, all of these peptides possess the same stereochemistries at OHVal-8, Val-11, and Val-12. We previously reported the total syntheses of 1a–c and 2a–d by assembling eight building blocks. This efficient strategy was applied to the syntheses of 2e–r by switching the fragments to their corresponding stereoisomers. Accordingly, sixteen fragments were necessary for the construction of 2e–r (NTA: 3a and 3b; OHIle-1-ΔIle-2: 4a, 4b, and 4c; Ala-3-ΔIle-4: 5a and 5b; Val-5: 6a and 6b; Ile-6: 7a and 7b; OHVal-7: 8a and 8b; OHVal-8-ΔIle-9: 9; Ala-10-Val-11-Val-12-ΔVal-13-CTA: 10a and 10b). While 3a/b, 4a, 5a, 6a/b, 7a/b, 8a/b, 9 and 10a/b were previously prepared on the way toward the total synthesis and structural elucidation of 1c and 2c,5 dipeptides 4b (enantiomer of 4a), 4c (diastereomer of 4a) and 5b (enantiomer of 5a) were newly obtained by using Cu-mediated C(sp2)–N bond formation from the corresponding monomers.9To produce the analogues 2e–r, the sixteen synthetic fragments were condensed according to the assembly procedure that was optimized for the route to 2a and 2b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5a (Scheme 1). The unified total syntheses of the known 2a/b and the new 2e–r started with the TFA-promoted removal of the Nα-Boc group from 4-mer derivatives 10a and 10b. The obtained two amines were separately treated with carboxylic acid 9 in the presence of PyBOP
5a (Scheme 1). The unified total syntheses of the known 2a/b and the new 2e–r started with the TFA-promoted removal of the Nα-Boc group from 4-mer derivatives 10a and 10b. The obtained two amines were separately treated with carboxylic acid 9 in the presence of PyBOP![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 10/HOAt
10/HOAt![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 11 to generate the protected 6-mer peptides 11a and 11b, respectively. Detachment of the two Boc groups of OHVal-8 and ΔIle-9 of 11a and 11b with TFA afforded the corresponding amines, which were amidated with 8a or 8b by the action of COMU,12 giving rise to the four 7-mer derivatives 12a/b and 12c/d, respectively. Stepwise elongation from 12a–d using the five building blocks completed the syntheses of 2a/b/e–r. Namely, two cycles of TFA-mediated Nα-deprotection and COMU-promoted condensation using 7a/b and 6a/b converted 12a–d to the seven 9-mer peptides (12a–d → 13a–e → 14a–g). The obtained compounds 14a–g were then transformed to fifteen 13-mer peptides 16a–o through repeated Boc removal and PyBOP/HOAt-promoted conjugation using 5a/b or 4a–c (14a–g → 15a–h → 16a–o). Finally, COMU enabled the coupling of the NTA unit 3a/b with 16a–o, delivering the sixteen yaku'amide analogues 2a, 2b, and 2e–r. As a result of the present divergent synthesis, the fourteen new peptides 2e–r were efficiently constructed in 14 steps from 9 in reasonable yields (2a, 2b, 2e, 2f, 2g, 2h, 2i, 2j, 2k, 2l, 2m, 2n, 2o, 2p, 2q, and 2r for 14, 11, 9.5, 8.7, 11, 9.5, 4.8, 3.4, 5.9, 4.2, 10, 12, 7.0, 14, 6.6, and 3.5%, respectively). These achievements corroborated the high adaptability of our synthetic strategy for the preparation of diverse yaku'amide isomers.
11 to generate the protected 6-mer peptides 11a and 11b, respectively. Detachment of the two Boc groups of OHVal-8 and ΔIle-9 of 11a and 11b with TFA afforded the corresponding amines, which were amidated with 8a or 8b by the action of COMU,12 giving rise to the four 7-mer derivatives 12a/b and 12c/d, respectively. Stepwise elongation from 12a–d using the five building blocks completed the syntheses of 2a/b/e–r. Namely, two cycles of TFA-mediated Nα-deprotection and COMU-promoted condensation using 7a/b and 6a/b converted 12a–d to the seven 9-mer peptides (12a–d → 13a–e → 14a–g). The obtained compounds 14a–g were then transformed to fifteen 13-mer peptides 16a–o through repeated Boc removal and PyBOP/HOAt-promoted conjugation using 5a/b or 4a–c (14a–g → 15a–h → 16a–o). Finally, COMU enabled the coupling of the NTA unit 3a/b with 16a–o, delivering the sixteen yaku'amide analogues 2a, 2b, and 2e–r. As a result of the present divergent synthesis, the fourteen new peptides 2e–r were efficiently constructed in 14 steps from 9 in reasonable yields (2a, 2b, 2e, 2f, 2g, 2h, 2i, 2j, 2k, 2l, 2m, 2n, 2o, 2p, 2q, and 2r for 14, 11, 9.5, 8.7, 11, 9.5, 4.8, 3.4, 5.9, 4.2, 10, 12, 7.0, 14, 6.6, and 3.5%, respectively). These achievements corroborated the high adaptability of our synthetic strategy for the preparation of diverse yaku'amide isomers.
The completion of the total syntheses of 2a–d and the fourteen new analogues 2e–r permitted us to conduct systematic functional analyses. Since the overall hydrophobicity influences the molecular behavior and interaction with the cell membrane and biomolecules, it is one of the most critical factors affecting the biological activity of a compound. Accordingly, the hydrophobicities of isomers 2a–r were estimated by determination of their octanol/water distribution coefficients (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D).13 To calculate the log
D).13 To calculate the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D values of 2a–r, the retention times of 2a–r and the standard samples with known log
D values of 2a–r, the retention times of 2a–r and the standard samples with known log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D values were compared under identical UHPLC conditions employing an ODS column.14–16 Although all the peptides possess the same planar structure, the numbers of log
D values were compared under identical UHPLC conditions employing an ODS column.14–16 Although all the peptides possess the same planar structure, the numbers of log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D varied significantly, from 3.39 to 5.32 (Table 1), where the values of the natural 2c (4.13) and NTA-epimer 2d (4.34) were close to the average. As the amino acid monomers at the same residue numbers of 2a–r are enantiomeric with the exception of several diastereomeric monomers (OHIle-1 of 2f/q and Ile-6 of 2i), the hydrophobicities of most individual monomers are identical. Therefore, the diverse log
D varied significantly, from 3.39 to 5.32 (Table 1), where the values of the natural 2c (4.13) and NTA-epimer 2d (4.34) were close to the average. As the amino acid monomers at the same residue numbers of 2a–r are enantiomeric with the exception of several diastereomeric monomers (OHIle-1 of 2f/q and Ile-6 of 2i), the hydrophobicities of most individual monomers are identical. Therefore, the diverse log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D values of 2a–r would originate from differences in the entire three-dimensional structure of each compound.17 Specifically, the main-chain stereochemistries of 2a–r would organize the locations and orientations of the hydrophobic side-chains of the molecules, thereby changing the hydrophobic interactions between the peptides and the ODS column. Thus, quantification of the log
D values of 2a–r would originate from differences in the entire three-dimensional structure of each compound.17 Specifically, the main-chain stereochemistries of 2a–r would organize the locations and orientations of the hydrophobic side-chains of the molecules, thereby changing the hydrophobic interactions between the peptides and the ODS column. Thus, quantification of the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D values demonstrated the significant effect of configurational alterations on the hydrophobicity and the molecular shape of this family of compounds.
D values demonstrated the significant effect of configurational alterations on the hydrophobicity and the molecular shape of this family of compounds.
| Compound | log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Da Da |
IC50![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (nM) b (nM) |
|---|---|---|
a log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D values were measured by UHPLC methods.
b IC50 values were determined from the results of growth inhibition assays (XTT method) performed on P388 mouse leukemia cells. XTT = 3′-[1-[(phenylamino)-carbonyl]-3,4-tetrazolium]bis(4-methoxy-6-nitro)-benzenesulfonic acid hydrate. D values were measured by UHPLC methods.
b IC50 values were determined from the results of growth inhibition assays (XTT method) performed on P388 mouse leukemia cells. XTT = 3′-[1-[(phenylamino)-carbonyl]-3,4-tetrazolium]bis(4-methoxy-6-nitro)-benzenesulfonic acid hydrate.
|
||
| 2a | 3.48 | 98 |
| 2b | 3.39 | 410 |
| 2c | 4.13 | 0.51 |
| 2d | 4.34 | 0.95 |
| 2e | 3.57 | 620 |
| 2f | 3.56 | 450 |
| 2g | 3.99 | 92 |
| 2h | 3.91 | 530 |
| 2i | 3.39 | 300 |
| 2j | 4.25 | 9.3 |
| 2k | 4.40 | 34 |
| 2l | 3.93 | 23 |
| 2m | 4.11 | 93 |
| 2n | 4.09 | 73 |
| 2o | 3.92 | 45 |
| 2p | 3.90 | 260 |
| 2q | 4.06 | 100 |
| 2r | 5.32 | 6.8 |
Next, the cytotoxicities of the synthetic analogues against P388 mouse leukemia cells were assessed using the XTT assay method (Table 1).18 The natural isomer 2c exhibited subnanomolar-level cytotoxicity, with an IC50 value of 0.51 nM. Whereas the stereochemical inversion of the C4 of NTA (2d) induced no drastic change in cytotoxicity (IC50 = 0.95 nM), alteration of the main chain stereochemistry decreased the cytotoxicities of the peptides by 13- (6.8 nM, 2r) to 1200-fold (620 nM, 2e). Interestingly, 2j and 2r that have the identical left half structure (NTA to OHVal-7) to the natural 2c are the only analogues among 2a/b/e–r with nanomolar-level toxicities. On the other hand, the toxicities of the originally proposed structures 2a and 2b were 190 and 800 times weaker than those of 2c, supporting the validity of the revised structure. Overall, the greater potency of 2c/d compared to the other fourteen isomers indicated the biological importance of the configurations of the thirteen amino-acid residues of 2c/d.
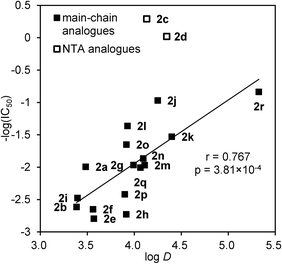
To investigate the relationship between the physicochemical and biological properties, the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D values of the eighteen yaku'amide isomers 2a–r were plotted against their IC50 numbers (Fig. 2). Consequently, these two values of the sixteen non-natural analogues 2a, 2b, and 2e–r were found to have a modest linear relationship with the more hydrophobic analogues among 2a, 2b and 2e–r generally exhibiting a higher cytotoxicity. Because of the variable stereostructures of 2a, 2b and 2e–r, the cytotoxicities of these non-natural analogues would relate to their non-specific hydrophobic interactions with membranes or biomolecules. In contrast, the naturally occurring isomer 2c and its NTA epimer 2d located well beyond the approximate straight line of the other sixteen analogues. For instance, the cytotoxicities of 2c and 2d were significantly higher than those of 2j and 2k, despite their similar log
D values of the eighteen yaku'amide isomers 2a–r were plotted against their IC50 numbers (Fig. 2). Consequently, these two values of the sixteen non-natural analogues 2a, 2b, and 2e–r were found to have a modest linear relationship with the more hydrophobic analogues among 2a, 2b and 2e–r generally exhibiting a higher cytotoxicity. Because of the variable stereostructures of 2a, 2b and 2e–r, the cytotoxicities of these non-natural analogues would relate to their non-specific hydrophobic interactions with membranes or biomolecules. In contrast, the naturally occurring isomer 2c and its NTA epimer 2d located well beyond the approximate straight line of the other sixteen analogues. For instance, the cytotoxicities of 2c and 2d were significantly higher than those of 2j and 2k, despite their similar log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D values. Taken together, these data suggested a mode of action of 2c and 2d distinct from that of 2a, 2b and 2e–r. Hence, it is likely that peptides 2c and 2d display their bioactivities through specific binding to a chiral biomolecule rather than through non-specific hydrophobic interactions. According to this hypothesis, the main chain stereochemistry of 2c and 2d would fold into the appropriate three dimensional structure, form a complementary surface to a target biomolecule, and thereby cause a potent toxic effect on cancer cells.
D values. Taken together, these data suggested a mode of action of 2c and 2d distinct from that of 2a, 2b and 2e–r. Hence, it is likely that peptides 2c and 2d display their bioactivities through specific binding to a chiral biomolecule rather than through non-specific hydrophobic interactions. According to this hypothesis, the main chain stereochemistry of 2c and 2d would fold into the appropriate three dimensional structure, form a complementary surface to a target biomolecule, and thereby cause a potent toxic effect on cancer cells.
Conclusions
The fourteen analogues 2e–r of the naturally occurring yaku'amide B (2c) were newly synthesized in a divergent fashion by assembling stereoisomers of the eight fragments 3–10. The physicochemical and biological properties of the prepared 2e–r were systematically evaluated and analyzed along with the originally proposed structures 2a/b, the natural 2c, and the C4-epimer of NTA 2d. Although all the isomers 2a–r share the same planar structure, they showed diverse hydrophobicities and cytotoxicities against P388 mouse leukemia cells. The natural 2c and the NTA-epimer 2d had average hydrophobicity values (log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D), yet they were 13–1200 times more cytotoxic compared to the sixteen non-natural isomers 2a, 2b, and 2e–r. The linear correlation between the log
D), yet they were 13–1200 times more cytotoxic compared to the sixteen non-natural isomers 2a, 2b, and 2e–r. The linear correlation between the log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D and IC50 values of the weaker analogues 2a/b/e–r suggested that the bioactivities of these analogues would be affected through non-specific hydrophobic interactions. On the other hand, the excellent potency of 2c and 2d would originate from a mode of action distinct from that of the other isomers. Thus, the main chain stereochemistries of 2c and 2d would be important for folding into a specific three-dimensional structure, which binds to a target biomolecule of the cell to display its cytotoxicity. Detailed biological studies to prove this hypothesis are currently underway in our laboratory.
D and IC50 values of the weaker analogues 2a/b/e–r suggested that the bioactivities of these analogues would be affected through non-specific hydrophobic interactions. On the other hand, the excellent potency of 2c and 2d would originate from a mode of action distinct from that of the other isomers. Thus, the main chain stereochemistries of 2c and 2d would be important for folding into a specific three-dimensional structure, which binds to a target biomolecule of the cell to display its cytotoxicity. Detailed biological studies to prove this hypothesis are currently underway in our laboratory.
Acknowledgements
This research was financially supported by Grant-in-Aids for Scientific Research (A) to M. I., for Young Scientists (B) to T. K., and for Research Activity Start-up to H. I. A fellowship to H. M. from Graduate Program for Leaders in Life Innovation (MEXT) is gratefully acknowledged.Notes and references
- R. Ueoka, Y. Ise, S. Ohtsuka, S. Okada, T. Yamori and S. Matsunaga, J. Am. Chem. Soc., 2010, 132, 17692 CrossRef CAS PubMed.
- (a) S. Yaguchi, Y. Fukui, I. Koshimizu, H. Yoshimi, T. Matsuno, H. Gouda, S. Hirono, K. Yamazaki and T. Yamori, J. Natl. Cancer Inst., 2006, 98, 545 CrossRef CAS PubMed; (b) T. Yamori, Cancer Chemother. Pharmacol., 2003, 52, S74 CrossRef CAS PubMed; (c) T. Yamori, A. Matsunaga, S. Sato, K. Yamazaki, A. Komi, K. Ishizu, I. Mita, H. Edatsugi, Y. Matsuba, K. Takezawa, O. Nakanishi, H. Kohno, Y. Nakajima, H. Komatsu, T. Andoh and T. Tsuruo, Cancer Res., 1999, 59, 4042 CAS.
- For a recent review on α,β-dehydroamino acids, see: J. Jiang, Z. Ma and S. L. Castle, Tetrahedron, 2015, 71, 5431 CrossRef CAS.
- For recent reviews on the application of natural products to drug discovery, see: (a) D. J. Newman and G. M. Cragg, J. Nat. Prod., 2016, 79, 629 CrossRef CAS PubMed; (b) M. S. Butler, A. A. B. Robertson and M. A. Cooper, Nat. Prod. Rep., 2014, 31, 1612 RSC; (c) J. W.-H. Li and J. C. Vederas, Science, 2009, 325, 161 CrossRef PubMed.
- (a) T. Kuranaga, H. Mutoh, Y. Sesoko, T. Goto, S. Matsunaga and M. Inoue, J. Am. Chem. Soc., 2015, 137, 9443 CrossRef CAS PubMed; (b) T. Kuranaga, Y. Sesoko, K. Sakata, N. Maeda, A. Hayata and M. Inoue, J. Am. Chem. Soc., 2013, 135, 5467 CrossRef CAS PubMed.
- For synthetic studies on yaku'amides from other groups, see: (a) Z. Ma, J. Jiang, S. Luo, Y. Cai, J. M. Cardon, B. M. Kay, D. H. Ess and S. L. Castle, Org. Lett., 2014, 16, 4044 CrossRef CAS PubMed; (b) C. J. Saavedra, A. Boto and R. Hernández, Org. Lett., 2012, 14, 3788 CrossRef CAS PubMed; (c) Z. Ma, B. C. Naylor, B. M. Loertscher, D. D. Hafen, J. M. Li and S. L. Castle, J. Org. Chem., 2012, 77, 1208 CrossRef CAS PubMed.
- For reviews on Cu-mediated enamide formation, see: (a) T. Kuranaga, Y. Sesoko and M. Inoue, Nat. Prod. Rep., 2014, 31, 514 RSC; (b) G. Evano, C. Theunissen and A. Pradal, Nat. Prod. Rep., 2013, 30, 1467 RSC; (c) D. S. Surry and S. L. Buchwald, Chem. Sci., 2010, 1, 13 RSC; (d) D. Ma and Q. Cai, Acc. Chem. Res., 2008, 41, 1450 CrossRef CAS PubMed; (e) G. Evano, N. Blanchard and M. Toumi, Chem. Rev., 2008, 108, 3054 CrossRef CAS PubMed.
- For recent accounts on the total synthesis and biological evaluation of peptidic natural products from our laboratory, see: antillatoxin. (a) M. Inoue, Proc. Jpn. Acad., Ser. B, 2014, 90, 56 CrossRef CAS; polytheonamide B. (b) H. Itoh and M. Inoue, Acc. Chem. Res., 2013, 46, 1567 CrossRef CAS PubMed.
- See the ESI† for details.
- J. Coste, D. Le-Nguyen and B. Castro, Tetrahedron Lett., 1990, 31, 205 CrossRef CAS.
- L. A. Carpino, J. Am. Chem. Soc., 1993, 115, 4397 CrossRef CAS.
- A. El-Faham, R. S. Funosas, R. Prohens and F. Albericio, Chem. – Eur. J., 2009, 15, 9404 CrossRef CAS PubMed.
- (a) D. Gaspar, A. S. Veiga and M. A. R. B. Castanho, Front. Microbiol., 2013, 4, 294 Search PubMed; (b) V. Teixeira, M. J. Feio and M. Bastos, Prog. Lipid Res., 2012, 51, 149 CrossRef CAS PubMed.
- OECD, Test No. 117. Partition Coefficient (n-octanol/water), HPLC Method, OECD Guidelines for the Testing of Chemicals, Section 1, OECD Publishing, Paris, 2004 Search PubMed.
- log
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) D values were measured in the presence of 0.05% TFA as a buffer, because protonation of the CTA moiety under acidic conditions was required for the reliability and reproducibility of UHPLC analyses of the analogues. Detailed conditions are described in the ESI.†.
D values were measured in the presence of 0.05% TFA as a buffer, because protonation of the CTA moiety under acidic conditions was required for the reliability and reproducibility of UHPLC analyses of the analogues. Detailed conditions are described in the ESI.†. - For recent examples, see: (a) A. Hayata, H. Itoh, S. Matsutaka and M. Inoue, Chem. – Eur. J., 2016, 22, 3370 CrossRef CAS PubMed; (b) H. Itoh, S. Matsutaka, T. Kuranaga and M. Inoue, Tetrahedron Lett., 2014, 55, 728 CrossRef CAS; (c) L. Ferrins, M. Gazdik, R. Rahmani, S. Varghese, M. L. Sykes, A. J. Jones, V. M. Avery, K. L. White, E. Ryan, S. A. Charman, M. Kaiser, C. A. S. Bergström and J. B. Baell, J. Med. Chem., 2014, 57, 6393 CrossRef CAS PubMed; (d) P. P. Bose, U. Chatterjee, I. Hubatsch, P. Artursson, T. Govender, H. G. Kruger, M. Bergh, J. Johansson and P. I. Arvidsson, Bioorg. Med. Chem., 2010, 18, 5896 CrossRef CAS PubMed; (e) E. H. Kerns, L. Di, S. Petusky, T. Kleintop, D. Huryn, O. McConnell and G. Carter, J. Chromatogr., B, 2003, 791, 381 CrossRef CAS.
- For a recent related example, see: Y. Huang, L. Pan, L. Zhao, C. T. Mant, R. S. Hodges and Y. Chen, Biomed. Chromatogr., 2014, 28, 511 CrossRef CAS PubMed.
- (a) D. A. Scudiero, R. H. Shoemaker, K. D. Paull, A. Monks, S. Tierney, T. H. Nofziger, M. J. Currens, D. Seniff and M. R. Boyd, Cancer Res., 1988, 48, 4827 CAS; (b) N. W. Roehm, G. H. Rodgers, S. M. Hatfield and A. L. Glasebrook, J. Immunol. Methods, 1991, 142, 257 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Experimental procedures and assay data. See DOI: 10.1039/c6ob00640j |
| ‡ Current address: Faculty of Pharmaceutical Sciences, Hokkaido University, Kita 12-jo Nishi 6-chome, Kita-ku, Sapporo, 060-0812, Japan. |
| This journal is © The Royal Society of Chemistry 2016 |