Yeojin Choi, Tanmay Chatterjee, Jun Kim, Jun Soo Kim and Eun Jin Cho
Org. Biomol. Chem., 2016,14, 6804-6810
DOI:
10.1039/C6OB01235C,
Paper
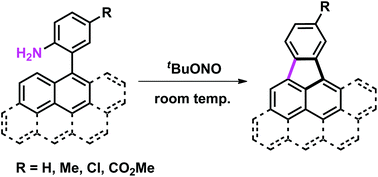
Cyclopenta-fused polycyclic aromatic hydrocarbons (CP-PAHs), potentially electronically and biologically highly active materials, were synthesized from readily available 2-aryl-substituted anilines. Reactions occur under extremely mild, room temperature conditions using tBuONO as the sole reagent. The use of a nitrite source generates a reactive diazonium intermediate in situ that then reacts with a tethered polycyclic aromatic moiety by intramolecular aromatic substitution. This protocol could be presented as one of the simplest methods to access CP-PAHs.