Structure, phase transition and negative thermal expansion in ammoniated ZrW2O8†
Weigang
Cao
a,
Qingzhen
Huang
b,
Yangchun
Rong
a,
You
Wang
a,
Jinxia
Deng
a,
Jun
Chen
a and
Xianran
Xing
*a
aDepartment of Physical Chemistry, University of Science and Technology Beijing, Beijing 100083, China. E-mail: xing@ustb.edu.cn; Fax: +86-10-62332525; Tel: +86-10-62334200
bNIST Center for Neutron Research, National Institute of Standards and Technology (NIST), Gaithersburg, Maryland 20899-6102, USA
First published on 22nd March 2016
Abstract
In the present work, we have developed an effective way to control the thermal expansion of ZrW2O8 by the insertion of NH3 into the void of the framework structure. Neutron powder diffraction (NPD) and XPS studies reveal that N is bonding to W in the ammoniated ZrW2O8 and retains the original structure with the space group P213. This kind of ammoniation improves the thermal stability, raised the phase transition temperature about 50 K, and weakens the negative thermal expansion (NTE) from −7.8 × 10−6 K−1 to −2.1 × 10−6 K−1. These behaviors account for bonding of NH3 to the neighboring atoms of ZrW2O8, and hinder the rocking motion of the corner shared polyhedral structure.
Introduction
Among the negative thermal expansion (NTE) materials, ZrW2O8 is one of the archetypes of NTE oxides, which exhibits isotropic negative thermal expansion from 0.3 K to 1050 K. Particularly, the magnitude of the overall thermal expansion coefficient is large (−8.7 × 10−6 K−1) from 0.3 K to 693 K.1,2 At around 430 K, ZrW2O8 undergoes an order–disorder phase transition from an acentric (α phase, space group P213) to a centric (β phase, space group Pa![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) ) structure, which is due to the disordering of the WO4 tetrahedra. Although ZrW2O8 exhibits outstanding NTE properties, its thermal stability and controlling of its thermal expansion are still big challenges for both fundamental studies and practical applications.3,4 For example, the interfacial stress of the composite between the filler (ZrW2O8) and the substrate can easily lead the tailor-made thermal expansion to failure.
) structure, which is due to the disordering of the WO4 tetrahedra. Although ZrW2O8 exhibits outstanding NTE properties, its thermal stability and controlling of its thermal expansion are still big challenges for both fundamental studies and practical applications.3,4 For example, the interfacial stress of the composite between the filler (ZrW2O8) and the substrate can easily lead the tailor-made thermal expansion to failure.
The character of the ZrW2O8 structure is that it contains corner sharing ZrO6 octahedra and WO4 tetrahedra, and the ZrO6 octahedra share all six corners with the WO4 tetrahedra, whereas the WO4 tetrahedra share only three of their four corners with ZrO6 octahedra.5 The mechanism to explain the negative thermal expansion is used by the rigid unit mode (RUM) model which is based on this unique structure.6,7 Meanwhile, this kind of framework structure permits us to tailor the thermal expansion coefficient and tune the related properties by two ways: chemical substitutions of Zr and/or W and the accommodation of small molecules into the void of the framework structure.
The effects of many chemical substitutions on the Zr site and/or the W site of ZrW2O8, which include the isovalent ion and the aliovalent ion doping, have been investigated. With the identical charge, the substitutions of Hf,2,8,9 Sn,10,11 and Ti,10,12 on the Zr site and Mo13–16 on the W site have been reported, and their solubility limits are different. The Sn and Ti substitutions show a limited range of solubility: Zr1−xSnxW2O8 (x ≤ 0.3),11 Zr1−xTixW2O8 (x ≤ 0.05),12 while the Zr and W can be completely substituted by Hf and Mo, respectively. Besides, the aliovalent ion can also be incorporated into the ZrW2O8. Substituting a number of trivalent ions (Sc, Y, In, Eu, Er, Yb, Lu)17–20 onto the Zr site and V (ref. 21, 22) onto the W site have been investigated by many groups, which also showed a limited solubility. With the exception of Hf, the other substitutions usually decrease the phase transition temperature.17 For example, the high temperature β phase of ZrMoWO8 is kinetically stable at room temperature.14 But the thermal contraction properties of these materials are almost unchanged with respect to the magnitude of the α phase.
The insertion of the small molecules is another way to tune the properties of ZrW2O8, which is significant to the potential use. This is because the insertion can not only tune the intrinsic properties of ZrW2O8, but also reduce the interfacial stress as a negative thermal filler in the composites. However, only one example has been investigated, which is the insertion of H2O into the framework of ZrW2O8. This makes the volume of ZrW2O8 contract without shortening the Zr–O and W–O bond lengths, and affects the thermal expansion.23,24 But the insertion makes the structure of ZrW2O8 change to a β phase. Meanwhile, this hydrated material doesn't have the thermal contraction. This is unfavourable to the applications.
In this paper, we insert NH3 into the framework of ZrW2O8 to tune its properties. We investigate the effects of the insertion on its structure, order–disorder phase transition and thermal expansion. The ammoniation opens a new avenue to tune the properties of frameworks like ZrW2O8.
Experimental section
ZrW2O8 samples were prepared through a hydrothermal method. Generally, 1.575 g of ZrOCl2·8H2O and 2.31 g of Na2WO4·2H2O were dissolved in 5 mL of distilled H2O separately. Then the dissolved solutions were added dropwise to 13 mL distilled water, and a white precipitate was formed. After another 2 h of stirring, 9.6 mL of concentrated HCl were added to the solution mixture. This stirring was continued for 1 h. The formed slurry was then transferred to a Teflon-lined Parr bomb (volume: 50 mL) and heated at 433 K for 12 h to carry out the hydrothermal reaction. The ZrW2O7(OH)2·2H2O precursor was centrifuged, washed with distilled H2O, and dried at 353 K for 12 h. The white powder was heat treated at 853 K for 6 h to form the crystalline cubic ZrW2O8. The synthesis of the ammoniated sample was carried out by a thermal reaction of the pristine ZrW2O8 under an NH3 atmosphere in a conventional tube furnace at 698 K for 30 h, with a flow rate of 300 ml min−1.Phase identification and variable temperature X-ray powder diffraction (XRD) were performed on the PANalytical diffractometer with Cu Kα radiation. Standard silicon was used to obtain room temperature XRD. The low-temperature XRD data were collected by using a HTK450, and recorded in the range from 173 K to 398 K. The range of each data is from 15° to 80°. The high-temperature XRD were collected by using a HTK1200 to investigate the phase transition. The neutron powder diffraction (NPD) patterns for the pristine ZrW2O8 and the ammoniated ZrW2O8 were collected on the BT-1 diffractometer at the Center for Neutron Research at the NIST with a Cu monochromator (λ = 1.53980 Å) at room temperature. The NPD data were collected with a step size of 0.05° in the 2θ range from 3° to 167°. The low-temperature XRD data and NPD data were analysed using GSAS25 to determine the structure. TG-DSC was performed on the NETZSCH STA 409 C/CD machine under an air atmosphere to investigate the weight loss and the phase transition from 298 K to 773 K at a rate of 10 K min−1. The average nitrogen content of the ammoniated ZrW2O8 was measured by using a HON analyzer (LECO HTC600). The infrared absorption spectra were recorded from 400 cm−1 to 4000 cm−1 with a FT spectrometer (Excalibur 3100 America Varian). The surface composition of the samples and the binding energy were determined by X-ray photo electron spectroscopy (XPS, Kratos Axis Ultra DLD).
Results and discussion
The ammoniated ZrW2O8 is confirmed by X-ray diffraction. Fig. 1 shows the reflections of the pristine ZrW2O8 and the ammoniated ZrW2O8. The XRD pattern of the ammoniated ZrW2O8 is consistent with ZrW2O8, which means that it retains the framework structure of the pristine ZrW2O8. To further check the influence of the ammoniation treatment on the structure of the ZrW2O8, a much slower scanning rate was adopted to collect the main diffraction peaks of (2 1 0) and (2 1 1), as given in the insert of Fig. 1. Both these diffraction peaks slightly shift to a smaller angle after the ammoniation treatment, indicating that the lattice constant of the crystal increases due to the incorporation of NH3.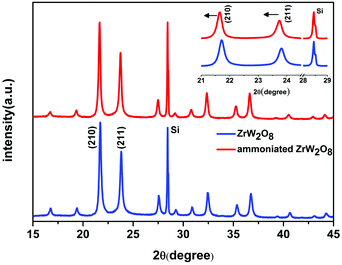 | ||
| Fig. 1 The XRD pattern of the pristine ZrW2O8 and the ammoniated ZrW2O8. The inset shows the diffraction peaks of (2 1 0) and (2 1 1) of the ammoniated ZrW2O8 and the pristine ZrW2O8. | ||
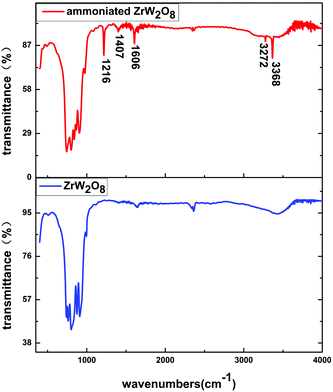
To identify changes in the ammoniated ZrW2O8, FTIR spectra of the ammoniated ZrW2O8 and the pristine ZrW2O8 in the range of 400–4000 cm−1 are exhibited in Fig. 2. Compared with the pristine ZrW2O8, five extra bands are obviously detected in the curve of the ammoniated ZrW2O8. The band at 1216 cm−1 is assigned to the symmetric bending vibration of N–H bonds of the coordinated NH3, while the band located at 1606 cm−1 is associated with the asymmetric bending mode of NH3.26–29 The weak peak at around 1407 cm−1 is attributed to NH4+, which is related to Brønsted acid sites.29,30 Furthermore, the bands at 3272 cm−1 and 3368 cm−1 can be ascribed to N–H stretching vibration modes of the coordinated NH3.31 Besides, the other peaks related to the WO4 vibrations also shift compared with the pristine ZrW2O8.
With the aim of obtaining a fine structure of the ammoniated ZrW2O8, NPD was performed. The NPD refinement results of the ammoniated ZrW2O8 are shown in Fig. 3(a). The observed and calculated results agree well. And a summary of the refinement statistics is presented in Tables S1 and S2 (see the ESI Tables S1 and S2†).
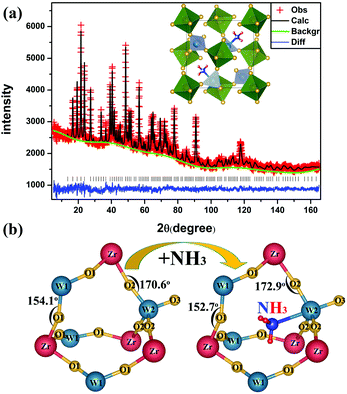 | ||
| Fig. 3 (a) Rietveld refinement of the neutron diffraction pattern of the ammoniated ZrW2O8. (b) The local structure of ZrW2O8 and the ammoniated ZrW2O8. | ||
The NPD results show the space group of the ammoniated ZrW2O8 is P213, and the lattice parameter is 9.1738 Å, which is slightly larger than that of ZrW2O8 (9.1501 Å). In consideration of the molecular size and the coordination number of the cations, we infer the location of NH3. And this judgement is confirmed by the NPD results. As shown in Fig. 3(b), NH3 indeed locates in the large void of ZrW2O8, and forms the weak coordinated bond to W2. The chemical formula of the ammoniated ZrW2O8 is ZrW2O7.91·0.64NH3. This formula is consistent with the result of 0.8 mol nitrogen/1 mol ZrW2O8 obtained by the LECO TC-600 nitrogen/oxygen determinator.
The local structural changes in the ammoniated ZrW2O8 are viewed in Fig. 3(b). The angle of Zr–O1–W1 decreases from 154.1° to 152.7°, while the angle of Zr–O2–W2 inverses from 170.6° to −172.9°. The other changes about bond distances and bond angles of the ZrW2O8 and ZrW2O7.91·0.64NH3 are presented in Table S2 in the ESI.† After the insertion of NH3, there is no apparent effect on the average bond distances of Zr–O1, Zr–O2, and W2–O2, while the average bond distances of W1–O1, and W2–O3 have obvious changes. The bond length of W1–O1 increases from 1.78 Å to 1.81 Å. And the W2–O3 changes from 1.63 Å to 1.74 Å. These marked changes above are owing to the insertion of NH3 which interacts with the neighbouring atoms. The coordinated N to W2 weakens the bond of W2–O3. And the repulsion of N–O2 makes the bond angle of Zr–O2–W2 inverse. The other changes of W1–O1 and Zr–O1–W1 may be due to the hydrogen bond formation between the H and O1. These changes about the local structure have some influence on the order–disorder phase transition and the thermal expansion of ZrW2O8.
Furthermore, the XPS spectra are incorporated to determine the changes of the ammoniated ZrW2O8. In Fig. S2,† an additional peak is observed which is corresponding to N 1s. High resolution scans were taken for N 1s, O 1s and W 4f. Fig. 4(a) shows N 1s spectra of the pristine ZrW2O8 and the ammoniated ZrW2O8. Compared with ZrW2O8, additional three peaks can obviously be distinguished. The peak at 397 eV corresponds to the bonding state of N–W.32,33 The peak at around 400 eV can be assigned to NH3–W and the adsorbed N2.34,35 And the peak centred at 402 eV is attributed to the NH4+.34,35 The compounds of the N 1s agree well with the spectra of FTIR, which also confirms our previous result of NPD. The spectra of O 1s are presented in Fig. 4(b). No difference is detected between the pristine ZrW2O8 and the ammoniated ZrW2O8. This indicates that the ammoniation does not significantly affect the coordination polyhedron of oxygen. In addition, the lower oxidation W5+ is observed in the curve of the ammoniated ZrW2O8, as shown in Fig. 4(c), which is consistent with the NPD results.
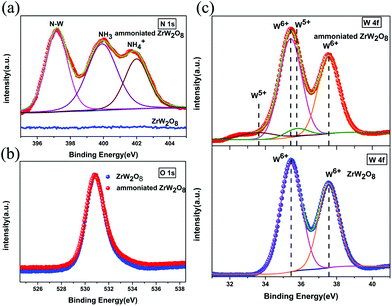 | ||
| Fig. 4 (a) The N 1s spectra, (b) the O 1s spectra and (c) the W 4f spectra of the pristine ZrW2O8 and the ammoniated ZrW2O8. | ||
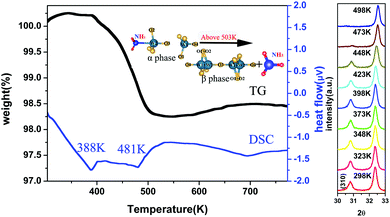
Fig. 5 shows the TG-DSC curves and the high-temperature XRD spectra of the ammoniated ZrW2O8. The TG curve indicates two regions of the mass loss. The first weight loss is not obvious around 388 K, which is attributed to desorption of H2O and NH3 adsorbed on the surface. The other is equivalent to 10.86 g mol−1 from 398 K to 503 K, which is due to the loss of NH3 which is inserted into the framework. There is almost no mass loss above 503 K. Meanwhile, the phase transition temperature is identified by the high temperature XRD measurement. The high temperature XRD measurement indicates that the ammoniation indeed increases the phase transition temperature of ZrW2O8 from 433 K to 481 K, which is also identified by the DSC curve. The DSC curve has two strong endothermic peaks corresponding to the mass loss. Among them, the second peak is due to the phase transition from P213 to Pa![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) besides desorption of NH3.
besides desorption of NH3.
The insertion of NH3 also has a great effect on the thermal contraction of ZrW2O8. Fig. 6 shows the lattice constant as a function of the temperature. In the temperature range from 173 K to 398 K, the ammoniated ZrW2O8 represents the isotropic negative thermal expansion with the magnitude of −2.1 × 10−6 K−1 which is almost one fourth of the ZrW2O8 (−7.8 × 10−6 K−1). This is the first example to show an obviously weakened negative thermal expansion with respect to the magnitude of the α phase of ZrW2O8. Below 398 K, one thermal cycle (heating and cooling) for the ammoniated ZrW2O8 is exhibited as shown in the inset of Fig. 6. There is nearly no difference between heating and cooling, which indicates that the sample is stable in the temperature range.
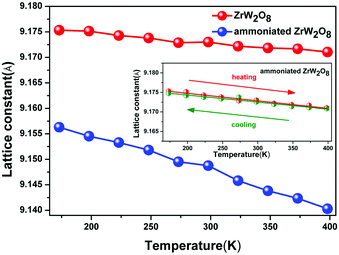 | ||
| Fig. 6 The lattice constant plots against the temperature of the pristine ZrW2O8 and the ammoniated ZrW2O8. The inset shows the thermal cycle for the ammoniated ZrW2O8. | ||
These changes about the phase transition temperature and the thermal expansion are closely related to the structural variation. The insertion of NH3 into the void of ZrW2O8 and the interaction of NH3 with the neighbouring atoms of ZrW2O8 are the main reasons for these changes. On the one hand, the insertion decreases the rotation space of the polyhedron since the void of the framework is occupied by the insertion, which makes the framework more rigid. On the other hand, NH3 interacts with the neighbouring atoms of ZrW2O8, such as the repulsion of N–O2 and the attraction of H–O1. To some degree, these interactions attenuate the dynamic rocking motions of the WO4 tetrahedra and ZrO6 octahedra and hinder the oxygen exchange, which is responsible for the negative thermal expansion and phase transition.
Conclusions
The small molecule NH3 can be successfully inserted into the framework of ZrW2O8 by ammoniation. NH3 locates at the interstitial void and interacts with the neighbouring atoms. The N atom of NH3 forms a weak bond with W2 and a repulsive force is produced between N and O2. Simultaneously, an attraction exists between the H atoms and O1. The insertion makes the framework structure more stable and rigid, which increases the phase transition temperature about 50 K, and decreases the NTE from −7.8 × 10−6 K−1 to −2.1 × 10−6 K−1. The investigation of this paper opens a new avenue to tune the properties of ZrW2O8 and other framework materials. In addition, this is the first example to show an obvious change in the thermal expansion of the α phase of ZrW2O8, which is helpful for further research about the mechanism of NTE.Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 91022016, 91422301, and 21231001), the Program for Changjiang Scholars and the Innovative Research Team in University (IRT1207), and the Fundamental Research Funds for the Central Universities, China (Grant No. FRF-SD-13-008A).Notes and references
- T. Mary, J. Evans, T. Vogt and A. Sleight, Science, 1996, 272, 90 CAS.
- J. Evans, T. Mary, T. Vogt, M. Subramanian and A. Sleight, Chem. Mater., 1996, 8, 2809–2823 CrossRef CAS.
- X. Yang, X. Cheng, X. Yan, J. Yang, T. Fu and J. Qiu, Compos. Sci. Technol., 2007, 67, 1167–1171 CrossRef CAS.
- S. Yilmaz, J. Phys.: Condens. Matter, 2002, 14, 365 CrossRef CAS.
- J. S. Evans, W. David and A. Sleight, Acta Crystallogr., Sect. B: Struct. Sci., 1999, 55, 333–340 CrossRef.
- A. K. Pryde, K. D. Hammonds, M. T. Dove, V. Heine, J. D. Gale and M. C. Warren, J. Phys.: Condens. Matter, 1996, 8, 10973 CrossRef CAS.
- A. K. Pryde, K. D. Hammonds, M. T. Dove, V. Heine, J. D. Gale and M. C. Warren, Phase Transitions, 1997, 61, 141–153 CrossRef CAS.
- Y. Yamamura, N. Nakajima, T. Tsuji, M. Koyano, Y. Iwasa, S. i. Katayama, K. Saito and M. Sorai, Phys. Rev. B: Condens. Matter, 2002, 66, 014301 CrossRef.
- Y. Yamamura, N. Nakajima and T. Tsuji, Phys. Rev. B: Condens. Matter, 2001, 64, 184109 CrossRef.
- K. D. Buysser, I. V. Driessche, B. V. Putte, P. Vanhee, J. Schaubroeck and S. Hoste, Inorg. Chem., 2008, 47, 736–741 CrossRef PubMed.
- C. De Meyer, F. Bouree, J. S. Evans, K. De Buysser, E. Bruneel, I. Van Driessche and S. Hoste, J. Mater. Chem., 2004, 14, 2988–2994 RSC.
- K. De Buysser, I. Van Driessche, B. V. Putte, J. Schaubroeck and S. Hoste, J. Solid State Chem., 2007, 180, 2310–2315 CrossRef CAS.
- S. Allen and J. S. Evans, J. Mater. Chem., 2004, 14, 151–156 RSC.
- J. Evans, P. Hanson, R. Ibberson, N. Duan, U. Kameswari and A. Sleight, J. Am. Chem. Soc., 2000, 122, 8694–8699 CrossRef CAS.
- C. Lind, A. P. Wilkinson, C. J. Rawn and E. A. Payzant, J. Mater. Chem., 2001, 11, 3354–3359 RSC.
- S. Allen and J. Evans, Phys. Rev. B: Condens. Matter, 2003, 68, 134101 CrossRef.
- Y. Yamamura, N. Nakajima, T. Tsuji, A. Kojima, Y. Kuroiwa, A. Sawada, S. Aoyagi and H. Kasatani, Phys. Rev. B: Condens. Matter, 2004, 70, 104107 CrossRef.
- T. Hashimoto, J. Kuwahara, T. Yoshida, M. Nashimoto, Y. Takahashi, K. Takahashi and Y. Morito, Solid State Commun., 2004, 131, 217–221 CrossRef CAS.
- N. Nakajima, Y. Yamamura and T. Tsuji, Solid State Commun., 2003, 128, 193–196 CrossRef CAS.
- T. Tsuji, Y. Yamamura and N. Nakajima, Thermochim. Acta, 2004, 416, 93–98 CrossRef CAS.
- X. Chen, X. Deng, H. Ma, J. Tao and X. Zhao, J. Solid State Chem., 2011, 184, 1090–1095 CrossRef CAS.
- X. Chen, J. Tao, H. Ma and X. Zhao, Acta Crystallogr., Sect. C: Cryst. Struct. Commun., 2009, 65, 74–76 Search PubMed.
- N. Duan, U. Kameswari and A. Sleight, J. Am. Chem. Soc., 1999, 121, 10432–10433 CrossRef CAS.
- N. A. Banek, H. I. Baiz, A. Latigo and C. Lind, J. Am. Chem. Soc., 2010, 132, 8278–8279 CrossRef CAS PubMed.
- B. H. Toby, J. Appl. Crystallogr., 2001, 34, 210–213 CrossRef CAS.
- L. Chen, J. Li and M. Ge, J. Phys. Chem. C, 2009, 113, 21177–21184 CAS.
- M. A. Larrubia, G. Ramis and G. Busca, Appl. Catal., B, 2000, 27, L145–L151 CrossRef CAS.
- L. Lietti, I. Nova, G. Ramis, L. Dall'Acqua, G. Busca, E. Giamello, P. Forzatti and F. Bregani, J. Catal., 1999, 187, 419–435 CrossRef CAS.
- Y. Peng, J. Li, W. Si, J. Luo, Q. Dai, X. Luo, X. Liu and J. Hao, Environ. Sci. Technol., 2014, 48, 13895–13900 CrossRef CAS PubMed.
- S. D. Lin, A. C. Gluhoi and B. E. Nieuwenhuys, Catal. Today, 2004, 90, 3–14 CrossRef CAS.
- L. Dall'Acqua, I. Nova, L. Lietti, G. Ramis and E. Giamello, Phys. Chem. Chem. Phys., 2000, 2, 4991–4998 RSC.
- Y.-C. Nah, I. Paramasivam, R. Hahn, N. K. Shrestha and P. Schmuki, Nanotechnology, 2010, 21, 105704 CrossRef PubMed.
- K. Nakagawa, N. Miura, S. Matsumoto, R. Nakano and H. Matsumoto, Jpn. J. Appl. Phys., 2008, 47, 7230–7235 CrossRef CAS.
- C. Guimon, A. Zouiten, A. Boreave, G. Pfister-Guillouzo, P. Schulz, F. Fitoussi and C. Quet, J. Chem. Soc., 1994, 90, 3461–3467 CAS.
- C. Guimon, A. Gervasini and A. Auroux, J. Phys. Chem. B, 2001, 105, 10316–10325 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available: Rietveld refinement of the neutron diffraction pattern of ZrW2O8, the crystallographic data and the explicit structure data, the full XPS spectra, and the cell parameters. See DOI: 10.1039/c5qi00292c |
| This journal is © the Partner Organisations 2016 |