New way to multi-shelled hollow spheres for robust battery electrode
Hong Jin
Fan
School of Physical and Mathematical Sciences, Nanyang Technological University, Singapore 637371, Singapore. E-mail: fanhj@ntu.edu.sg
First published on 22nd June 2016
Abstract
A new and generic synthesis approach has been developed for a range of metal oxide multi-shelled hollow microspheres that are useful in fast and stable battery electrodes and more.
Lithium-ion batteries are something we have been living with in daily life and will most likely live with for a quite long while, being used in devices from your mobile phone, laptops, electric scooters and drones, to future cars. However, it is obvious to us that the mediocre performance and limited lifespan of batteries has been the bottleneck to the advance of modern electronics. The basic criteria for a high-performance Li-ion battery electrode include high specific capacity, high-rate capability, and long-cycle stability. From an industrial point of view, they also require safety and low cost. To meet these criteria, materials scientists and engineers are putting enormous efforts into designing nanostructured electrode materials that can simultaneously exhibit high specific surface areas, good electrical conductivity, and structural robustness.
Multi-shelled hollow nano- or microstructures are truly useful for a wide range of electrochemical processes including those in batteries and electrocatalysis.1–3 For Li-ion batteries, which work on the basis on repeated insertion/extraction of lithium ions into the crystals, a hollow microstructure is extra favorable. A multi-shelled hollow microsphere (MS-HMS) has the advantages of large surface area and large number of active sites, easy access for electrolytes and shortened diffusion paths for both ions and electrons. In addition, it can also buffer volume expansion and alleviate stress. With all these merits, MS-HMSs can be a model candidate in the nano-energy community. There has indeed been widespread interest to develop HMS electrodes for batteries.4–6
V2O5 is an ideal cathode material because of its high theoretical capacity (294 mA h g−1 based on two Li per formula unit), high voltage (4.0–2.0 V vs. Li/Li+), and earth abundance. Unfortunately, there are also obvious drawbacks; it has a relatively low diffusion coefficient of Li+ (∼10−12 cm2 s−1) and poor structural stability upon deep discharge, which results in poor rate-capability and cycling stability. Various types of low-dimensional V2O5 materials have been developed and demonstrated to be effective in improving the specific capacity and long-cycle stability.7–9 One of them is made from hollow nanoparticles with multiple shells.
However, fabricating multi-shelled hollow V2O5 is particularly challenging. In the conventional way of fabricating MS-HMSs, researchers rely on electrostatic attraction between negatively-charged carbonaceous microsphere templates and positively-charged metal cations, followed by removal of the carbon sphere templates by combustion.10 This textbook electrostatic attraction cannot be more familiar to both physicists and chemists. However, in the case of V2O5, the V ions usually exist as anions in the form of VO3−. This makes the above conventional approach (electrostatic attraction) fail for V2O5 MS-HMSs.
Ranbo Yu, Yu Zhang, Dan Wang and co-workers did not take this convention for granted. They believed that the commonly-adopted cation-adsorption process in solution is actually insufficiently understood. They asked themselves, why not anion adsorption? In the 5th issue of Nature Energy, the team made a successful attempt.11 By quantum chemistry calculations based on density functional theory, they validated their proposal that, under suitable conditions (precursor concentration and temperature), metal anions (e.g., VO3−) can bind strongly to the OH groups on the carbon sphere templates via coordination interactions, and the binding is strong enough to overcome the electrostatic repulsion. As a result, thermodynamically the metal anions can also be adsorbed and penetrate into the negatively-charged carbon sphere templates. Based on this new anion-adsorption mechanism, the team designed two workable routes towards MS-HMSs (see Fig. 1): (i) preferential adsorption of OH− ions followed by electrostatic attraction of metal cations; (ii) competitive adsorption and penetration of both OH− and VO3−, followed by electrostatic attraction of NH4+ cations to promote further adsorption of anions. Finally, the sacrificial carbon sphere templates are removed by a subtle catalytic combustion, leading to the final product of multi-shelled hollow spheres. It is noteworthy that while both processes towards MS-HMSs based on negatively-charged carbon spheres are reported therein for the first time, only the second one applies to V2O5 for obvious reasons.
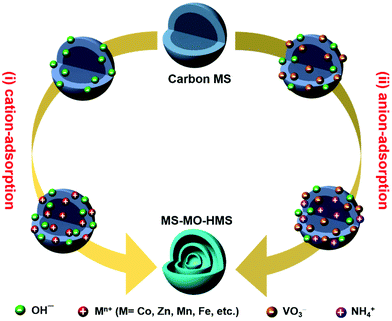 | ||
| Fig. 1 Two synthesis routes toward multi-shelled metal oxide hollow microspheres (MS-MO-HMSs) using negatively-charged carbon MS as a sacrificial template. | ||
In addition to V2O5, which is obtained by the second route and selected for detailed characterization in this work, it is nice to see that the synthesis protocol developed by the team is generic and also applicable to a series of other multi-shelled metal oxide hollow microspheres, such as MnO2, MoO3, Cr2O3 and WO3. This greatly enriches the MS-HMS family, and certainly creates a new playground for researchers working on nanomaterials in energy.
The rationally designed MS-HMSs developed in this work add a new member to the microstructured V2O5 family. Not just good-looking, they indeed show unprecedentedly outstanding Li-ion storage performance. In particular, the triple-shelled HMS electrode outperforms single- or double-shelled HMSs and normal nanosheets. Intuitively, one may feel the three shells are more fragile than a single shell, but it is found that the truth is the other way round – the multiple thin shells work together in harmony. First, as the three-shells have the largest specific surface area and pore volume, the number of active sites for lithium-storage will be increased and the access for electrolyte penetration will be significantly enhanced. Therefore, it is not surprising that they exhibit the largest specific capacity, as the authors observed. Second, the three shells are found not separated as individuals, but in close contact and supporting each other. Hence, one can expect a good electrical contact for efficient electron collection. This is nontrivial because it ensures that the electrode can work equally well at different current rates, meaning that it can sustain fast charging and discharging when necessary. Finally, the synergy between the shells is also beneficial to preserving the structural integrity of the microspheres after more than 100 cycles. Compared to solid nanostructures and HMSs with thick shells, the HMS with multiple thin shells allows Li insertion into individual shells and also into the tiny gaps between the shells, thus effectively buffering the volume expansion and eliminating mechanical strain.
How are these MHSs useful in other processes? There is no boundary to the imagination. Thinking of any electrochemical process, such as supercapacitive charge storage, the catalytic oxygen reduction reaction (ORR) in fuel cells, the hydrogen or oxygen evolution reaction (HER, OER) in water splitting, photocatalytic organic pollutant reduction, dye-sensitized solar cells, sensors, nano-reactors and drug-delivery (a summary is provided in Fig. 2), the MS-MHSs of metal oxides developed by the team are for you to choose.
References
- G. Zhang and X. W. Lou, Angew. Chem., Int. Ed., 2014, 53, 9041 CrossRef CAS PubMed.
- X. Wang, J. Feng, Y. Bai, Q. Zhang and Y. Yin, Chem. Rev., 2016 DOI:10.1021/acs.chemrev.5b00731.
- J. Qi, X. Lai, J. Wang, H. Tang, H. Ren, Y. Yang, Q. Jin, L. Zhang, R. Yu, G. Ma, Z. Su, H. Zhao and D. Wang, Chem. Soc. Rev., 2015, 44, 6749 RSC.
- A. Pan, H. B. Wu, L. Yu and X. W. D. Lou, Angew. Chem., Int. Ed., 2013, 125, 2282 CrossRef.
- L. Shen, L. Yu, H. B. Wu, X. Y. Yu, X. Zhang and X. W. Lou, Nat. Commun., 2015, 6, 6694 CrossRef CAS PubMed.
- L. Shen, L. Yu, X. Y. Yu, X. Zhang and X. W. Lou, Angew. Chem., Int. Ed., 2015, 54, 1868 CrossRef CAS PubMed.
- Y. Zhao, J. Feng, X. Liu, F. Wang, L. Wang, C. Shi, L. Huang, X. Feng, X. Chen, L. Xu, M. Yan, Q. Zhang, X. Bai, H. Wu and L. Mai, Nat. Commun., 2014, 5 Search PubMed.
- M. Yan, F. Wang, C. Han, X. Ma, X. Xu, Q. An, L. Xu, C. Niu, Y. Zhao, X. Tian, P. Hu, H. Wu and L. Mai, J. Am. Chem. Soc., 2013, 135, 18176 CrossRef CAS PubMed.
- D. Chao, X. Xia, J. Liu, Z. Fan, C. F. Ng, J. Lin, H. Zhang, Z. X. Shen and H. J. Fan, Adv. Mater., 2014, 26, 5794 CrossRef CAS PubMed.
- M. Chen, J. Wang, H. Tang, Y. Yang, B. Wang, H. Zhao and D. Wang, Inorg. Chem. Front., 2016 10.1039/C6QI00083E.
- J. Wang, H. Tang, L. Zhang, H. Ren, R. Yu, Q. Jin, J. Qi, D. Mao, M. Yang, Y. Wang, P. Liu, Y. Zhang, Y. Wen, L. Gu, G. Ma, Z. Su, Z. Tang, H. Zhao and D. Wang, Nat. Energy, 2016, 1, 16050 CrossRef CAS.
| This journal is © the Partner Organisations 2016 |