Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Correction: Alkoxide-intercalated NiFe-layered double hydroxides magnetic nanosheets as efficient water oxidation electrocatalysts
Jose A.
Carrasco
a,
Jorge
Romero
a,
María
Varela
b,
Frank
Hauke
c,
Gonzalo
Abellán
*ac,
Andreas
Hirsch
c and
Eugenio
Coronado
*a
aInstituto de Ciencia Molecular (ICMol), Universidad de Valencia, Catedrático José Beltrán 2, 46980, Paterna, Valencia, Spain. E-mail: gonzalo.abellan@fau.de
bUniversidad Complutense de Madrid, Dpt. Fisica Aplicada III & Instituto Pluridisciplinar, Madrid 28040, Spain
cDepartment of Chemistry and Pharmacy and Institute of Advanced Materials and Processes (ZMP), University Erlangen-Nürnberg, Henkestr. 42, 91054 Erlangen and Dr.-Mack Str. 81, 90762 Fürth, Germany
First published on 30th March 2016
Abstract
Correction for ‘Alkoxide-intercalated NiFe-layered double hydroxides magnetic nanosheets as efficient water oxidation electrocatalysts’ by Jose A. Carrasco, et al., Inorg. Chem. Front., 2016, DOI: 10.1039/c6qi00009f.
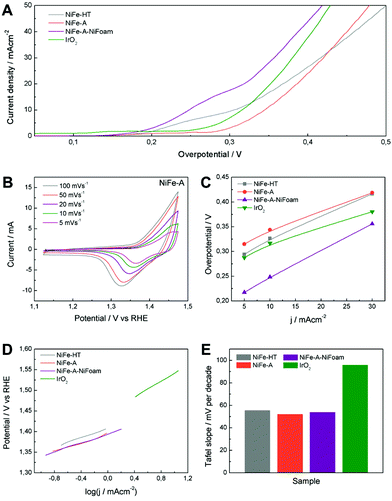
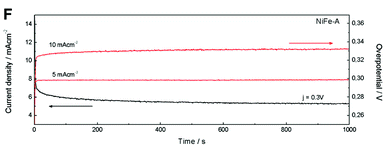
The authors regret that the caption for Fig. 7 was provided incorrectly. The correct version of Fig. 7 and the associated caption are provided below.
Fig. 7 (A) Polarization curves of NiFe-LDHs and commercial IrO2. (B) Cyclic voltammetry at different scan rates of NiFe-A in 1 M KOH solution. (C) Overpotential required at different current densities. (D) Tafel plots and (E) the histogram of corresponding values of Tafel slopes. (F) The potentiostatic and galvanostatic stability testing under a certain potential or current density.
In addition, the authors regret that any citations to Fig. 7 within the article text are incorrect. Correct versions of the affected text are provided below.
For page 7 of the article:
For NiFe-A the best fit of our data to a linear form of the eqn (3) (Fig. 6F) was obtained for Tg = 3.8 K, τ0 = 1.99 × 10−9 s and a zν = 13.7.
For page 8 of the article:
The cyclic voltammetry at different scan rates of NiFe-A (Fig. 7B) reveals the presence of a redox peak around 1.35 V vs. RHE, that can be assigned to the Ni(II)/Ni(III or IV) redox processes, probably related with the transformation between Ni1−xFex(OH)2 and Ni1−xFexOOH (see ESI 7† for additional CVs of the other samples).14,33,58 Moreover, the anodic wave of the NiFe-A catalyst is nearly merged with the catalytic wave but a distinct cathodic feature is evident, in excellent accordance with previous reports (see ESI 7† for pristine Ni-foam blank experiments and additional CVs of the other sample).58,59
The catalytic materials were measured by linear sweep voltammetry, showing the lowest onset potential for the NiFe-A-NiFoam (1.51 V vs. RHE), followed by the NiFe-HT and the pristine NiFe-A (Fig. 7A and ESI 7†). Different parameters were calculated to quantify the improvements of activity: the overpotential (η) at different current densities (5, 10 and 30 mA cm−2), the current density (j) at η = 300 mV and the Tafel slopes (the performance of the different samples have been summarized in ESI 8†). The current density of 10 mA cm−2 was chosen because it represents the current density from a device with 12% solar to hydrogen efficiency, considered as a realistic measure of the catalytic activity.15
As presented in Fig. 7C, an overpotential of ca. 0.249 V is required at j = 10 mA cm−2 for NiFe-A-NiFoam, a value much smaller than that of NiFe-HT (more than 0.32 V) or NiFe-A (0.34 V) and similar to that previously published by Lu and co- workers.33 In Fig. 7C it can be seen that the overpotential is decreased by 60–80 mV by growing the LDH directly on the Ni-foam. The excellent catalytic activity of the as-synthesized NiFe-LDHs is also reflected in the Tafel slopes, showing values in the range of 52–55 mV per decade, much smaller than that exhibited by the commercial IrO2 (we should indicate that the Tafel slopes not solely reflect the kinetic information of water oxidation but a combined process of redox reactions and OER).
To further evaluate their electrocatalytic activity, the values of the turnover frequency (TOF) of the powdered samples were calculated by assuming that all the transition metal ions in the catalysts are contributing to the reaction, which also confirm that NiFe-A has the highest TOF of 0.01 s−1 at an overpotential of 0.3 V, nearly of NiFe-HT (0.007). It is worth to keep in mind that these TOF values compete favourably with those recently reported for others NiFe-LDHs.26,33
The stability and durability of the NiFe-A powdered catalyst was tested at constant current densities j of 5 and 10 mA cm−2 and with a constant overpotential of η 300 mV for 1000 s. In Fig. 7F, we can see a very high stability in both cases. When increasing the current density from 5 to 10 mA cm−2 the overpotential correspondingly increases to ca. 0.03 V (see ESI 7† for additional measurements).
The Royal Society of Chemistry apologises for these errors and any consequent inconvenience to authors and readers.
| This journal is © the Partner Organisations 2016 |