Facile synthesis of amorphous aluminum vanadate hierarchical microspheres for supercapacitors†
Yan
Yan
a,
Hao
Xu
a,
Wei
Guo
b,
Qingli
Huang
a,
Mingbo
Zheng
a,
Huan
Pang
*a and
Huaiguo
Xue
*a
aCollege of Chemistry and Chemical Engineering, Yangzhou University, Yangzhou, 225002, Jiangsu, China. E-mail: huanpangchem@hotmail.com; panghuan@yzu.edu.cn; chhgxue@yzu.edu.cn
bCollege of Chemistry and Chemical Engineering, Anyang Normal University, Anyang, 455002, P. R. China
First published on 25th April 2016
Abstract
Micro-nanostructured mixed metal vanadates have recently garnered enormous attention owing to their remarkable performances in catalysis, energy storage and conversion. In this work, we report the synthesis of amorphous aluminum vanadate hierarchical microspheres via a simple hydrothermal approach with polyvinylpyrrolidone as a surface directing agent. Amorphous aluminum vanadate hierarchical microspheres are firstly described as a kind of electrode material for supercapacitors. The measured specific capacitance of the amorphous aluminum vanadate electrode is 497 F g−1 at 1 A g−1 with good stability and a retention capacity of 89% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles. In addition, the fabricated asymmetric supercapacitor device delivered better performance with an extended operating voltage window of 1.5 V, excellent cycle stability (10
000 cycles. In addition, the fabricated asymmetric supercapacitor device delivered better performance with an extended operating voltage window of 1.5 V, excellent cycle stability (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles, 85% capacitance retention), high energy density (37.2 W h kg−1 at 1124.4 W kg−1) and high power density (11
000 cycles, 85% capacitance retention), high energy density (37.2 W h kg−1 at 1124.4 W kg−1) and high power density (11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 W kg−1 at 25 W h kg−1). This study essentially offers a new kind of vanadate as an electrochemical active material for the development of supercapacitors.
250 W kg−1 at 25 W h kg−1). This study essentially offers a new kind of vanadate as an electrochemical active material for the development of supercapacitors.
1. Introduction
Supercapacitors (SCs), also called electrochemical capacitors (ECs), have a higher power density, longer cycling life and shorter charge/discharge time than other electrochemical energy storage devices, such as dielectric capacitors, secondary cells, fuel cells, and lithium-ion batteries. Supercapacitors can not only reach thousands of watts per kilogram and a long cycle life but also enhance the efficiency of energy utilization.1–8As pseudocapacitor materials, metal oxides can provide higher energy density in comparison with others such as carbon-based active materials and conducting polymers.9–24 Recently, binary metal oxides have aroused people's widespread interest by dint of their better performance than single-component oxides which can be attributed to their feasible oxidation states and high electrical conductivity.25–29 Metal vanadium oxides and vanadates have been widely investigated as novel active materials for lithium-ion batteries.30–38 Very recently, Yang et al. have reported the successful preparation of aluminum vanadium oxide (AlV3O9) 3D hierarchical microspheres which exhibit an eminent reversible capacity and excellent rate performance for lithium storage.39 However, the exploitation of AlV3O9 for supercapacitors has few developments.
Materials with amorphous phases or poor crystallinity may exhibit unique physical and chemical properties with more active sites and isotropic nature.40–42 The amorphous transition metal oxide materials show a broad prospect in the applications of supercapacitors as their disorder structures are better to accommodate repeated volume changes associated with doping–undoping without breaking.43–46 The continuous redox reaction of an amorphous composite occurs not only on the surface but also in the bulk of the powder, leading to better performance compared with crystallized structures.46–49
In this work, we, for the first time, investigate amorphous aluminum vanadate hierarchical microspheres for supercapacitors. We provide a facile and hydrothermal method approaching with PVP as a surfactant to synthesize amorphous aluminum vanadate hierarchical microspheres by the modified method of Yang et al.39 Interestingly, the obtained amorphous aluminum vanadate hierarchical microsphere electrode shows a specific capacitance (497 F g−1 at 1 A g−1) with good stability and a retention capacity of 89% after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles.
000 cycles.
2. Experimental section
Materials
All chemicals, aluminum chloride hexahydrate (AlCl3·6H2O, 99%), ammonium metavanadate (NH4VO3, 99%), hydrochloric acid and polyvinyl pyrrolidone (PVP), were purchased from Shanghai Sinopharm Chemical Reagent Co. and used without further purification. All aqueous solutions were freshly prepared with high purity water (18 MΩ cm−1).Synthesis of amorphous aluminum hierarchical microspheres
In a typical synthesis, 105.3 mg NH4VO3 was dissolved in 40 mL deionized water at 80 °C. Under severe stirring, hydrochloric acid was added dropwise to the ammonium metavanadate solution until the final pH of the solution was about 3.0. Then, 434.6 mg mmol AlCl3·6H2O and 500 mg PVP were added to the solution under stirring. After continuous stirring for 10 min, the resulting yellow precursor suspension was transferred into a 50 mL Teflon-lined autocalve and maintained at 160 °C for 6 hours. After reaction, dark green precipitates were obtained. The precipitates were completely washed with deionized water and ethanol to remove ions possibly remaining in the final products, and dried at room temperature in air.Characterization
The morphological features were characterized by field emission scanning electron microscopy (FESEM, Zeiss-Supra 55), high resolution transmission electron microscopy (HRTEM, Tecnai G2 F30 S-TWIN), selected area electron diffraction (SAED) and energy-dispersive energy dispersive X-ray spectrometry (EDS) mapping. X-ray diffraction (XRD) patterns were examined on a Bruker D8 Advanced X-ray diffractometer (CuKα radiation: λ = 0.15406 nm). The chemical states were analyzed using X-ray photoelectron spectroscopy (XPS) using an XPS (ESCAL AB250) system with monochromatic AlKα excitation under vacuum higher than 1 × 10−7 Pa. Thermogravimetric measurements were performed via a Perkinelmer Pyris 1 TGA instrument. V and Al contents were analyzed by inductively coupled plasma optical emission spectrometry (ICP-OES, PE-3300DV) after the sample was dissolved.Electrochemical measurements
An electrochemical study on amorphous aluminum vanadate hierarchical microsphere electrodes, including cyclic voltammetry (CV), charge–discharge tests, and electrochemical impedance spectroscopy (EIS), was carried out on a CHI 660D electrochemical working station (Shanghai Chenhua Instrument, Inc.). All electrochemical performances were carried out respectively in a conventional three-electrode system equipped with a platinum electrode and a saturated calomel electrode (SCE) as counter and reference electrodes. The working electrode was made by mixing active materials (amorphous aluminum vanadate hierarchical microspheres), acetylene black, and PTFE (polytetrafluoroethylene) at a weight ratio of 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 15
15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5, this mixture was coated on a piece of foamed nickel foam of about 1 cm2, and pressed it to be a thin foil at the pressure of 5.0 MPa. The asymmetric supercapacitors (ASCs) were assembled with amorphous aluminum vanadate hierarchical microspheres as a positive electrode, and active carbon (AC) as a negative electrode. AC was purchased from Fuzhou Yihuan Carbon Co., Ltd (China), with a specific surface area of more than 2100 m2 g−1 and a particle size of about 10 μm. For the ASCs, the method of preparation of the AC negative electrode was the same as the preparation of amorphous aluminum vanadate hierarchical microspheres as a positive electrode. The electrolyte used was 1.0 M Na2SO4 solution. The EIS plots were obtained in the frequency range from 100 kHz to 0.1 Hz at the open-circuit potential with a sinusoidal ac perturbation of 5 mV.
5, this mixture was coated on a piece of foamed nickel foam of about 1 cm2, and pressed it to be a thin foil at the pressure of 5.0 MPa. The asymmetric supercapacitors (ASCs) were assembled with amorphous aluminum vanadate hierarchical microspheres as a positive electrode, and active carbon (AC) as a negative electrode. AC was purchased from Fuzhou Yihuan Carbon Co., Ltd (China), with a specific surface area of more than 2100 m2 g−1 and a particle size of about 10 μm. For the ASCs, the method of preparation of the AC negative electrode was the same as the preparation of amorphous aluminum vanadate hierarchical microspheres as a positive electrode. The electrolyte used was 1.0 M Na2SO4 solution. The EIS plots were obtained in the frequency range from 100 kHz to 0.1 Hz at the open-circuit potential with a sinusoidal ac perturbation of 5 mV.
3. Results and discussion
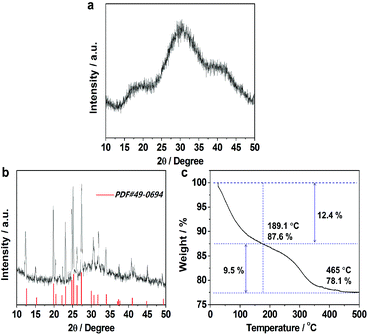
XRD patterns of the as-prepared samples were obtained to reveal the phase of the as-prepared samples as shown in Fig. 1a. All XRD patterns of the sample indicate that it is amorphous. The crystalline materials can be obtained after calcination at 500 °C for 2 h in air (Fig. 1b), whose XRD patterns are indexed to be in agreement with AlV3O9 (JCPDS no. 49-0649). Thermogravimetric analysis (TGA) (Fig. 1c) under the same conditions can confirm that it is composed of AlV3O9·nH2O.To gain more insight into the purity of hierarchical microspheres, we further analyzed the sample with the aid of XPS. Fig. 2a shows the complete scan for detection of the elements present in the sample. The deconvoluted peaks of O 1s at 533.5 eV (Fig. 2b) can be assigned to the adsorbed oxygen species such as H2O on the surface of amorphous aluminum vanadate hierarchical microspheres and other two peaks of O 1s at 532 eV and 530.5 eV can belong to Al2O3 and V2O5, respectively.50,51Fig. 2c shows the Al 2p spectrum. Previous studies indicate that Al 2p3/2 and Al 2p1/2 peaks here belong to Al2O3.52,53Fig. 2d shows the V 2p spectrum. The deconvolution of the XPS peak of V 2p1/2 at 524.8 eV was carried out to observe that the oxidation state of vanadium is +5 explicitly.54 Other V 2p3/2 peaks at positions 517.3 and 517.4 eV, which belong to the V5+ state, are also observed.55,56
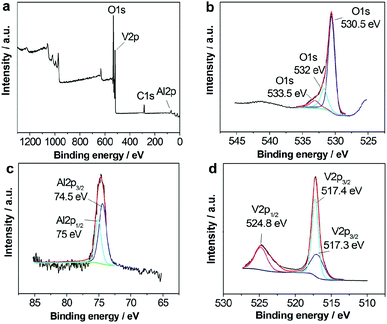 | ||
| Fig. 2 XPS spectra of amorphous aluminum vanadate hierarchical microspheres. (a) Survey spectrum, (b) O 1S, (c) Al 2p, and (d) V 2p peaks, respectively. | ||
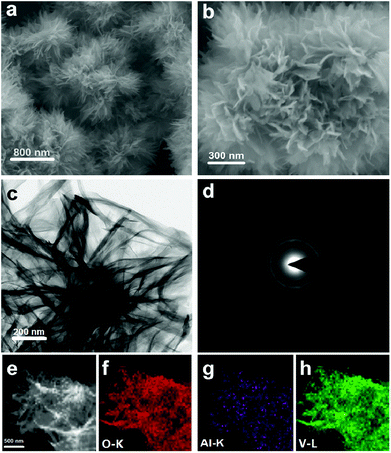
Field-emission scanning electron microscopy (FESEM) was used to study the morphological characterization of aluminum vanadate hierarchical microspheres. It can be seen in Fig. 3a and b that the nanospheres are composed of primary substructures (nanosheet-like), which are the primary building blocks. Fig. 3c shows the TEM image of the hierarchical microspheres. Fig. 3d shows the SAED pattern of the hierarchical microspheres indicating the amorphous nature of the samples. Energy dispersive X-ray spectrometry (EDS) mapping analysis (Fig. 3e–h) of amorphous aluminum vanadate hierarchical microspheres unambiguously confirms the sea urchin-like structure and the existence of V and Al element. In addition, the V and Al contents in the hierarchical microspheres were measured by ICP-OES. The ratio of contents for V and Al elements that was determined from the ICP-OES quantitative analysis is about 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, which is consistent with the EDS quantitative analysis result.
1, which is consistent with the EDS quantitative analysis result.
In order to first study the influence of other surfactants on the morphology and structure of aluminum vanadate, we prepared the sample under the same conditions, only using two other kinds of surfactants of sodium dodecyl sulfate (SDS) and hexadecyl trimethyl ammonium bromide (CTAB) instead of PVP. The amount of the two surfactants is also 0.5 g. The FESEM images and XRD patterns of samples which were prepared with additions of SDS or CTAB are shown in Fig. S1 and S2,† respectively. It is seen that the morphology of the sample which was modified with SDS and CTAB is the same as that of PVP (Fig. S1†) and the XRD patterns also proved their amorphous structure (Fig. S2†).
The N2 adsorption/desorption measurement indicated that the as-prepared amorphous aluminum vanadate hierarchical microspheres had a Brunauer–Emmett–Teller (BET) surface area of 56.7 m2 g−1 (Fig. 4a), which could provide a large interface to facilitate the electrochemical uptake and release of ions with respect to the bulk materials. From the pore distribution curve, it can be found that the amorphous aluminum vanadate hierarchical microspheres possess a bimodal pore distribution calculated using the Barrett–Joyner–Halenda (BJH) method, the average pore size is about 22 nm, revealing that the sample includes mesopores (Fig. 4b). Considering their larger surface area, porous structure, and the synergy effect between the macroporous and mesoporous layers, the as-synthesized amorphous aluminum vanadate hierarchical microspheres are beneficial to enhance the diffusion of the ions and electrolyte, and can improve the electrochemical performance, which make them promising to be used in many potential applications for supercapacitors.
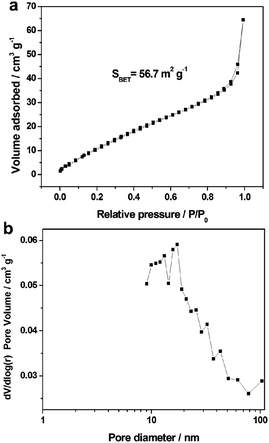 | ||
| Fig. 4 (a) N2 adsorption/desorption isotherms and (b) pore size distribution curves of amorphous aluminum vanadate hierarchical microspheres. | ||
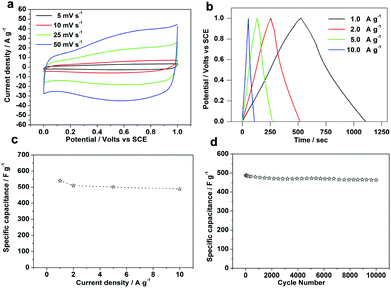
The CVs of amorphous aluminum vanadate hierarchical microsphere electrodes (a mass loading of 1 mg) in 1.0 M Na2SO4 at different scan rates (5–50 mV s−1) are shown in Fig. 5a. As is seen in Fig. 5a, the shapes are different from that of electric double-layer capacitance, suggesting that the capacity mainly results from pseudocapacitive capacitance. Chronopotentiometry (CP) curves at different current densities are shown in Fig. 5b. Different charge–discharge current densities and times can be clearly seen. On increasing current densities, short charge–discharge times have been obtained. No obvious IR drop is observed at the beginning of the discharge curve, reflecting a very small ESR of the amorphous aluminum vanadate hierarchical microsphere electrodes.
The specific capacitance of the electrode material can be calculated from the charge–discharge curves according to the equation:
| C = Q/(m × ΔV) = I × tdischarge/(m × ΔV) | (1) |
It is believed that galvanostatic charge–discharge technique is a more accurate technique for measuring supercapacitance, so the specific capacitances (C) were calculated from galvanostatic charge–discharge curves according to the equation. The relationship of the specific capacitance against the cycling number of amorphous aluminum vanadate hierarchical microsphere electrodes is shown in Fig. 5d, which shows its good specific capacitance retention at 1 A g−1. After about 300 continuous charge–discharge cycles, amorphous aluminum vanadate hierarchical microsphere electrodes almost retain the same specific capacitance as its initial value. More importantly, amorphous aluminum vanadate hierarchical microsphere electrodes still retain more than 89% of their specific capacitance after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 continuous charge–discharge cycles. After the testing of the cycle life, we have measured the morphology of the electrode materials as shown in Fig. S3.† There are few changes of amorphous aluminum vanadate hierarchical microspheres after 10
000 continuous charge–discharge cycles. After the testing of the cycle life, we have measured the morphology of the electrode materials as shown in Fig. S3.† There are few changes of amorphous aluminum vanadate hierarchical microspheres after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 charge–discharge cycles as shown in Fig. S3.†
000 charge–discharge cycles as shown in Fig. S3.†
| AlV3O9 + xNa+ + xe− ↔ NaxAlV3O9 | (2) |
We have tried to propose the possible reaction mechanism of aluminum vanadate in eqn (2).
EIS measurement in the frequency range of 0.01 Hz to 105 Hz was performed to clearly understand the ion diffusion of the electrodes (Fig. 6). Obviously, the EIS plot of the amorphous aluminum vanadate hierarchical microsphere electrode is composed of a semicircle in the high frequency region and a straight line in the low-frequency region. The internal resistance (Rb) can be obtained from the intercept of the plot with the real axis in the high-frequency region, which is 3.5 Ω. The charge-transfer resistance (Rct) of the electrode is 1.0 Ω from the size of the semicircle.
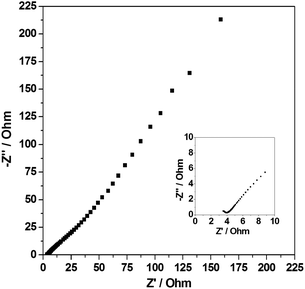 | ||
| Fig. 6 The EIS of the electrodes (amorphous aluminum vanadate hierarchical microspheres) at room temperature from 0 to 225 ohm, and in the inset from 0 to 10 ohm. | ||
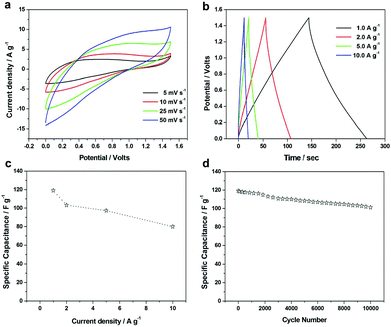
To further evaluate the amorphous aluminum vanadate hierarchical microsphere electrode for practical applications, we fabricated an ASC device based on the amorphous aluminum vanadate hierarchical microsphere cathode and the AC anode with a voltage window of 1.5 V (1 M Na2SO4 was used as the electrolyte). Fig. 7a shows the CV curves of the amorphous aluminum vanadate hierarchical microspheres//AC ASC device collected at varied scan rates (5–50 mV s−1) in a voltage range between 0 and 1.5 V. The rectangular shape of the CV curve is still well retained, presenting good capacitive behavior and fast charge–discharge capability of the as-assembled ASC device in the voltage window of 0–1.5 V. The galvanostatic charge–discharge curves of the ASCs at various current densities are shown in Fig. 7b. The charge and discharge curves retain good symmetry at a cell voltage as high as 1.5 V, implying that the device has excellent electrochemical reversibility and capacitive characteristics. Specific capacitances at different current densities were calculated and are plotted in Fig. 7c. The highest value of specific capacitance of 119.1 F g−1, was achieved at a 1 A g−1 current density and showed a gradual specific capacitance reduction of up to 80 F g−1 when the current density was increased from 1 A g−1 to 10 A g−1. The excellent cycling stability of the device was also verified by 85% retention in specific capacitance after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 continuous cycles at a current density of 1 A g−1 as shown in Fig. 7d.
000 continuous cycles at a current density of 1 A g−1 as shown in Fig. 7d.
The energy density (E) and power density (P) of the ASCs (expressed in W h kg−1 and W kg−1, respectively) were calculated from charge–discharge curves according to the following formulae:
| E = C × (ΔV)2/7.2 | (3) |
| P = 3600 × E/Δt | (4) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 W kg−1 at 25 W h kg−1). As efficient and powerful energy storage devices, supercapacitors could, in principle, be charged and then discharged to drive various electronic units. To demonstrate the practical application of the assembled full-cell supercapacitors, two ASC devices based on amorphous aluminum vanadate hierarchical microsphere nanoparticles and AC were connected in series. Then, they were connected with a rotating motor. They can easily drive the rotating motor, as shown in the inset of Fig. 8. It indicates that these ASC devices based on amorphous aluminum vanadate hierarchical microsphere nanoparticles and AC are very promising candidates for various practical applications.
250 W kg−1 at 25 W h kg−1). As efficient and powerful energy storage devices, supercapacitors could, in principle, be charged and then discharged to drive various electronic units. To demonstrate the practical application of the assembled full-cell supercapacitors, two ASC devices based on amorphous aluminum vanadate hierarchical microsphere nanoparticles and AC were connected in series. Then, they were connected with a rotating motor. They can easily drive the rotating motor, as shown in the inset of Fig. 8. It indicates that these ASC devices based on amorphous aluminum vanadate hierarchical microsphere nanoparticles and AC are very promising candidates for various practical applications.
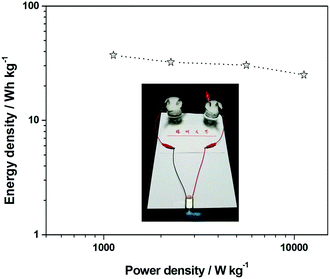 | ||
| Fig. 8 Ragone plot of the amorphous aluminum vanadate hierarchical microspheres//AC ASC device, the inset is a rotating motor powered by two ASC devices linked in series. | ||
4. Conclusions
In summary, we present a facile method to synthesize amorphous aluminum vanadate hierarchical microspheres through a hydrothermal process by adding a PVP surfactant. The capacitive behavior of the electrode materials was investigated by cyclic voltammetry and galvanostatic charging and discharging in 1 M Na2SO4. The amorphous aluminum vanadate hierarchical microspheres showed a specific capacitance of up to 497 F g−1 and can be cycled for 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 cycles with 89% capacitance retention. The ASCs were able to deliver a high energy density of 37.2 W h kg−1 at a power density of 1124.4 W kg−1 and a high power density of 11
000 cycles with 89% capacitance retention. The ASCs were able to deliver a high energy density of 37.2 W h kg−1 at a power density of 1124.4 W kg−1 and a high power density of 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 250 W kg−1 at an energy density of 25 W h kg−1. The device can retain 85% of the capacitance after 10
250 W kg−1 at an energy density of 25 W h kg−1. The device can retain 85% of the capacitance after 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 continuous charge–discharge cycles, demonstrating the importance and great potential of aluminum vanadate in the development of energy storage systems. Our research shows that aluminum vanadate has a broad application prospect which points toward further application of aluminum vanadate for supercapacitors.
000 continuous charge–discharge cycles, demonstrating the importance and great potential of aluminum vanadate in the development of energy storage systems. Our research shows that aluminum vanadate has a broad application prospect which points toward further application of aluminum vanadate for supercapacitors.
Acknowledgements
This work is supported by the Program for New Century Excellent Talents of the University in China (grant no. NCET-13-0645), the National Natural Science Foundation of China (NSFC-21201010, 21173183, 51202106), the Innovation Scientists and Technicians Troop Construction Projects of Henan Province, the Program for the Innovative Research Team (in Science and Technology) in the University of Henan Province (14IRTSTHN004), the Science & Technology Foundation of Henan Province (122102210253 and 13A150019), the 333 Project (Grant BRA2011188), the Six Talent Plan (2015-XCL-030) of Jiangsu Province, and the China Postdoctoral Science Foundation (2012M521115). We also acknowledge the Priority Academic Program Development of Jiangsu Higher Education Institutions and the technical support received at the Testing Center of Yangzhou University.References
- C. Liu, F. Li, L. Ma and H. Cheng, Adv. Mater., 2010, 22, E28 CrossRef CAS PubMed.
- S. Chu and A. Majumdar, Nature, 2012, 488, 294 CrossRef CAS PubMed.
- D. Lindley, Nature, 2010, 463, 18 CrossRef CAS PubMed.
- P. Simon and Y. Gogotsi, Nat. Mater., 2008, 7, 845 CrossRef CAS PubMed.
- J. Chmiola, C. Largeot, P.-L. Taberna, P. Simon and Y. Gogotsi, Science, 2010, 328, 480 CrossRef CAS PubMed.
- P. Simon, Y. Gogotsi and B. Dunn, Science, 2014, 343, 1210 CrossRef CAS PubMed.
- M. Winter and R. J. Brodd, Chem. Rev., 2004, 104, 4245 CrossRef CAS PubMed.
- Y. Gogotsi and P. Simon, Science, 2011, 334, 917 CrossRef CAS PubMed.
- C. Wu, F. Feng and Y. Xie, Chem. Soc. Rev., 2013, 42, 5157 RSC.
- Y. Zhang, Y. Wang, T. Chen, W. Lai, H. Pang and W. Huang, Chem. Soc. Rev., 2015, 44, 7484 RSC.
- C. Z. Yuan, H. B. Wu, Y. Xie and X. W. Lou, Angew. Chem., Int. Ed., 2014, 53, 1488 CrossRef CAS PubMed.
- V. Ozolins, F. Zhou and M. Asta, Acc. Chem. Res., 2013, 46, 1084 CrossRef CAS PubMed.
- H. Wang and H. Dai, Chem. Soc. Rev., 2013, 42, 3088 RSC.
- C. Zhong, Y. Deng, W. Hu, J. Qiao, L. Zhang and J. Zhang, Chem. Soc. Rev., 2015, 44, 7484 RSC.
- J. Daubert, N. Lewis, H. Gotsch, J. Mundy, D. Monroe, E. Dickey, M. Losego and G. Parsons, Chem. Mater., 2015, 27, 6524 CrossRef CAS.
- S. D. Perera, B. Patel, N. Nijem, K. Roodenko, O. Seitz, J. P. Ferraris, Y. J. Chabal and K. J. Balkus, Adv. Energy Mater., 2011, 1, 936 CrossRef CAS.
- M. Sathiya, A. S. Prakash, K. Ramesha, J.-M. Tarascon and A. K. Shukla, J. Am. Chem. Soc., 2011, 133, 16291 CrossRef CAS PubMed.
- A. Ghosh, E. Ra, M. Jin, H. Jeong, T. Kim, C. Biswas and Y. Lee, Adv. Funct. Mater., 2011, 21, 2541 CrossRef CAS.
- Q. Qu, Y. Zhu, X. Gao and Y. Wu, Adv. Energy Mater., 2012, 2, 950 CrossRef CAS.
- B. Li, M. Zheng, H. Xue and H. Pang, Inorg. Chem. Front., 2016, 3, 175 RSC.
- W. Li, Q. Liu, Y. Sun, J. Sun, R. Zou, G. Li, X. Hu, G. Song, G. Ma, J. Yang, Z. Chen and J. Hu, J. Mater. Chem., 2012, 22, 14864 RSC.
- X. Zhang, Y. Zhao and C. Xu, Nanoscale, 2014, 6, 3638 RSC.
- S. Jin and C. Wang, Nano Energy, 2014, 7, 63 CrossRef CAS.
- P.-Y. Tang, L.-J. Han, A. Genç, Y.-M. He, X. Zhang, L. Zhang, J. R. Galán-Mascarós, J. R. Morante and J. Arbiol, Nano Energy, 2016, 22, 189 CrossRef CAS.
- F. Butt, M. Tahir, C. Cao, F. Idrees, R. Ahmed, W. Khan, Z. Ali, N. Mahmood, M. Tanveer, A. Mahmood and I. Aslam, ACS Appl. Mater. Interfaces, 2014, 6, 13635 CAS.
- M. Liu, L. Kong, L. Kang, X. Li, F. Walsh, M. Xing, C. Lu, X. Ma and Y. Luo, J. Mater. Chem. A, 2014, 2, 4919 CAS.
- Y. Zhang, Y. Liu, J. Chen, Q. Guo, T. Wang and H. Pang, Sci. Rep., 2014, 4, 5678 Search PubMed.
- W. Zhang, L. Kong, X. Ma, Y. Luo and L. Kang, RSC Adv., 2014, 4, 41772 RSC.
- S. Vijayakumar, S. Lee and K. Ryu, RSC Adv., 2015, 5, 91822 RSC.
- H. Ma, S. Zhang, W. Ji, Z. Tao and J. Chen, J. Am. Chem. Soc., 2008, 130, 5361 CrossRef CAS PubMed.
- F. Cheng and J. Chen, J. Mater. Chem., 2011, 21, 9841 RSC.
- S. Zhang, R. Hu, L. Liu and D. Wang, Mater. Lett., 2014, 124, 57 CrossRef CAS.
- L. Xiao, Y. Zhao, J. Yin and L. Zhang, Chem. – Eur. J., 2009, 15, 9442 CrossRef CAS PubMed.
- L. Zeng, F. Xiao, J. Wang, S. Gao, X. Ding and M. Wei, J. Mater. Chem., 2012, 22, 14284 RSC.
- C. Zheng, L. Zeng, M. Wang, H. Zheng and M. Wei, CrystEngComm, 2014, 16, 10309 RSC.
- F. Wu, C. Yu, W. Liu, T. Wang, J. Feng and S. Xiong, J. Mater. Chem. A, 2015, 3, 16728 CAS.
- G. Yang, H. Cui, G. Yang and C. Wang, ACS Nano, 2014, 8, 4474 CrossRef CAS PubMed.
- Y. Sun, C. Li, L. Wang, Y. Wang, X. Ma, P. Ma and M. Song, RSC Adv., 2012, 2, 8110 RSC.
- G. Yang, H. Song, G. Yang, M. Wu and C. Wang, Nano Energy, 2015, 15, 281 CrossRef CAS.
- H. Li, M. Yu, F. Wang, P. Liu, Y. Liang, J. Xiao, C. Wang, Y. Tong and G. Yang, Nat. Commun., 2013, 4, 1894 CrossRef CAS PubMed.
- S. Yang, X. Wu, C. Chen, H. Dong, W. Hu and X. Wang, Chem. Commun., 2012, 48, 2773 RSC.
- H. Du, L. Jiao, K. Cao, Y. Wang and H. Yuan, ACS Appl. Mater. Interfaces, 2013, 5, 6643 CAS.
- H. Li, Y. Gao, C. Wang and G. Yang, Adv. Energy Mater., 2014, 5, 1401767 Search PubMed.
- W. Jiang, D. Yu, Q. Zhang, K. Goh, L. Wei, Y. Yong, R. Jiang, J. Wei and Y. Chen, Adv. Funct. Mater., 2015, 25, 1063 CrossRef CAS.
- Y. Zhang, W. Sun, X. Rui, B. Li, H. T. Tan, G. Guo, S. Madhavi, Y. Zong and Q. Yan, Small, 2015, 11, 3694 CrossRef CAS PubMed.
- P. Yang, Y. Li, Z. Lin, Y. Ding, S. Yue, C. P. Wong, X. Cai, S. Tan and W. Mai, J. Mater. Chem. A, 2014, 2, 595 CAS.
- Z. Jiang, W. Lu, Z. Li, K. H. Ho, X. Li, X. Jiao and D. Chen, J. Mater. Chem. A, 2014, 2, 8603 CAS.
- Y. Fu, J. Song, Y. Zhu and C. Cao, J. Power Sources, 2014, 262, 344 CrossRef CAS.
- L. Hu, Z. Yu, Z. Hu, Y. Song, F. Zhang, H. Zhu and S. Jiao, Electrochim. Acta, 2015, 174, 273 CrossRef CAS.
- A. K. Dua, V. C. George and R. P. Agarwala, Thin Solid Films, 1988, 165, 163 CrossRef CAS.
- R. Gopalakrishnan, B. V. R. Chowdari and K. L. Tan, Solid State Ionics, 1992, 53, 1168 CrossRef.
- P. H. Bolt, E. Ten Grotenhuis, J. W. Geus and F. H. P. M. Habraken, Surf. Sci., 1995, 329, 227 CrossRef CAS.
- Y. C. Kim, H. H. Park, J. S. Chun and W. J. Lee, Thin Solid Films, 1994, 237, 57 CrossRef CAS.
- J. L. G. Fierro, L. A. Arrua, J. M. L. Nieto and G. Kremenic, Appl. Catal., 1988, 37, 323 CrossRef CAS.
- L. M. Cornaglia and E. A. Lombardo, Appl. Catal., A, 1995, 127, 125 CrossRef CAS.
- A. Meisel, K. H. Hallmeier, R. Szargan, J. Muller and W. Schneider, Phys. Scr., 1990, 41, 513 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6qi00089d |
| This journal is © the Partner Organisations 2016 |