Novel photo- and/or thermochromic MOFs derived from bipyridinium carboxylate ligands†
Chenghui
Zhang
,
Libo
Sun
,
Chuanqi
Zhang
,
Song
Wan
,
Zhiqiang
Liang
* and
Jiyang
Li
*
State Key Lab of Inorganic Synthesis and Preparative Chemistry, Jilin University, Changchun, 130012, P. R. China. E-mail: lijiyang@jlu.edu.cn; liangzq@jlu.edu.cn
First published on 3rd March 2016
Abstract
By utilizing the photo-active bipyridinium carboxylate ligand 1-(3,5-dicarboxyphenyl)-4,4′-bipyridinium bromide (H2ipbpBr) and varying the solvents, two new Zn-MOFs with photo- and/or thermochromic as well as photocontrolled luminescent properties have been successfully synthesized. [Zn(ipbp)(H2O)]·NO3·H2O (1) has a three-dimensional (3D) interpenetrated cationic framework, while [Zn(ipbp)(H2O)2]·NO3·H2O (2) possesses a 2D layered cationic network. Interestingly, 2 and its dehydrated phase 2′ can undergo reversible structure transformation upon removal and uptake of guest and coordinated water molecules. 1 and 2 are photo-active to UV and visible light, and undergo an obvious color transformation from light yellow to green as a result of the formation of ipbp radicals. Compared with 2, 1 is more photosensitive due to its close donor–acceptor interaction and dense arrangement of interpenetrated frameworks. Meanwhile, 1 is thermochromic with a lower thermal-response temperature (90 °C). These two compounds also exhibit tuneable luminescence in response to light. This work provides a facile method to synthesize novel chromic MOFs using the photosensitive organic linkers and the conformational adjustment.
Introduction
Metal–organic frameworks (MOFs) are a class of materials constructed from metal ions or clusters linked by organic ligands. The study of MOFs has received immense attention due to their variety of fascinating structures and their potential applications in gas separation,1 gas storage or capture,2 luminescence,3 catalysis,4 sensing,5 biomedicine6 and magnetism.7 Recently, chromism as a new emerging property has also been found in MOF materials.8 Such a color change can be caused by photo-irradiation or heating treatment, correspondingly called photochromism and thermochromism, respectively. Generally, inorganic photochromic compounds have considerable thermal stability, high strength and diverse coordination chemistry, while organic photochromic materials are varied and easy to modify or process.9 Different from them, hybrid materials can preserve or even improve the respective features of the inorganic and organic components; meanwhile, they may produce new properties originating from the synergy between them.10 So by taking advantage of inorganic and organic components, MOFs would exhibit promising perspectives in photochromic and thermochromic materials.11In the syntheses of chromic MOFs, the utilization of photo-active organic linkers with specific structures is of great importance. In the past few years, bipyridinium (also called viologen) derivatives as functional organic ligands have attracted more interest due to their photoelectrochromism, electron-accepting ability and redox activity.12 By now, a few MOF materials based on viologen derivatives have been prepared and display interesting properties in photochromism, photoswitching, colorimetric detection/sensing, gas adsorption and separation.11
Viologen-based ligands with aromatic polycarboxylate substituents possess multiple potential coordination sites including terminal N and O atoms. The diverse coordination modes between such organic linkers and metal species not only offer more opportunities to construct various framework structures, but also can tune the chromic properties of the resultant MOFs. However, studies of the chromic MOFs involving this kind of ligand are limited. For example, studies of the 1-(3,5-dicarboxyphenyl)-4,4′-bipyridinium bromide (H2ipbpBr, Scheme 1) ligand are quite rare, and the only known example of studies on this ligand does not explore its chromic properties.11b,c
Here, we present chromic MOF materials constructed from an ipbp photo-sensitive linker. By adjustment of the coordination modes between the organic linker and metal ions through varying the solvents, two photochromic and/or thermochromic Zn-MOFs have been successfully synthesized. [Zn(ipbp)(H2O)]·NO3·H2O (1) possesses a three dimensional (3D) interpenetrated cationic framework, and [Zn(ipbp)(H2O)2]·NO3·H2O (2) is a 2D layered cationic network. Their different structures result in their distinct chromic behaviours. 1 is photochromic and highly photosensitive and thermochromic with a low thermal-response temperature, while 2 is only photochromic. Both compounds 1 and 2 are photo-active in response to visible and UV light. In addition, they also exhibit photo-modulated photoluminescent properties.
Experimental section
Materials and methods
The reagents and solvents employed were commercially available and used without further purification except for the H2ipbpBr (H2ipbp+ stands for 1-(3,5-dicarboxyphenyl)-4,4′-bipyridinium) ligand. All fluorescence measurements were carried out on a Shimadzu RF-5301 PC fluorescence spectrophotometer. The electron paramagnetic resonance (EPR) spectra were recorded on a JEOL JES-FA200 EPR spectrometer. A 500 W high-pressure mercury lamp was employed as an irradiation light source for in situ EPR measurements. The IR absorption spectra were recorded in the range of 400–4000 cm−1 on a Nicolet Impact 410 FTIR spectrometer with KBr pellets. The CHN elemental analyses were obtained by using a Perkin-Elmer 2400 elemental analyzer. Thermogravimetric analyses (TGA) were performed on a Perkin-Elmer TGA-7 thermogravimetric analyzer from room temperature to 800 °C under air atmosphere with a heating rate of 10 °C min−1. Powder X-ray diffraction (PXRD) patterns were collected on a Rigaku D-Max 2550 diffractometer using Cu-Kα radiation (λ = 0.15418 nm) in a 2θ range of 4–40° at room temperature.Crystal structure determination
The single crystal X-ray diffraction measurements for compounds 1 and 2 were carried out on a Rigaku RAXIS-RAPID diffractometer with graphite monochromated Mo Kα (λ = 0.71073 Å) radiation at 293 K. Data processing was performed by using the SAINT processing program.14 The structures were solved by direct methods and refined on F2 by full-matrix least-squares with the SHELX-97 program.15 All the non-hydrogen atoms were refined with anisotropic thermal parameters and hydrogen atoms on the aromatic rings were placed geometrically with isotropic thermal parameters 1.2 times that of the attached carbon atoms. The guest solvent molecules were located directly by considering the charge balance. A summary of the related crystallographic data and structure refinement parameters is given in Table S1 (ESI†) for 1 and 2. The selected bond lengths and angles of compounds 1 and 2 are presented in Table S2 (ESI†) and Table S3 (ESI†), respectively.Synthesis of 1-(3,5-dicarboxyphenyl)-4,4′-bipyridinium bromide (H2ipbpBr)
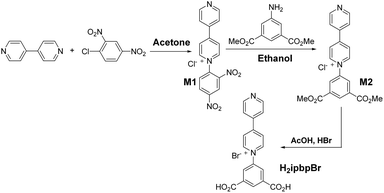
The synthesis process of the H2ipbpBr ligand is shown in Scheme 1. 4′,4-Bipyridine (1.92 g, 10 mmol) and 2,4-dinitrochlorobenzene (2.03 g, 10 mmol) were dissolved in acetone (15 mL). The solution was refluxed for 24 h under a N2 atmosphere. After cooling to room temperature, by filtration and washing twice with acetone, a dark green precipitate was collected. The product M1 was dried in a vacuum. Yield: 1.588 g (44% yield). 1H (D2O): δ 9.41 (d, J = 2.4 Hz, 1H), 9.26 (d, J = 7.2 Hz, 2H), 8.95 (dd, J1 = 2.4 Hz, J2 = 8.7 Hz, 1H), 8.85 (d, J1 = 1.5 Hz, J2 = 5.1 Hz, 2H), 8.70 (d, J = 7.2 Hz, 2H), 8.29 (d, J = 8.7 Hz, 1H), 8.04 (d, J1 = 1.5 Hz, J2 = 5.1 Hz, 2H).The product M1 (1.588 g, 4.42 mmol) and 5-amino-isophthalic acid dimethyl ester (0.925 g, 4.42 mmol) were dissolved in ethanol (80 mL). Such a solution was refluxed for 12 h followed by rotary evaporation. Then the appropriate ethyl acetate was added and stirred for 0.5 h under 80 °C, giving the yellow powder M2, which was collected by filtration and washed twice with ethyl acetate after cooling to room temperature. The product M2 was dried in a vacuum. Yield: 1.2 g (71% yield). 1H (D2O): δ 9.32 (d, J = 7.5 Hz, 2H); 8.95 (t, J = 1.5 Hz, 1H); 8.84 (dd, J1 = 1.8 Hz, J2 = 4.5 Hz, 2H); 8.70–8.65 (m, 4H); 8.03 (dd, J1 = 1.8 Hz, J2 = 4.5 Hz, 2H), 4.04 (s, 6H).
Finally, acetic acid and hydrobromic acid (48%) in the ratio 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (v
1 (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v) were added to the product M2 (1.2 g, 3.12 mmol), and this solution was refluxed for 12 h. Then an appropriate amount of ice water was slowly added after cooling, giving the raw product H2ipbpBr, which was filtered and washed twice with ice water, followed by recrystallization with acetonitrile and drying in a vacuum to give the pure target product H2ipbpBr. Yield: 1.56 g (45% yield). 1H (D2O): δ 9.46 (d, J = 7.2 Hz, 2H); 9.08 (dd, J1 = 1.5 Hz, J2 = 5.7 Hz, 2H); 8.87 (t, J = 1.5 Hz, 1H); 8.77 (d, J = 6.6 Hz, 2H); 8.65 (d, J = 1.5 Hz, 2H); 8.62 (dd, J1 = 1.5 Hz, J2 = 5.4 Hz, 2H).
v) were added to the product M2 (1.2 g, 3.12 mmol), and this solution was refluxed for 12 h. Then an appropriate amount of ice water was slowly added after cooling, giving the raw product H2ipbpBr, which was filtered and washed twice with ice water, followed by recrystallization with acetonitrile and drying in a vacuum to give the pure target product H2ipbpBr. Yield: 1.56 g (45% yield). 1H (D2O): δ 9.46 (d, J = 7.2 Hz, 2H); 9.08 (dd, J1 = 1.5 Hz, J2 = 5.7 Hz, 2H); 8.87 (t, J = 1.5 Hz, 1H); 8.77 (d, J = 6.6 Hz, 2H); 8.65 (d, J = 1.5 Hz, 2H); 8.62 (dd, J1 = 1.5 Hz, J2 = 5.4 Hz, 2H).
Results and discussion
Crystal structure of [Zn(ipbp)(H2O)]·NO3·H2O (1)
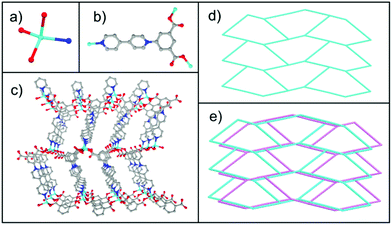
Single-crystal X-ray structural analysis reveals that 1 crystallizes in the orthorhombic Pbcn (no. 60) space group. The asymmetric unit contains one Zn center, one deprotonated ipbp ligand, one coordinated H2O molecule, one free H2O molecule and one NO3− anion to achieve the charge balance (Fig. S3a, ESI†). The zinc center is four-coordinated with two oxygen atoms from two different carboxylates of ipbp ligands, one oxygen atom from H2O, and one nitrogen atom from the other ipbp ligand (Fig. 1a). The Zn–O distances range from 1.949 to 1.984 Å, and the Zn–N distance is 2.041 Å, which are close to previously reported data.16 In the structure of 1, the carboxylate of the ligand adopts the monodentate coordination mode (Fig. 1b). The dihedral angle between the central pyridine and isophthalic moieties is 45.0°, while that between the central pyridine and edge pyridine is 13.1° and that between the isophthalic moieties and edge pyridine is 32.0°. The adjacent four-coordinated zinc atoms are linked together through three-connected ipbp ligands to construct a 3D cationic framework (Fig. 1c). Interestingly, there exist two such independent equivalent networks described above, so compound 1 is a 3D interpenetrated cationic framework (Fig. S4, ESI†).The topological approach can help us to understand better the 3D framework of 1. The ipbp ligand linking three zinc atoms can be considered to be a three-connected node. Each zinc atom connected by three ipbp ligands can be viewed as a three-connected node. Thus, the framework of 1 could be simplified to a 3,3-connected 3D topological net with a point symbol of 103 (Fig. 1d, e and S5, ESI†). Topological analysis suggests that 1 possesses a 3,3-connected uninodal net with an utp topology. As far as we know, three-connected 3D MOFs are relatively rare.17
Crystal structure of [Zn(ipbp)(H2O)2]·NO3·H2O (2)
Single-crystal X-ray structural analysis reveals that 2 crystallizes in the triclinic P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (no. 2) space group. Its asymmetric unit contains one zinc atom, one deprotonated ipbp ligand, two coordinated H2O molecules, one free H2O molecule and one NO3− anion to balance charge (Fig. S3b, ESI†). As shown in Fig. 2a, each Zn2+ ion is five-coordinated by four O atoms from two H2O molecules and two carboxylate groups from different ipbp ligands, as well as one N atom from other ipbp ligands. The carboxylate groups of the ligand also adopt the monodentate coordination mode (Fig. 2b). The Zn–O bond lengths range between 1.986 and 2.167 Å, and the Zn–N distance is 2.079 Å, which are close to previously reported data.16
(no. 2) space group. Its asymmetric unit contains one zinc atom, one deprotonated ipbp ligand, two coordinated H2O molecules, one free H2O molecule and one NO3− anion to balance charge (Fig. S3b, ESI†). As shown in Fig. 2a, each Zn2+ ion is five-coordinated by four O atoms from two H2O molecules and two carboxylate groups from different ipbp ligands, as well as one N atom from other ipbp ligands. The carboxylate groups of the ligand also adopt the monodentate coordination mode (Fig. 2b). The Zn–O bond lengths range between 1.986 and 2.167 Å, and the Zn–N distance is 2.079 Å, which are close to previously reported data.16
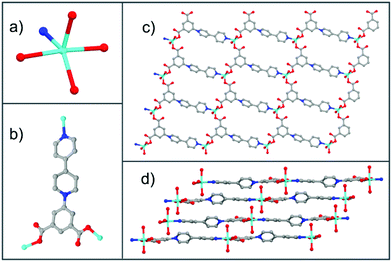 | ||
| Fig. 2 (a) Coordination environment of the zinc atom. (b) Coordination modes of the ipbp ligand. (c) The 2D layer of 2. (d) The stacking 2D layers of 2 (color modes: Zn cyan, O red, N blue, C gray). | ||
The connection of ipbp ligands and Zn atoms forms the 2D layered cationic network of 2 (Fig. 2c). The stacking 2D layers are shown in Fig. 2d. It is worth noting that the dihedral angles between the central pyridine and isophthalic moieties and edge pyridine are 38.4° and 23.2°, respectively, while the dihedral angle between the isophthalic moieties and edge pyridine is 4.5°.
In order to understand better this framework, a topological analysis was performed. The Zn unit coordinated to three ligands could be viewed as a three-connected node and the ipbp ligand could be considered to be a three-connected node. The connection of these two nodes results in a 3,3-connected hcb uninodal network (Fig. S6, ESI†) with the point symbol of 63. Such a network has also been constructed in a previous study.18
In the previous report on Cu-ipbp, the ipbq ligand adopts bridging bismonodentate coordination modes for the carboxylate group (Fig. 3a), to coordinate with a three paddlewheel copper ion unit.13 In Cu-ipbp, the dihedral angles between the central pyridine and isophthalic moieties and edge pyridine are 44.5° and 11.5°, respectively, and the dihedral angle between the isophthalic moieties and edge pyridine is 33.0°. However, the ipbp ligand adopts the monodentate coordination mode in compounds 1 and 2 with different dihedral angles (Fig. 3b). This indicates that the ipbp ligand can adopt different coordination modes with diverse dihedral angles to facilitate the construction of MOFs with various networks.
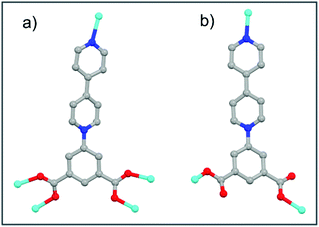 | ||
| Fig. 3 Summary of diverse coordination modes of ipbq in the synthesized MOFs: (a) Cu-ipbp and (b) 1 or 2 (colour modes: metal, cyan; oxygen, red; N, blue; carbon, gray). | ||
Photochromic and/or thermochromic properties
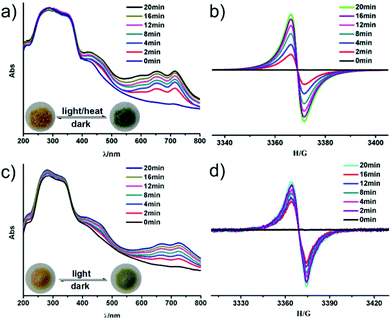
It is well known that bipyridinium derivatives as functional organic ligands have fascinating photoelectrochromic properties due to their electron-accepting ability and redox activity, and have attracted increasing attention in recent years.12 Both compounds 1 and 2 display interesting photo and/or thermochromic properties like other viologen-based compounds.19 Compound 1 undergoes a rapid visible color change from light yellow to dark green upon UV (365 nm, 24 W) or visible light (≥400 nm) irradiation at room temperature within 5 min (Fig. 4a). The dark green samples of 1 are stable in air, which can reverse to light yellow when they are kept in the dark for one day. Such a reversible color transformation can be repeated several times without a noticeable color loss.UV-vis and EPR measurements have been used to explore the photochromic process of 1. As shown in Fig. 4a, two new absorption bands centered at 652 nm and 713 nm appear in the UV-vis spectrum of 1 after UV/visible light irradiation, which are the characteristic absorption of viologen radicals.20 In addition, in situ time dependent EPR studies show that no EPR signal is observed for the as-synthesized sample of 1, while a signal at g = 2.0038 appears after the irradiation (Fig. 4b). This signal becomes gradually stronger with increasing irradiation. Such an EPR signal disappears again after color conversion from dark green to light yellow in the dark (Fig. S9a, ESI†). This suggests that the discoloration of 1 arises from the generation of the ipbp radicals. The fact is that photoinduced electron transfer in viologen-based MOFs occurs from the O atoms of carboxylate groups to the N atoms of pyridinium units to generate ipbp radicals after irradiation, and ipbp radicals recover to ipbp after the color pales in the dark in air.21–23
Strikingly, such a chromic process can also occur when 1 is heated at 90 °C for 30 min under air atmosphere or vacuum. To the best of our knowledge, thermochromic MOFs based on bipyridinium derivatives are quite rare,19,23a because high temperature facilitates charge-recombination.24b UV-Vis spectra and EPR analyses of 1 show the characteristic signals of ipbp radicals (Fig. S8, ESI†), indicating that thermochromism also arises from the electron transfer process.
Interestingly, compound 2 is photochromic, but not thermochromic. As with 1, 2 can also respond to both UV and visible light. A color change from light yellow to light green can be observed upon continuous irradiation with UV light (365 nm, 24 W) for 10 min in air at room temperature. A similar photochromism process under visible light needs about 20 min, which is slower than that of 1 under visible light irradiation. The light green sample can be stable in the dark for several days, which means that the photochromic transformation of compound 2 is not easily reversible. The EPR study indicates that the signal intensity is slightly decreased when the chromic 2 is kept in the dark for 21 days (Fig. S9b, ESI†).
It is worth noting that the light green color of 2 can be restored to light yellow when heated at 100 °C for 30 min under air atmosphere. A powder X-ray diffraction study shows that a dehydrated phase 2′ is formed (Fig. S1b, ESI†) through the removal of one free and one coordinated water molecules upon heating at 100 °C. This result is consistent with TG analysis (Fig. S7, ESI†). Interestingly, 2′ can undergo a reversible structural transformation to 2 when 2′ is kept in moisture (RH: 33%) for 5 minutes or under air atmosphere for about 7 days (Fig. S1b, ESI†).
Both UV-Vis spectra and EPR studies of 2 indicate the generation of viologen radicals during the UV/visible irradiation. As shown in Fig. 4c, two new absorption bands centered at 668 nm and 724 nm appear after irradiation, which are also the characteristic of viologen radicals.20 Besides, in situ time dependent EPR studies show that no EPR signal is observed for the as-synthesized sample of 2, while a signal at g = 2.0037 appears after the irradiation (Fig. 4d). This confirms that the photochromic behavior of 2 is also originated from the photo-induced electron transfer and generating ipbp radicals.
Extending photoinduced energy to the visible spectrum is very important for the utilization of solar energy.25 Strikingly, 1 and 2 are not only photosensitive to UV light, but also to visible light. It is well known that the photochromic behavior of MOFs may be influenced by many factors, such as metal, metal–ligand coordination, crystal packing, guest species, and so on. But the most important factor is the electron transfer pathway between the donor and acceptor in the resulting structure. As previously reported, the photoinduced electron transfer in viologen-based MOFs may occur from the O atoms of carboxylate groups to the N atoms of pyridinium units.21–23 Structural analysis reveals that the shortest distances between adjacent carboxylate groups and pyridinium ring of 1 and 2 are 2.979 Å (O3⋯N1) and 3.126 Å (O4⋯N1), and the dihedral angle between O–N-pyridinium plane are 135.1° and 140.5°, which provide a suitable pathway for the electron transfer (Fig. S10 and S11, ESI†).26 It is noticeable that such electron transfer distances are shorter than other observed values in photochromic MOFs (3.2–3.7 Å),27 and the resulting 1 and 2 are both photosensitive to UV and visible light. This suggests that the photoinduced energy may be related with the electron transfer pathway of the donor–acceptor as was observed in the photochromic zeolite-like materials.24 In addition, the ipbp ligands with uncoordinated carboxyl oxygen in 1 and 2 are also thought to favor the photoinduced electron transfer from carboxylate oxygen to pyridinium units.27a On the other hand, the electron transfer distance of 1 is much shorter than that of 2. The 3D interpenetrated framework of 1 causes more dense packing arrangement of networks, which also facilitates the donor–accepter interaction.27b,28 As a result, 1 is more photosensitive upon photo-irradiation, and also thermochromic with a lower thermal-response temperature of 90 °C. An XRD study shows that the structures of 1 and 2 remain intact after the photochromism and/or thermochromism. The frameworks can be stable up to 320 °C for compound 1, indicating that it has good thermal stability for the practical application of photochromic crystalline materials (Fig. S1 and S7, ESI†).
Photocontrolled luminescence
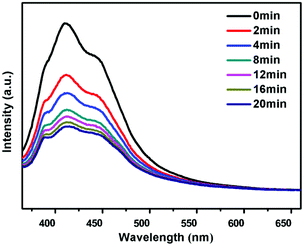
Compounds 1 and 2 show blue emission with the maximum centered at 411 nm and 387 nm when excited under 352 nm and 344 nm, respectively (Fig. 5), which is assigned to the luminescence of the viologen component in those networks (Fig. S12, ESI†). The blue-shift of the luminescence in 1 and 2 may be caused by the synergistic effect between the neighboring ligands and the zinc unit.29,30 Interestingly, the strength of the blue emission is gradually reduced after photo-irradiation, and can be recovered after the color bleached in the dark for compound 1 (Fig. S13, ESI†). Note that the blue emission of 1 does not change after the loss of guest H2O molecules upon heating, which illustrates that the guest H2O does not influence the luminescence of compound 1, whereas compound 2′ shows blue emission with the maximum centered at 410 nm when excited under 344 nm, exhibiting a red shift compared with 2 (Fig. S12, ESI†). As with 1, the luminescence of 2′ can also be modulated by photo-irradiation and heating (Fig. 6 and S13, ESI†). Such a luminescence tuning has also been observed in the reported chromic MOFs and zeolitic materials, and has potential application in photoswitching.27a,31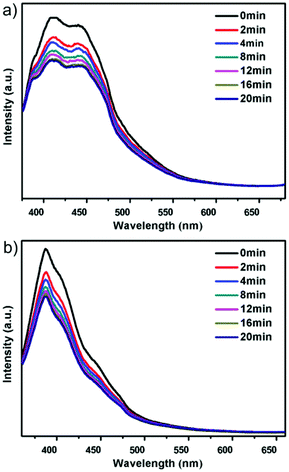 | ||
| Fig. 5 Solid-state fluorescence emission spectral changes of these two compounds upon light irradiation: (a) compound 1; (b) compound 2. | ||
Conclusions
In summary, two new photochromic and/or thermochromic Zn-MOFs based on the bipyridinium ipbp ligand have been synthesized. The different coordination modes of Zn-ipbp controlled by the solvents lead to their distinct structures: a 3D interpenetrated cationic framework for 1 and a 2D layered cationic network for 2. Their chromic behaviors both originate from the photo/heating induced formation of viologen radicals. Interestingly, 1 is photochromic and thermochromic with a rapid colour change from light yellow to dark green and a low chromic temperature, while 2 is only photochromic with a slower photosensitivity due to their different electron transfer distances and framework arrangements. In addition, they also exhibit photoluminescence tuning in response to light. This work will provide abundant scope to search for novel MOF materials with useful photochromic and photoswitching functionalities and the phase transition mechanism.Acknowledgements
We thank the National Natural Science Foundation of China (Grants 21271081, 21320102001 and 21471064) for the support of this work.Notes and references
- (a) B. Van de Voorde, B. Bueken, J. Denayer and D. De Vos, Chem. Soc. Rev., 2014, 43, 5766 RSC; (b) Q. Y. Yang, D. H. Liu, C. L. Zhong and J.-R. Li, Chem. Rev., 2013, 113, 8261 CrossRef CAS PubMed; (c) Z. X. Zhao, X. L. Ma, A. Kasik, Z. Li and Y. S. Lin, Ind. Eng. Chem. Res., 2013, 52, 1102 CrossRef CAS; (d) M. W. Anjum, F. Vermoortele, A. L. Khan, B. Bueken, D. E. De Vos and I. F. J. Vankelecom, ACS Appl. Mater. Interfaces, 2015, 7, 25193 CrossRef CAS PubMed; (e) J.-R. Li, J. Sculley and H.-C. Zhou, Chem. Rev., 2012, 112, 869 CrossRef CAS PubMed.
- (a) R. B. Getman, Y.-S. Bae, C. E. Wilmer and R. Q. Snurr, Chem. Rev., 2012, 112, 703 CrossRef CAS PubMed; (b) N. C. Burtch, H. Jasuja and K. S. Walton, Chem. Rev., 2014, 114, 10575 CrossRef CAS PubMed; (c) M. P. Suh, H. J. Park, T. K. Prasad and D.-W. Lim, Chem. Rev., 2012, 112, 782 CrossRef CAS PubMed; (d) D. Wu, J. J. Gassensmith, D. Gouvêa, S. Ushakov, J. F. Stoddart and A. Navrotsky, J. Am. Chem. Soc., 2013, 135, 6790 CrossRef CAS PubMed; (e) J. Liu, P. K. Thallapally, B. P. McGrail, D. R. Brown and J. Liu, Chem. Soc. Rev., 2012, 41, 2308 RSC.
- (a) Y. J. Cui, Y. F. Yue, G. D. Qian and B. L. Chen, Chem. Rev., 2012, 112, 1126 CrossRef CAS PubMed; (b) Y. Lu and B. Yan, J. Mater. Chem. C, 2014, 2, 7411 RSC; (c) K. A. White, D. A. Chengelis, K. A. Gogick, J. Stehman, N. L. Rosi and S. Petoud, J. Am. Chem. Soc., 2009, 131, 18069 CrossRef CAS PubMed; (d) M. D. Allendorf, C. A. Bauer, R. K. Bhakta and R. J. T. Houk, Chem. Soc. Rev., 2009, 38, 1330 RSC; (e) M.-S. Wang, S.-P. Guo, Y. Li, L.-Z. Cai, J.-P. Zou, G. Xu, W.-W. Zhou, F.-K. Zheng and G.-C. Guo, J. Am. Chem. Soc., 2009, 131, 13572 CrossRef CAS PubMed.
- (a) A. H. Chughtai, N. Ahmad, H. A. Younus, A. Laypkovc and F. Verpoort, Chem. Soc. Rev., 2015, 44, 6804 RSC; (b) A. Corma, H. García and F. X. Llabrés i Xamena, Chem. Rev., 2010, 110, 4606 CrossRef CAS PubMed; (c) L. Q. Ma, C. Abney and W. B. Lin, Chem. Soc. Rev., 2009, 38, 1248 RSC; (d) J. Lee, O. K. Farha, J. Roberts, K. A. Scheidt, S. T. Nguyen and J. T. Hupp, Chem. Soc. Rev., 2009, 38, 1450 RSC; (e) M. Yoon, R. Srirambalaji and K. Kim, Chem. Rev., 2012, 112, 1196 CrossRef CAS PubMed.
- (a) L. C. He, Y. Liu, J. Z. Liu, Y. S. Xiong, J. Z. Zheng, Y. L. Liu and Z. Y. Tang, Angew. Chem., Int. Ed., 2013, 52, 3741 CrossRef CAS PubMed; (b) Y. Li, S. S. Zhang and D. T. Song, Angew. Chem., Int. Ed., 2013, 52, 710 CrossRef CAS PubMed; (c) W. T. Yang, J. Feng and H. J. Zhang, J. Mater. Chem., 2012, 22, 6819 RSC; (d) B. L. Chen, Y. Yang, F. Zapata, G. N. Lin, G. D. Qian and E. B. Lobkovsky, Adv. Mater., 2007, 19, 1693 CrossRef CAS; (e) B. Zhao, X.-Y. Chen, P. Cheng, D.-Z. Liao, S.-P. Yan and Z.-H. Jiang, J. Am. Chem. Soc., 2004, 126, 15394 CrossRef CAS PubMed; (f) Z. C. Hu, B. J. Deibert and J. Li, Chem. Soc. Rev., 2014, 43, 5815 RSC.
- (a) P. Horcajada, R. Gref, T. Baati, P. K. Allan, G. Maurin, P. Couvreur, G. Férey, R. E. Morris and C. Serre, Chem. Rev., 2012, 112, 1232 CrossRef CAS PubMed; (b) D. Cunha, M. Ben Yahia, S. Hall, S. R. Miller, H. Chevreau, E. Elkaïm, G. Maurin, P. Horcajada and C. Serre, Chem. Mater., 2013, 25, 2767 CrossRef CAS.
- (a) M. Kurmoo, Chem. Soc. Rev., 2009, 38, 1353 RSC; (b) D.-F. Weng, Z.-M. Wang and S. Gao, Chem. Soc. Rev., 2011, 40, 3157 RSC; (c) P. Dechambenoit and J. R. Long, Chem. Soc. Rev., 2011, 40, 3249 RSC.
- (a) L. S. Meriwether, E. C. Breitner and C. L. Sloan, J. Am. Chem. Soc., 1965, 87, 4441 CrossRef CAS; (b) M.-S. Wang, G.-C. Guo, W.-Q. Zou, W.-W. Zhou, Z.-J. Zhang, G. Xu and J.-S. Huang, Angew. Chem., Int. Ed., 2008, 47, 3565 CrossRef CAS PubMed; (c) R. Pardo, M. Zayat and D. Levy, Chem. Soc. Rev., 2011, 40, 672 RSC.
- M.-S. Wang, G. Xu, Z.-J. Zhang and G.-C. Guo, Chem. Commun., 2010, 46, 361 RSC.
- C. Sanchez, B. Julián, P. Belleville and M. Popall, J. Mater. Chem., 2005, 15, 3559 RSC.
- (a) L.-Z. Cai, Q.-S. Chen, C.-J. Zhang, P.-X. Li, M.-S. Wang and G.-C. Guo, J. Am. Chem. Soc., 2015, 137, 10882 CrossRef CAS PubMed; (b) J.-K. Sun and J. Zhang, Dalton Trans., 2015, 44, 19041 RSC; (c) F. Luo, C. B. Fan, M. B. Luo, X. L. Wu, Y. Zhu, S. Z. Pu, W.-Y. Xu and G.-C. Guo, Angew. Chem., Int. Ed., 2014, 53, 9298 CrossRef CAS PubMed.
- (a) T. Okada and M. Ogawa, Chem. Commun., 2003, 1378 RSC; (b) H. Yoshikawa, S. Nishikiori, K. Suwinska, R. Luboradzki and J. Lipkowski, Chem. Commun., 2001, 1398 RSC.
- J.-B. Lin and G. K. H. Shimizu, Inorg. Chem. Front., 2014, 1, 302 RSC.
- E. C. P. Bruker, AXS Inc., Madison, WI 53711-5373, USA, 2000.
- G. M. Sheldrick, Acta Crystallogr., Sect. A: Fundam. Crystallogr., 2008, 64, 112 CrossRef CAS PubMed.
- X. Q. Zhang, Y. F. Gao, H. T. Liu and Z. L. Liu, CrystEngComm, 2015, 17, 6037 RSC.
- (a) R. R. Yun, Z. Y. Lu, Y. Pan, X. Z. You and J. F. Bai, Angew. Chem., Int. Ed., 2013, 52, 11282 CrossRef CAS PubMed; (b) Y.-K. Lv, C.-H. Zhan, Z.-G. Jiang and Y.-L. Feng, Inorg. Chem. Commun., 2010, 13, 440 CrossRef CAS; (c) X. Li, B.-L. Wu, C.-Y. Niu, Y.-Y. Niu and H.-Y. Zhang, Cryst. Growth Des., 2009, 9, 3423 CrossRef CAS; (d) M. Oh, L. Rajput, D. Kim, D. Moon and M. S. Lah, Inorg. Chem., 2013, 52, 3891 CrossRef CAS PubMed.
- S. A. Sotnik, R. A. Polunin, M. A. Kiskin, A. M. Kirillov, V. N. Dorofeeva, K. S. Gavrilenko, I. L. Eremenko, V. M. Novotortsev and S. V. Kolotilov, Inorg. Chem., 2015, 54, 5169 CrossRef CAS PubMed.
- O. Toma, N. Mercier, M. Allain, A. A. Kassiba, J.-P. Bellat, G. Weber and I. Bezverkhyy, Inorg. Chem., 2015, 54, 8923 CrossRef CAS PubMed.
- (a) E. M. Kosower and J. L. Cotter, J. Am. Chem. Soc., 1964, 86, 5524 CrossRef CAS; (b) M. Kaneko, J. Motoyoshi and A. Yamada, Nature, 1980, 285, 468 CrossRef CAS; (c) P. M. S. Monk, The Viologens: Physicochemical Properties, Synthesis and Applications of the Salts of 4,4′-Bipyridine, John Wiley & Sons, New York, 1998 Search PubMed.
- (a) J.-K. Sun, P. Wang, Q.-X. Yao, Y.-J. Chen, Z.-H. Li, Y.-F. Zhang, L.-M. Wu and J. Zhang, J. Mater. Chem., 2012, 22, 12212 RSC; (b) Q.-X. Yao, L. Pan, X.-H. Jin, J. Li, Z.-F. Ju and J. Zhang, Chem. – Eur. J., 2009, 15, 11890 CrossRef CAS PubMed.
- (a) N. Leblanc, W. H. Bi, N. Mercier, P. Auban-Senzier and C. Pasquier, Inorg. Chem., 2010, 49, 5824 CrossRef CAS PubMed; (b) R.-G. Lin, G. Xu, M.-S. Wang, G. Lu, P.-X. Li and G.-C. Guo, Inorg. Chem., 2013, 52, 1199 CrossRef CAS PubMed.
- (a) Q.-X. Yao, Z.-F. Ju, X.-H. Jin and J. Zhang, Inorg. Chem., 2009, 48, 1266 CrossRef CAS PubMed; (b) M.-S. Wang, C. Yang, G.-E. Wang, G. Xu, X.-Y. Lv, Z.-N. Xu, R.-G. Lin, L.-Z. Cai and G.-C. Guo, Angew. Chem., Int. Ed., 2012, 51, 3432 CrossRef CAS PubMed; (c) P.-X. Li, M.-S. Wang, M.-J. Zhang, C.-S. Lin, L.-Z. Cai, S.-P. Guo and G.-C. Guo, Angew. Chem., Int. Ed., 2014, 53, 11529 CrossRef CAS PubMed.
- (a) J. B. Wu, C. Y. Tao, Y. Li, Y. Yan, J. Y. Li and J. H. Yu, Chem. Sci., 2014, 5, 4237 RSC; (b) J. B. Wu, C. Y. Tao, Y. Li, J. Y. Li and J. H. Yu, Chem. Sci., 2015, 6, 2922 RSC.
- L. Wu, J. C. Yu and X. Z. Fu, J. Mol. Catal. A: Chem., 2006, 244, 25 CrossRef CAS.
- P.-C. Jhang, N.-T. Chuang and S.-L. Wang, Angew. Chem., Int. Ed., 2010, 49, 4200 CrossRef CAS PubMed.
- (a) X.-H. Jin, J.-K. Sun, X.-M. Xu, Z.-H. Li and J. Zhang, Chem. Commun., 2010, 46, 4695 RSC; (b) Y. Zeng, S. J. Liao, J. C. Dai and Z. Y. Fu, Chem. Commun., 2012, 48, 11641 RSC; (c) J.-K. Sun, L.-X. Cai, Y.-J. Chen, Z.-H. Li and J. Zhang, Chem. Commun., 2011, 47, 6870 RSC.
- G. R. Hutchison, M. A. Ratner and T. J. Marks, J. Am. Chem. Soc., 2005, 127, 16866 CrossRef CAS PubMed.
- X. D. Guo, G. S. Zhu, Q. R. Fang, M. Xue, G. Tian, J. Y. Sun, X. T. Li and S. L. Qiu, Inorg. Chem., 2005, 44, 3850 CrossRef CAS PubMed.
- J. Xu, L. B. Sun, H. Z. Xing, Z. Q. Liang, J. H. Yu and R. R. Xu, Inorg. Chem. Commun., 2011, 14, 978 CrossRef CAS.
- J. B. Wu, Y. Yan, B. K. Liu, X. L. Wang, J. Y. Li and J. H. Yu, Chem. Commun., 2013, 49, 4995 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: The crystal data and structure refinement, and the selected bond lengths and angles for 1 and 2. PXRD patterns, TGA, EPR, UV, fluorescence and IR spectra. The asymmetric units, topos of 1 and 2. CCDC 1438481 and 1438482. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6qi00013d |
| This journal is © the Partner Organisations 2016 |