Enantiodiscrimination of carboxylic acids using the diphenylprolinol NMR chiral solvating agents†
Gaowei
Li
a,
Jiangming
Cao
a,
Wen
Zong
b,
Xinxiang
Lei
*b and
Renxiang
Tan
*a
aInstitute of Functional Biomolecules, State Key Laboratory of Pharmaceutical Biotechnology, Nanjing University, Nanjing 210093, P. R. China. E-mail: rxtan@nju.edu.cn
bCollege of Chemistry & Materials Engineering, Wenzhou University, Wenzhou 325035, P. R. China. E-mail: xinxianglei@gmail.com
First published on 26th November 2015
Abstract
Enantiopure diphenylprolinols were synthesized from a commercially available starting material. The utility of 1H NMR spectroscopy for the differentiation of enantiomers using these chiral compounds as CSAs is stated, and their capacity acting as receptors for various carboxylic acids via hydrogen bonding is exploited. A linear relationship has been observed between the experimental and observed values of ee indicating the possible use of these compounds for quick and reliable analysis of enantiomerically enriched samples of various mandelic acids. From the experiments performed a preliminary conclusion indicated that the diphenylprolinol 1b with the free NH and OH is most effective in the chiral discrimination of carboxylic acids in 1H NMR.
Chiral molecular recognition is an essential phenomenon involved in a wide variety of areas, ranging from chiral separations and analysis, asymmetric catalysis, to biological and host–guest chemistry.1,2 However, among the large numbers of analytical techniques, NMR spectroscopy has proven to be a powerful and versatile tool for the discrimination of chiral molecules and the precise measurement of enantiomeric contents in the last few decades.3 And, if all things go well, microcoil and microchip NMR are becoming more widely available with the rapid development of miniaturization of NMR systems in the near future.4 NMR spectra of enantiomers are identical in achiral medium, and usually have no difference between the spectra of a racemic mixture and that of a single isomer. However, in the classical approach, enantiodiscrimination is achieved by converting substrates to diastereomers by utilizing an enantiopure chiral solvating agent (CSA), chiral lanthanide shift reagent (CLSR), chiral derivatizing agent (CDA) and/or by aligning in chiral anisotropic medium such as PBLG liquid crystal.5 In a rare but ideal case, a catalytic amount of reagent is enough for chiral discrimination in solution NMR. Therefore, the use of CSAs for enantiodiscrimination can be attractive as there is no need for derivatization and/or further purification step as in CDA, no paramagnetic signal broadening as in LSR appears, and no isotopical labeling as in chiral alignment media is required. Meanwhile, the CSAs can differentiate enantiomers due to the formation of diastereomeric complexes held together by weak intermolecular interactions such as van der Waals forces and/or hydrogen bonds following a large enough chemical shift non-equivalence to give clear baseline separation of the NMR signals. Therefore, the CSA is usually a better alternative for enantiodiscrimination owing to its fast and simple approach.6
Chiral carboxylic acids and their derivatives are organic molecules involved in a wide variety of biological processes and the investigation of the chiral recognition of carboxylic acids by artificial receptors was of critical importance in the preparation, separation, and analysis of enantiomerically pure carboxylic acids and disclosing the mechanism of interaction of the carboxylic acids with biological systems.7 The growing use of optically pure carboxylic acids has given rise to the need for the development of fast and accurate methodologies for the determination of the enantiomeric composition of chiral carboxylic acids. In the past few decades, a variety of chiral NMR solvating agents has been the focus of extensive investigation to determine the enantiomeric purity and understand the basic mechanism of host–guest complexation, particularly for carboxylic acids, such as chiral amines,8 amino alcohols,9 macrocyclic amines and amides,10 calixarenes,11 crown or aza-crown ethers,12 BINOL and their derivatives,13 chiral thiourea moieties,14etc. To date, however, there are only a few examples of optically active proline-derived receptors for the enantiomeric recognition of carboxylic acids.15 Therefore, the development of structurally simple yet efficient receptors for the enantioselective recognition of carboxylic acids still remains a challenging goal.
Among the variety of commercially available amino alcohol derivatives well documented in the literature, proline-derived diphenylprolinols have usually been widely employed as chiral ligands in the field of asymmetric synthesis and catalysis over the past decade. Because of the existence of multiple H-bonding and rigid heterocyclic ring of diphenylprolinols, they have mostly been used to catalyze the enantioselective alkylation,16 organocatalytic Michael addition,17 direct Aldol reaction18 and acyloin condensation,19 as well as promising applications, especially in efficient construction of axially chiral allenes or allenols.20 However, to the best of our knowledge, there is no report on the use of structurally simple diphenylprolinols as CSAs for the analysis of chiral carboxylic acids. In recent years, we have paid continuing attention to develop new and effective CSAs for the determination of enantiomeric excess and the application of chiral discrimination.21 Therefore, in continuation of our work on the development of structurally simple and effective CSAs in this aspect, we herein report L-proline-based diphenylprolinols and their recognition ability for carboxylic acids by 1H NMR spectroscopy.
The chiral N-benzyl-(S)-diphenyl(pyrrolidin-2-yl)methanol 1a was synthesized starting from L-proline according to the literature procedure,22 and the subsequent hydrogenolysis of 1a gave (S)-diphenyl(pyrrolidin-2-yl)methanol 1b in good yield. Their structures are shown in Scheme 1.
In order to explore the binding properties of chiral diphenylprolinols (S)-1a and (S)-1b, they were then screened to see their efficacy in binding with a test sample of DL-mandelic acid in 1H NMR analysis. The NMR experiments were performed with stoichiometric amounts of mandelic acid and CSA (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) (10 mM, in CDCl3). The upfield change in the position of the signal of CαH proton of mandelic acid upon treatment with CSA was measured (Δδ) while the degree of splitting was measured by the differences in the separated peaks in terms of chemical shift non-equivalences as chemical shift change (ΔΔδ). The two entries of Table 1 indicate that the N-Bn, OH derivative CSA 1a showed poor recognition (entry 1), while the basic unit (S)-CSA 1b with free NH and OH groups showed very good recognition (entry 2). This observation perhaps indicates that the presence of the complex system is sufficient to offer multiple intermolecular hydrogen-bonding between the free NH and OH of the CSA and the test substrate. Therefore, the (S)-CSA 1b was determined as the most effective receptor, and conducted a further study.
1) (10 mM, in CDCl3). The upfield change in the position of the signal of CαH proton of mandelic acid upon treatment with CSA was measured (Δδ) while the degree of splitting was measured by the differences in the separated peaks in terms of chemical shift non-equivalences as chemical shift change (ΔΔδ). The two entries of Table 1 indicate that the N-Bn, OH derivative CSA 1a showed poor recognition (entry 1), while the basic unit (S)-CSA 1b with free NH and OH groups showed very good recognition (entry 2). This observation perhaps indicates that the presence of the complex system is sufficient to offer multiple intermolecular hydrogen-bonding between the free NH and OH of the CSA and the test substrate. Therefore, the (S)-CSA 1b was determined as the most effective receptor, and conducted a further study.
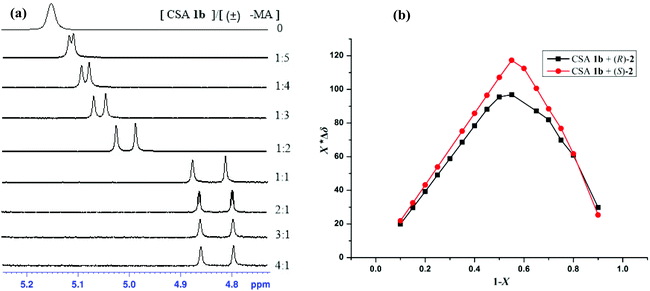
To further test the ability for enantiomeric discriminating of chiral diphenylprolinol 1b as CSA for carboxylic acids, we first recorded 1H NMR of 10 mM (R)-, (S)- and racemic mandelic acid (MA). The addition of (S)-CSA 1b to a solution of racemic MA in CDCl3 caused CαH resonance in the 1H NMR spectrum of MA to shift upfield and, in most cases, to split into two equal-intensity singlets that drift downfield slightly. Representative spectra for the most effective (S)-CSA 1b are presented in Fig. 1a. The two singlets were assigned to the MA enantiomers, which become desymmetrized through their interaction with the diphenylprolinol.
The stoichiometry was determined according to the Job's method of continuous variation. The Job plots of X*Δδ versus the molar fraction (X) of (R)- or (S)-mandelic acid in the mixture were obtained, which showed a maximum at X = 0.5, and the best baseline resolution was both achieved with a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio of (S)-1b/MA-2, as is shown in Fig. 1b. This indicated that (S)-1b and the acid bind in a 1
1 ratio of (S)-1b/MA-2, as is shown in Fig. 1b. This indicated that (S)-1b and the acid bind in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 complex under these conditions.
1 complex under these conditions.
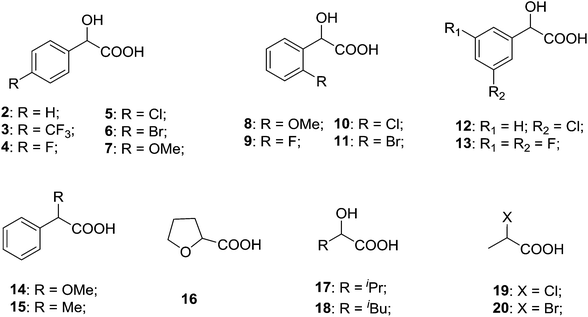
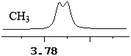
To further explore the practical applications in the general ability of discrimination of (S)-CAS 1b for a variety of analytes by 1H NMR spectroscopy, the following carboxylic acids were chosen as guests (Fig. 2). These carboxylic acids vary from one another in their substituent group. The ΔΔδ values of α-H signals were appropriate to give a good baseline resolution for most of the tested analytes which ranges from 0.005 to 0.096 ppm, the results are summarized in Table 2. The p-substituted aromatic hydroxy acids (Table 2, entries 2–6) almost showed a more bigger ΔΔδ value compared with the o-substituted aromatic hydroxy acids (Table 2, entries 7–10). In particular, p-CF3-substituted aromatic carboxylic acid (±)-3 showed the biggest ΔΔδ value as 0.076 ppm (Table 2, entry 2), while the o-halogen-substituted aromatic hydroxy acid showed the lowest ΔΔδ value as 0.005 ppm (Table 2, entry 10). Similar results can be obtained for the m-substituted aromatic hydroxy acids (Table 2, entries 11 and 12), the above results indicated that the enantiodifferentiation of (S)-CAS 1b could be possibly weakened due to steric hindrance of the o-substituted group. It is also interesting to note that chiral discrimination was observed for the OMe signals of (±)-7 (Table 2, entry 6). The aromatic carboxylic acid with no α-OH chiral discrimination was also observed for the Me/OMe and methine proton (CαH) signals (Table 2, entries 13 and 14).
| Entry | Carboxylic acids | ΔΔδb (ppm) | ΔΔδb (Hz) | Spectrumb |
|---|---|---|---|---|
a Typical conditions: concentration of the guest and the (S)-CSA 1b is 10 mM (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and the spectra are recorded at 25 °C on a 500 MHz spectrometer.
b ΔΔδ values and spectra for α-H or CH3 are shown. 1) and the spectra are recorded at 25 °C on a 500 MHz spectrometer.
b ΔΔδ values and spectra for α-H or CH3 are shown.
|
||||
| 1 | (±)-2 | 0.062 | 31 |
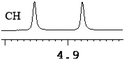
|
| 2 | (±)-3 | 0.076 | 38 |
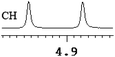
|
| 3 | (±)-4 | 0.056 | 28 |
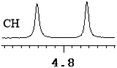
|
| 4 | (±)-5 | 0.058 | 29 |
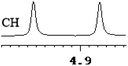
|
| 5 | (±)-6 | 0.058 | 29 |
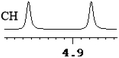
|
| 6 | (±)-7 | 0.060 | 30 |
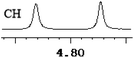
|
| 0.003 | 1.5 |
|
||
| 7 | (±)-8 | 0.022 | 11 |
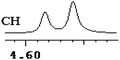
|
| 8 | (±)-9 | 0.008 | 4 |
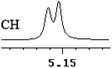
|
| 9 | (±)-10 | 0.012 | 6 |
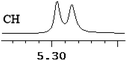
|
| 10 | (±)-11 | 0.005 | 2.5 |
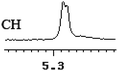
|
| 11 | (±)-12 | 0.044 | 22 |
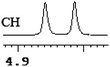
|
| 12 | (±)-13 | 0.050 | 25 |
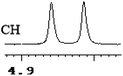
|
| 13 | (±)-14 | 0.023 | 11.5 |
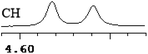
|
| 0.003 | 1.5 |
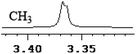
|
||
| 14 | (±)-15 | 0.052 | 26 |
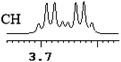
|
| 0.014 | 7 |
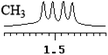
|
||
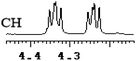
| 15 | (±)-16 | 0.096 | 48 |
|
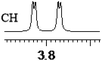
| 16 | (±)-17 | 0.057 | 28.5 |
|
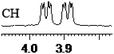
| 17 | (±)-18 | 0.061 | 30.5 |
|
| 18 | (±)-19 | 0.007 | 3.5 |
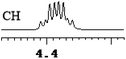
|
| 0.003 | 1.5 |
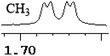
|
||
| 19 | (±)-20 | 0.006 | 3 |
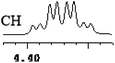
|
| 0.014 | 7 |
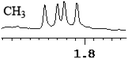
|
||
What's more, (S)-CAS 1b can even successfully discriminate the enantiomers of aliphatic carboxylic acids (Table 2, entries 15–19), especially for the pyromucic acid (±)-16 showed the biggest ΔΔδ value as 0.096 ppm (Table 2, entry 15), while the propionic acid derivatives (±)-19–20 gave poor results (Table 2, entries 18 and 19).
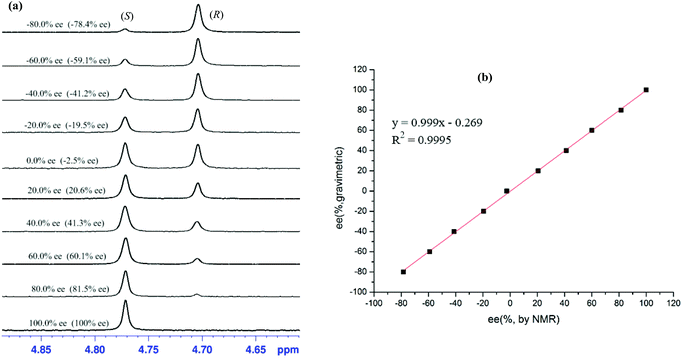
To explore the quantitative analysis ability of (S)-1b as a CSA for enantiomeric determination, the ee values of non-racemic mandelic acid 7 samples were determined by integration of the α-H signal of 7 in 1H NMR. The results shown in Fig. 3 were calculated based on the integrations of the NMR signals, and were within ±1% of the actual enantiopurity of the samples. The linear relationship between the NMR-determined values and those gravimetry determined values is excellent with R2 = 0.9995.
Having demonstrated enantiodiscrimination of the above carboxylic acids, we then tried to investigate the applicability of CSA 1b for the determination of absolute configuration of chiral carboxylic acids by a suitable method.14d,23 The NMR spectral behavior of a series of chiral carboxylic acids of known absolute configuration was studied using (S)-CSA 1b and its enantiomer (R)-CSA 1b to find the existence of any correlation between the absolute configuration and the NMR chemical shifts (Table 3). The results indicate that negative ΔδR,Sα-H correlates with (S)-carboxylic acids and positive ΔδR,Sα-H correlates with (R)-carboxylic acids.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) a
a
| Entry | Chiral carboxylic acids | ΔδR,Sα-H![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) b (ppm) b (ppm) |
|---|---|---|
| a ΔδR,Sα-H(ΔδR − ΔδS) values for α-H chiral carboxylic acids are shown. b Assigned configurations are labeled in parentheses. | ||
| 1 | (R)-Mandelic acid | +0.026(R) |
| 2 | (S)-Mandelic acid | −0.027(S) |
| 3 | (R)-4-Chloromandelic acid | +0.022(R) |
| 4 | (S)-4-Chloromandelic acid | −0.030(S) |
| 5 | (R)-4-Methoxymandelic acid | +0.027(R) |
| 6 | (S)-4-Methoxymandelic acid | −0.031(S) |
| 7 | (R)-3-Chloromandelic acid | +0.014(R) |
| 8 | (R)-2-Phenylpropionic acid | +0.006(R) |
| 9 | (S)-2-Tetrahydrofuroic acid | −0.163(S) |
| 10 | (S)-2-Hydroxy-3-methylbutanoic acid | −0.069(S) |
| 11 | (S)-2-Hydroxy-4-methylpentanoic acid | −0.070(S) |
| 12 | (S)-2-Hydroxy-3,3-dimethylbutyric acid | −0.149(S) |
We have developed structurally simple diphenylprolinols and tested their efficacy as CSAs in 1H NMR spectroscopy to discriminate carboxylic acids. A linear relationship has been observed between the experimental and observed values of ee indicating the possible use of these compounds for quick and reliable analysis of enantiomerically enriched samples of mandelic acid. From the experiments performed a preliminary conclusion indicated that the diphenylprolinol 1b with the free NH and OH is most effective in the chiral discrimination of carboxylic acids in 1H NMR.
Acknowledgements
We thank the Natural Science Foundation of China (Grants 21572164, 81421091, 81330079 and 91313303). We are also grateful for the financial support from the Zhejiang Provincial Natural Science Foundation of China (Grant no. LY14B020010).References
- (a) K. Soai and S. Niwa, Chem. Rev., 1992, 92, 833 CrossRef CAS; (b) P. Knochel and R. D. Singer, Chem. Rev., 1993, 93, 2117 CrossRef CAS; (c) A. P. Davis and R. S. Wareham, Angew. Chem., Int. Ed., 1999, 38, 2978 CrossRef; (d) C. M. Binder and B. Singaram, Org. Prep. Proced. Int., 2011, 43, 139 CrossRef CAS; (e) S. Yu and L. Pu, Tetrahedron, 2015, 71, 745 CrossRef CAS.
- (a) Y. Kubo, S. Maeda, S. Tokita and M. Kubo, Nature, 1996, 382, 522 CrossRef CAS; (b) G. M. Coppola and H. F. Schuster, R-Hydroxy Acids in Enantioselective Syntheses, Wiley-VCH, Weinheim, 1997 CrossRef.
- For reviews, see: (a) D. Parker, Chem. Rev., 1991, 91, 1441 CrossRef CAS; (b) N. Giraud, M. Joos, J. Courtieu and D. Merlet, Magn. Reson. Chem., 2009, 47, 300 CrossRef CAS PubMed; (c) D. Leung, S. O. Kang and E. V. Anslyn, Chem. Soc. Rev., 2012, 41, 448 RSC.
- S. S. Zalesskiy, E. Danieli, B. Blümich and V. P. Ananikov, Chem. Rev., 2014, 114, 5641 CrossRef CAS PubMed.
- (a) T. H. Webb and C. S. Wilcox, Chem. Soc. Rev., 1993, 22, 383 RSC; (b) B. Luy, J. Indian Inst. Sci., 2010, 90, 119 CAS; (c) T. J. Wenzel and C. D. Chisholm, Prog. Nucl. Magn. Reson. Spectrosc., 2011, 59, 1 CrossRef CAS PubMed.
- (a) T. J. Wenzel, Discrimination of Chiral Compounds Using NMR Spectroscopy, John Wiley & Sons, Inc., Hoboken, New Jersey, 2007 Search PubMed; (b) G. Uccello-Barretta and F. Balzano, Chiral NMR Solvating Additives for Differentiation of Enantiomers, in Topics in Current Chemistry, Springer, Berlin, Heidelberg, 2013 Search PubMed.
- Q. Ren, K. Ruth, L. Thöny-Meyer and M. Zinn, Appl. Microbiol. Biotechnol., 2010, 87, 41 CrossRef CAS PubMed.
- See, for examples: (a) D. Enders, C. R. Thomas and J. Runsink, Tetrahedron: Asymmetry, 1999, 10, 323 CrossRef CAS; (b) A. Port, A. Virgili, A. Alvarez-Larena and J. F. Piniella, Tetrahedron: Asymmetry, 2000, 11, 3747 CrossRef CAS; (c) X. Yang, G. Wang, C. Zhong, X. Wu and E. Fu, Tetrahedron: Asymmetry, 2006, 17, 916 CrossRef CAS; (d) W. Wang, F. Ma, X. Shen and C. Zhang, Tetrahedron: Asymmetry, 2007, 18, 832 CrossRef CAS; (e) C. Peña, J. González-Sabín, I. Alfonso, F. Rebolledo and V. Gotor, Tetrahedron: Asymmetry, 2007, 18, 1981 CrossRef; (f) C. Peña, J. González-Sabín, I. Alfonso, F. Rebolledo and V. Gotor, Tetrahedron, 2008, 64, 7709 CrossRef.
- (a) F. Cuevas, P. Ballester and P. Ma, Org. Lett., 2005, 7, 5485 CrossRef CAS PubMed; (b) W. Wang, X. Shen, F. Ma, Z. Li and C. Zhang, Tetrahedron: Asymmetry, 2008, 19, 1193 CrossRef CAS; (c) Z. Luo, C. Zhong, X. Wu and E. Fu, Tetrahedron Lett., 2008, 49, 3385 CrossRef CAS; (d) A. R. Chaudhary, P. Yadav and A. V. Bedekar, Tetrahedron: Asymmetry, 2014, 25, 767 CrossRef CAS.
- (a) Y. Quan, E. Fu, X. Wu, M. Fang, X. Peng, C. Wu and J. Chen, Tetrahedron Lett., 2002, 43, 3935 CrossRef; (b) T. Ema, D. Tanida and T. Sakai, Org. Lett., 2006, 8, 3773 CrossRef CAS PubMed; (c) T. Ema, D. Tanida and T. Sakai, J. Am. Chem. Soc., 2007, 129, 10591 CrossRef CAS PubMed; (d) F. Ma, L. Ai, X. Shen and C. Zhang, Org. Lett., 2007, 9, 125 CrossRef CAS PubMed; (e) F. Ma, X. Shen, X. Ming, J. Wang, O. Y. Jie and C. Zhang, Tetrahedron: Asymmetry, 2008, 19, 1576 CrossRef CAS; (f) T. Ema, D. Tanida, K. Hamada and T. Sakai, J. Org. Chem., 2008, 73, 9129 CrossRef CAS PubMed; (g) F. Ma, X. Shen, J. Ou-Yang, Z. Deng and C. Zhang, Tetrahedron: Asymmetry, 2008, 19, 31 CrossRef CAS; (h) K. Tanaka and N. Fukuda, Tetrahedron: Asymmetry, 2009, 20, 111 CrossRef CAS; (i) E. Busto, A. González-Álvarez, V. Gotor-Fernández, I. Alfonso and V. Gotor, Tetrahedron, 2010, 66, 6070 CrossRef CAS; (j) B. Altava, M. I. Burguete, N. Carbó and S. V. Luis, Tetrahedron: Asymmetry, 2010, 21, 982 CrossRef CAS; (k) A. Gualandi, S. Grilli, D. Savoia, M. Kwit and J. Gawronski, Org. Biomol. Chem., 2011, 9, 4234 RSC; (l) S. Guo, G. Wang and L. Ai, Tetrahedron: Asymmetry, 2013, 24, 480 CrossRef CAS; (m) X. F. Yang, R. Ning, L. J. Xie, Y. Cui, Y. L. Zhang and L. Y. Zheng, Bull. Chem. Soc. Jpn., 2013, 86, 987 CrossRef CAS; (n) B. Altava, M. I. Burguete, N. Carbó, S. V. Luis, V. Martí-Centelles and C. Vicent, Tetrahedron Lett., 2013, 54, 72 CrossRef CAS; (o) J. Labuta, Z. Futera, S. Ishihara, H. Kourilova, Y. Tateyama, K. Ariga and J. P. Hill, J. Am. Chem. Soc., 2014, 136, 2112 CrossRef CAS PubMed; (p) K. Tanaka, T. Iwashita, C. Sasaki and H. Takahashi, Tetrahedron: Asymmetry, 2014, 25, 602 CrossRef CAS.
- (a) Y. S. Zheng and C. Zhang, Org. Lett., 2004, 6, 1189 CrossRef CAS PubMed; (b) X. X. Liu and Y. S. Zheng, Tetrahedron Lett., 2006, 47, 6357 CrossRef CAS; (c) A. Karakucuk, M. Durmaz, A. Sirit, M. Yilmaz and A. S. Demir, Tetrahedron: Asymmetry, 2006, 17, 1963 CrossRef CAS; (d) M. Durmaz, S. Alpaydin, A. Sirit and M. Yilmaz, Tetrahedron: Asymmetry, 2006, 17, 2322 CrossRef CAS; (e) M. Durmaz, S. Alpaydin, A. Sirit and M. Yilmaz, Tetrahedron: Asymmetry, 2007, 18, 900 CrossRef CAS; (f) S. Shirakawa, A. Moriyama and S. Shimizu, Org. Lett., 2007, 9, 3117 CrossRef CAS PubMed; (g) S. Bozkurt, M. Durmaz, M. Yilmaz and A. Sirit, Tetrahedron: Asymmetry, 2008, 19, 618 CrossRef CAS; (h) S. Shirakawa, A. Moriyama and S. Shimizu, Eur. J. Org. Chem., 2008, 5957 CrossRef CAS; (i) H. N. Demirtas, S. Bozkurt, M. Durmaz, M. Yilmaz and A. Sirit, Tetrahedron: Asymmetry, 2008, 19, 2020 CrossRef CAS; (j) E. Kocabas, Chirality, 2008, 20, 26 CrossRef CAS PubMed; (k) H. N. Demirtas, S. Bozkurt, M. Durmaz, M. Yilmaz and A. Sirit, Tetrahedron, 2009, 65, 3014 CrossRef CAS; (l) M. Durmaz, M. Yilmaz and A. Sirit, Org. Biomol. Chem., 2011, 9, 571 RSC; (m) N. H. Pham and T. J. Wenzel, J. Org. Chem., 2011, 76, 986 CrossRef CAS PubMed; (n) K. Yang, S.-Z. Li, Y. H. Wang, W. Z. Zhang, Z. H. Xu, X. Y. Zhou, R. X. Zhu, J. Luo and Q. Wan, RSC Adv., 2014, 4, 6517 RSC.
- (a) N. Voyer, Chem. Commun., 1997, 2329 RSC; (b) T. J. Wenzel, J. Org. Chem., 2000, 65, 1243 CrossRef CAS PubMed; (c) N. Voyer, S. Côté, E. Biron, M. Beaumont, M. Chaput and S. Levac, J. Supramol. Chem., 2001, 1, 1 CrossRef CAS; (d) T. P. Quinn, P. D. Atwood, J. M. Tanski, T. F. Moore and J. F. Folmer-Andersen, J. Org. Chem., 2011, 76, 10020 CrossRef CAS PubMed.
- For recent examples, see: (a) S. Sambasivan, D. S. Kim and K. H. Ahn, Chem. Commun., 2010, 46, 541 RSC; (b) S. R. Chaudhari and N. Suryaprakash, Org. Biomol. Chem., 2012, 10, 6410 RSC; (c) I. Pal, S. R. Chaudhari and N. Suryaprakash, New J. Chem., 2014, 38, 4908 RSC; (d) S. K. Mishra, S. R. Chaudhari and N. Suryaprakash, Org. Biomol. Chem., 2014, 12, 495 RSC; (e) F. Yuste, R. Sánchez-Obregón, E. Díaz and M. A. García-Carrillo, Tetrahedron: Asymmetry, 2014, 25, 224 CrossRef CAS; (f) A. Couffin, O. Thillaye du Boullay, M. Vedrenne, C. Navarro, B. Martin-Vaca and D. Bourissou, Chem. Commun., 2014, 50, 5997 RSC; (g) I. Pal, S. R. Chaudhari and N. R. Suryaprakash, Magn. Reson. Chem., 2015, 53, 142 CrossRef CAS PubMed.
- (a) M. Hernández-Rodríguez and E. Juaristi, Tetrahedron, 2007, 63, 7673 CrossRef; (b) M. B. Foreiter, H. N. Gunaratne, P. Nockemann, K. R. Seddon, P. J. Stevenson and D. F. Wassell, New J. Chem., 2013, 37, 515 RSC; (c) G. Bian, H. Fan, S. Yang, H. Yue, H. Huang, H. Zong and L. Song, J. Org. Chem., 2013, 78, 9137 CrossRef CAS PubMed; (d) G. Bian, H. Fan, H. Huang, S. Yang, H. Zong, L. Song and G. Yang, Org. Lett., 2015, 17, 1369 CrossRef CAS PubMed.
- (a) D. J. Bailey, D. O'Hagan and M. Tavasli, Tetrahedron: Asymmetry, 1997, 8, 149 CrossRef CAS; (b) X. Yang, X. Wu, M. Fang, Q. Yuan and E. Fu, Tetrahedron: Asymmetry, 2004, 15, 2491 CrossRef CAS; (c) H. N. Naziroglu, M. Durmaz, S. Bozkurt and A. Sirit, Chirality, 2011, 23, 463 CrossRef CAS PubMed; (d) N. H. Pham and T. J. Wenzel, Tetrahedron: Asymmetry, 2011, 22, 641 CrossRef CAS.
- (a) Y. Takemoto, Y. Baba, I. Noguchi and C. Iwata, Tetrahedron Lett., 1996, 37, 3345 CrossRef CAS; (b) Y. Takemoto, Y. Baba, A. Honda and S. Nakao, Tetrahedron, 1998, 54, 15567 CrossRef CAS; (c) M. Lombardo, M. Chiarucci and C. Trombini, Chem. – Eur. J., 2008, 14, 11288 CrossRef CAS PubMed.
- (a) J. W. Xie, L. Yue, D. Xue, X. L. Ma, Y. C. Chen, Y. Wu, J. Zhu and J. G. Deng, Chem. Commun., 2006, 1563 RSC; (b) S. Wang, X. Li, H. Liu, L. Xu, J. Zhuang, J. Li, H. Li and W. Wang, J. Am. Chem. Soc., 2015, 137, 2303 CrossRef CAS PubMed.
- Y. Hayashi, T. Itoh, S. Aratake and H. Ishikawa, Angew. Chem., Int. Ed., 2008, 47, 2082 CrossRef CAS PubMed.
- (a) M. Y. Jin, S. M. Kim, H. Han, D. H. Ryu and J. W. Yang, Org. Lett., 2011, 13, 880 CrossRef CAS PubMed; (b) M. B. Schmid, K. Zeitler and R. M. Gschwind, J. Am. Chem. Soc., 2011, 133, 7065 CrossRef CAS PubMed.
- (a) J. Ye, W. Fan and S. Ma, Chem. – Eur. J., 2013, 19, 716 CrossRef CAS PubMed; (b) J. Ye, R. Lv, W. Fan and S. Ma, Tetrahedron, 2013, 69, 8959 CrossRef CAS; (c) R. Lu, J. Ye, T. Cao, B. Chen, W. Fan, W. Lin, J. Liu, H. Luo, B. Miao, S. Ni, X. Tang, N. Wang, Y. Wang, X. Xie, Q. Yu, W. Yuan, W. Zhang, C. Zhu and S. Ma, Org. Lett., 2013, 15, 2254 CrossRef CAS PubMed; (d) X. Tang, X. Huang, T. Cao, Y. Han, X. Jiang, W. Lin, Y. Tang, J. Zhang, Q. Yu, C. Fu and S. Ma, Org. Chem. Front., 2015, 2, 688 RSC.
- (a) X. X. Lei, L. Liu, X. J. Chen, X. C. Yu, L. S. Ding and A. J. Zhang, Org. Lett., 2010, 12, 2540 CrossRef CAS PubMed; (b) L. Liu, M. D. Ye, X. G. Hu, X. C. Yu, L. X. Zhang and X. X. Lei, Tetrahedron: Asymmetry, 2011, 22, 1667 CrossRef CAS; (c) Q. Z. Ma, M. S. Ma, H. Y. Tian, X. X. Ye, H. P. Xiao, L. H. Chen and X. X. Lei, Org. Lett., 2012, 14, 5813 CrossRef CAS PubMed.
- (a) J. Luis Olivares-Romero and E. Juaristi, Tetrahedron, 2008, 64, 9992 CrossRef; (b) N. Hosoda, H. Kamito, M. Takano, Y. Takebe, Y. Yamaguchi and M. Asami, Tetrahedron, 2013, 69, 1739 CrossRef CAS; (c) K. Nakano, K. Nozaki and T. Hiyama, J. Am. Chem. Soc., 2003, 125, 5501 CrossRef CAS PubMed.
- (a) J. M. Seco, E. Quinoa and R. Riguera, Chem. Rev., 2004, 104, 17 CrossRef CAS; (b) L. S. Moon, M. Pal, Y. Kasetti, P. V. Bharatam and R. S. Jolly, J. Org. Chem., 2010, 75, 5487 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5qo00264h |
| This journal is © the Partner Organisations 2016 |