Gold(I) catalyzed [1,3] O → C rearrangement of benzylvinyl ethers†
Chandrababu Naidu
Kona
,
Mahesh N.
Patil
and
Chepuri V.
Ramana
*
Division of Organic Chemistry, CSIR-National Chemical Laboratory, Pune – 411 008, India. E-mail: vr.chepuri@ncl.res.in
First published on 26th January 2016
Abstract
A simple procedure for the preparation of 3-(hetero)arylpropionaldehydes has been developed employing a gold-catalyzed [1,3]-rearrangement of vinyl ethers. Using this protocol, Canthoxal, an aromatic aldehyde used in perfumery, has been prepared on a gram scale.
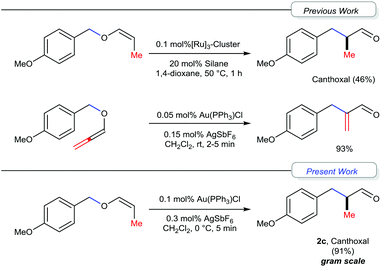
Gold catalysis has received significant attention over the past decade and many novel and efficient methodologies have been developed.1 Generally, gold complexes act as tunable soft π-acids.2 In particular, the selective activation of alkynes3a and allenes3b by gold-complexes, followed by the subsequent inter and intramolecular nucleophilic additions and [3,3]-sigmatropic rearrangements (such as Claisen and Cope rearrangements), has received substantial attention.3 Recently, we reported a gold(I) catalyzed [1,3] O → C rearrangement of allenyl ethers leading to α-substituted acryl aldehydes.4–6 We have proposed a mechanism that proceeds via the initial coordination of the Au-center with the oxygen of the allenyl ether resulting in elongation of the carbinol C–O bond and, if the pendant substituent of the oxygen is sufficiently electrophilic, the cleavage of the carbinol C–O bond, leading to the formation of a contact-ion pair that subsequently undergoes the [1,3]-rearrangement.6,7 Extending this concept, we were interested in exploring the [1,3]-rearrangement reaction of vinyl ethers to understand the mechanism in general and especially in the context of the synthesis of the fragrance ingredient Canthoxal and related 3-arylpriopionaldehydes.8 The generally adopted approaches for the synthesis of Canthoxal include mainly either Pd-based coupling of 4-haloanisole or hydroformylation of 4-allylanisole and subsequent functional group modifications.9–11 Recently, Nagashima and co-workers revealed the [1,3] O → C rearrangement as a possible alternative while studying the Ru-catalyzed cationic polymerization of vinyl ethers, albeit, in moderate yield (Fig. 1).12
To start in this direction, as a preliminary experiment, we have synthesized a set of three vinyl ethers of alkyl 1a, benzyl 1b and 4-methoxybenzyl 1c groups from the corresponding allyl derivatives by treating with KOtBu in DMSO for 15 min [ESI†].13
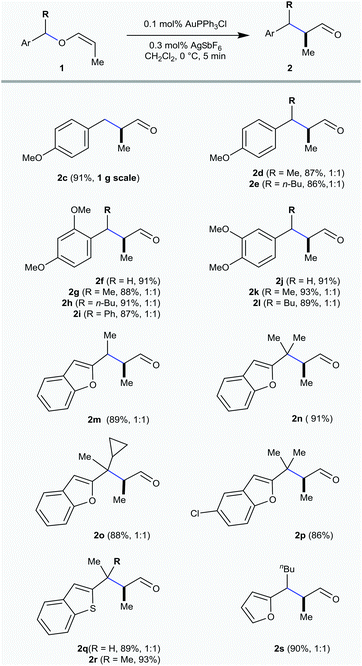
All these vinyl ethers are screened for the initial optimization with Au(PPh3)Cl alone and in combination with several silver salts like AgNTf2, AgOTf, AgSbF6, AgOAc and AgBF4 in different concentrations from 1 mol% to 0.1 mol%. Several gold complexes are also screened for this purpose at different temperatures from 0 °C to rt [ESI†]. After extensive optimization studies, 0.1 mol% Au(PPh3)Cl in combination with 0.3 mol% AgSbF6 has been identified as the catalyst of choice and only the rearrangement of 4-methoxybenzylvinyl ether 1c to the corresponding α-methylated aldehyde 2c was observed in 5 min at 0 °C with 92% isolated yield (Table 1). Whereas with the other two vinyl ethers having alkyl 1a and benzyl 1b groups, hydrolysis was the major event. This revealed that, analogous to allenyl ethers, the reaction progress with vinyl ethers was also significantly affected by the stability of the in situ generated benzylic carbocation and the basicity/coordinating ability of the counter anion.6
With the optimal catalytic conditions in hand, the scope of the rearrangement was investigated employing a wide range of vinyl ethers. The 4-methoxybenzylvinyl ethers having methyl 1d and n-butyl 1e substitution at the benzylic position delivered the corresponding aldehydes 2d and 2e in 87% and 86% yield, respectively, as 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 diastereomeric mixtures (Scheme 1).
1 diastereomeric mixtures (Scheme 1).
 | ||
| Scheme 1 Substrate scope for the gold(I) catalyzed [1,3] O → C rearrangement. Crude vinyl ethers resulting from KOtBu mediated olefin-migration of the corresponding allyl ethers have been used directly for the [1,3]-rearrangement. Selected vinyl ethers were purified and characterized (see the ESI†). | ||
A similar trend has been noticed in the case of 2,4-dimethoxy and 3,4-dimethoxybenzyl derived vinyl ethers 1f–1l and the corresponding [1,3] O → C rearranged products 2f–2l were procured in excellent yield. Not only the benzylvinyl ethers, but some of the heteroarylmethylvinyl ethers are also facile for this rearrangement purpose. The rearrangement was successfully achieved by the benzofuran derived vinyl ethers 1m–1p and afforded the α-substituted aldehydes 2m–2p in good yield (86–91%). The vinyl ethers 1m and 1o afforded a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 diastereomeric mixture of aldehydes 2m (89%) and 2o (88%). On the other hand, the vinyl ethers 1n and 1p gave the substituted C2-homologated benzofuran aldehydes 2n and 2p in 91% and 86%, respectively. Gratifyingly, the benzothiophene derived vinyl ether 1q, having the methyl- substitution at the benzylic position gave a 1
1 diastereomeric mixture of aldehydes 2m (89%) and 2o (88%). On the other hand, the vinyl ethers 1n and 1p gave the substituted C2-homologated benzofuran aldehydes 2n and 2p in 91% and 86%, respectively. Gratifyingly, the benzothiophene derived vinyl ether 1q, having the methyl- substitution at the benzylic position gave a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 diastereomeric mixture of the rearranged product 2q in 89% yield and 1r with gem-dimethyl substitution at the benzylic position delivered 2r in 93% yield. The rearrangement was quite clean with the furyl-derived vinyl ether 1s and the C2-homologated aldehyde 2s was obtained in 90% yield.
1 diastereomeric mixture of the rearranged product 2q in 89% yield and 1r with gem-dimethyl substitution at the benzylic position delivered 2r in 93% yield. The rearrangement was quite clean with the furyl-derived vinyl ether 1s and the C2-homologated aldehyde 2s was obtained in 90% yield.
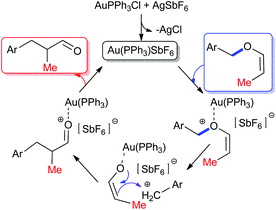
Coming to the mechanism, in the case of the [1,3] O → C rearrangement of allenyl ethers, with the help of trapping experiments with O-nucleophiles, we have proposed a reaction path comprising an initial gold(I) complexation with the oxygen lone pair that results in the cleavage of the C–O bond with the simultaneous formation of a contact ion pair intermediate depending upon the stability of the benzylic cation.14 If the resulting contact ion pair is sufficiently stable, the subsequent alkylation of the benzylic cation with gold enolate results in the formation of a new C–C bond with a net [1,3] O → C rearrangement of the allenyl ether unit.6,15a A similar mechanism is apparent in the present case of vinyl ethers rearrangement (Fig. 2).
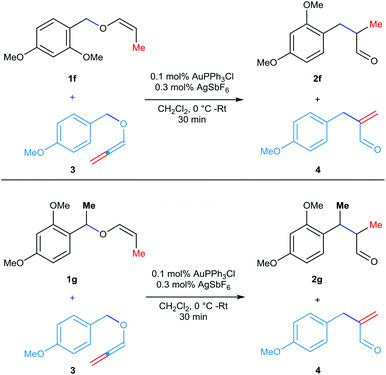
At this point, in search of conclusive proof for the nature of the contact ion-pair intermediate [either tight contact ion-pair or dispersed in the solvent media] involved in the rearrangement, the following two crossover experiments have been conducted. An equimolar mixture of 4-methoxybenzylallenyl ether 3 and vinyl ether 1f or 1g was subjected to the gold-catalyzed rearrangement at 0 °C for 30 min (Scheme 2). In general, the rearrangement of allenyl ether 3 was facile even with 0.05 mol% catalyst (half of what vinyl ethers need). On the other hand, the hydrolysis of vinyl ethers 1f/1g and the stability of the resulting counter cations are better than that of the PMB group present in the allenyl ether 3. Hence, if the 1,3-rearrangement of these substrates is proceeding via a solvent-dispersed contact ion-pair, the scrambling of these counter cations is expected and thus, a mixture with 4 products is expected. Whereas, if the reaction proceeds through a tight-contact pair, only two separate products are expected without any scrambling. In the case of the reaction employing a mixture of 4-methoxybenzylallenyl ether 3 and vinyl ether 1f, only the rearranged products 4 and 2f were noticed in the LCMS analysis. A similar trend has been noticed in the case of the LCMS analysis of the second cross-over experiment employing a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 mixture of 4 and 2g. Thus, these experiments provided a clear evidence of the involvement of a tight contact ion-pair intermediate in these gold-catalyzed [1,3] O → C rearrangements and thus ruled out an operative ion-pairing effect.
1 mixture of 4 and 2g. Thus, these experiments provided a clear evidence of the involvement of a tight contact ion-pair intermediate in these gold-catalyzed [1,3] O → C rearrangements and thus ruled out an operative ion-pairing effect.
Conclusions
In conclusion, gold(I) catalyzed [1,3] O → C rearrangement of benzylvinyl ethers has been developed as a practical method for the preparation of 2-methyl-3-(hetero)arylpropionaldehydes. The rearrangement is facile with the substrates having the benzylic aryl ring substituted with +M groups. The crossover experiments between vinyl- and allenyl ethers provided indirect evidence for the involvement of a tight contact ion-pair intermediate in the reaction path. As an off-shoot, a gram-scale process was established for the synthesis of the fragrance ingredient Canthoxal.Acknowledgements
We thank the CSIR for funding this project under the 12 FYP NICE Program (CSC0109) and for a research fellowship to CNK.Notes and references
- (a) G. Dyker, Angew. Chem., Int. Ed., 2000, 39, 4237 CrossRef CAS; (b) A. Hoffmann-Röder and N. Krause, Org. Biomol. Chem., 2005, 3, 387 RSC; (c) A. S. K. Hashmi and G. J. Hutchings, Angew. Chem., Int. Ed., 2006, 45, 7896 CrossRef PubMed; (d) A. Fürstner and P. W. Davies, Angew. Chem., Int. Ed., 2007, 46, 3410 CrossRef PubMed; (e) A. S. K. Hashmi, Chem. Rev., 2007, 107, 3180 CrossRef CAS PubMed; (f) Z. G. Li, C. Brouwer and C. He, Chem. Rev., 2008, 108, 3239 CrossRef CAS PubMed; (g) E. Jiménez-Núñez and A. M. Echavarren, Chem. Rev., 2008, 108, 3326 CrossRef PubMed; (h) A. S. K. Hashmi and M. Rudolph, Chem. Soc. Rev., 2008, 37, 1766 RSC; (i) N. D. Shapiro and F. D. Toste, Synlett, 2010, 675 CAS; (j) A. Corma, A. Leyva-Pérez and M. J. Sabater, Chem. Rev., 2011, 111, 1657 CrossRef CAS PubMed; (k) C. G. Freyschlag and R. J. Madix, Mater. Today, 2011, 14, 134 CrossRef CAS; (l) D. Garayalde and C. Nevado, ACS Catal., 2012, 2, 1462 CrossRef CAS; (m) M. Rudolph and A. S. K. Hashmi, Chem. Soc. Rev., 2012, 41, 2448 RSC; (n) A. S. K. Hashmi, Acc. Chem. Res., 2014, 47, 864 CrossRef CAS PubMed; (o) D. Weber and M. R. Gagne, in Homogeneous Gold Catalysis, 2015, pp. 167–211 Search PubMed.
- (a) M. Pernpointner and A. S. K. Hashmi, J. Chem. Theory Comput., 2009, 5, 2717 CrossRef CAS PubMed; (b) A. C. Jones, Top. Curr. Chem., 2015, 357, 133 CrossRef PubMed , and references cited there in.
- Selected reviews: (a) A. S. K. Hashmi, T. M. Frost and J. W. Bats, J. Am. Chem. Soc., 2000, 122, 11553 CrossRef CAS; (b) A. S. K. Hashmi, L. Schwarz, J. H. Choi and T. M. Frost, Angew. Chem., Int. Ed., 2000, 39, 2285 CrossRef CAS; (c) D. J. Gorin, B. D. Sherry and F. D. Toste, Chem. Rev., 2008, 108, 3351 CrossRef CAS PubMed; (d) Z. Li, C. Brouwer and C. He, Chem. Rev., 2008, 108, 3239 CrossRef CAS PubMed; (e) M. Brasholz, H. U. Reissig and R. Zimmer, Acc. Chem. Res., 2009, 42, 45 CrossRef CAS PubMed; (f) A. S. K. Hashmi, Angew. Chem., Int. Ed., 2010, 49, 5232 CrossRef CAS PubMed; (g) N. Krause and C. Winter, Chem. Rev., 2011, 111, 1994 CrossRef CAS PubMed; (h) D. Tejedor, G. Méndez-Abt, L. Cotos and F. GarcíaTellado, Chem. Soc. Rev., 2013, 42, 458 RSC.
- Selected reviews on [1,3]-rearrangement: (a) S. J. Meek and J. P. A. Harrity, Tetrahedron, 2007, 63, 3081 CrossRef CAS; (b) C. G. Nasveschuk and T. Rovis, Org. Biomol. Chem., 2008, 6, 240 RSC.
- Some selected examples of [1,3]-rearrangement catalyzed by Lewis acids (a) P. A. Grieco, J. D. Clark and C. T. Jagoe, J. Am. Chem. Soc., 1991, 113, 5488 CrossRef CAS; (b) B. du Roizel, M. Sollogoub, A. J. Pearce and P. Sinaÿ, Chem. Commun., 2000, 1507 RSC; (c) M. F. Buffet, D. J. Dixon, G. L. Edwards, S. V. Ley and E. W. Tate, J. Chem. Soc., Perkin Trans. 1, 2000, 1815 RSC; (d) Y. D. Zhang, N. T. Reynolds, K. Manju and T. Rovis, J. Am. Chem. Soc., 2002, 124, 9720 CrossRef CAS PubMed; (e) C. G. Nasveschuk and T. Rovis, Angew. Chem., Int. Ed., 2005, 44, 3264 CrossRef CAS PubMed. [Pd]: (f) B. M. Trost and J. Xie, J. Am. Chem. Soc., 2006, 128, 6044 CrossRef CAS PubMed; (g) D. M. D'Souza, F. Rominger and T. J. J. Müller, Chem. Commun., 2006, 4096 RSC; (h) S. Zhu, L. Wu and X. Huang, RSC Adv., 2012, 2, 132 RSC. [Co]: (i) S. J. Meek, F. Pradaux, D. R. Carbery, E. H. Demont and J. P. A. Harrity, J. Org. Chem., 2005, 70, 10046 CrossRef CAS PubMed. [Ir]: (j) H.-Y. Wang, D. S. Mueller, R. M. Sachwani, R. Kapadia, H. N. Londino and L. L. Anderson, J. Org. Chem., 2011, 76, 3203 CrossRef CAS PubMed.
- C. N. Kona and C. V. Ramana, Chem. Commun., 2014, 50, 2152 RSC.
- (a) B. D. Sherry and F. D. Toste, J. Am. Chem. Soc., 2004, 126, 15978 CrossRef CAS PubMed; (b) Y. Liu, J. Qian, S. Louand and Z. Xu, Synlett, 2009, 2971 CrossRef; (c) J. R. Vyvyan, H. E. Dimmitt, J. K. Griffith, L. D. Steffens and R. A. Swanson, Tetrahedron Lett., 2010, 51, 6666 CrossRef CAS; (d) M. E. Krafft, K. M. Hallal, D. V. Vidhani and J. W. Cran, Org. Biomol. Chem., 2011, 9, 7535 RSC.
- L. Kjeldmand, L. T. H. Salazar and M. Laska, J. Comp. Physiol., A, 2011, 197, 15 CrossRef CAS PubMed.
- Pd-based coupling approaches: (a) Palladium-catalyzed vinylation of organic halides: R. F. Heck, Org. React., 1982, 27, 345–390 CAS; (b) R.-H. Hu, X.-L. Liu and M.-Z. Cai, Jiangxi Shifan Daxue Xuebao, Ziran Kexueban, 2001, 25, 246 CAS; (c) V. Calo, A. Nacci, A. Monopoli and M. Spinelli, Eur. J. Org. Chem., 2003, 1382 CrossRef CAS; (d) S. A. Forsyth, H. Q. N. Gunaratne, C. Hardacre, A. McKeown, D. W. Rooney and K. R. Seddon, J. Mol. Catal. A: Chem., 2005, 231, 61 CrossRef CAS; (e) T. Yamada, S. Sakaguchi and Y. Ishii, J. Org. Chem., 2005, 70, 5471 CrossRef CAS PubMed; (f) M. Barbero, S. Cadamuro and S. Dughera, Synthesis, 2006, 3443 CrossRef CAS; (g) A. Scrivanti, M. Bertoldini, V. Beghetto and U. Matteoli, Tetrahedron, 2008, 64, 543 CrossRef CAS; (h) A. G. Panda, M. D. Bhor, S. S. Ekbote and B. M. Bhanage, Catal. Lett., 2009, 131, 649 CrossRef CAS.
- Hydroformylation: (a) The hydroformylation reaction: I. Ojima, C.-Y. Tsai, M. Tzamarioudaki and D. Bonafoux, Org. React., 2000, 56, 1–354 CAS; (b) R. Abu-Reziq, D. Avnir and J. Blum, Eur. J. Org. Chem., 2005, 3640 CrossRef CAS; (c) M. R. Axet, S. Castillon and C. Claver, Inorg. Chim. Acta, 2006, 359, 2973 CrossRef CAS; (d) L. G. Melean, M. Rodriguez, M. Romero, M. L. Alvarado, M. Rosales and P. J. Baricelli, Appl. Catal., A, 2011, 394, 117 CrossRef CAS; (e) M. Alhaffar, R. Suleiman, S. M. Shakil Hussain and B. El Ali, React. Kinet., Mech. Catal., 2011, 104, 323 CrossRef CAS; (f) R. Franke, M. Beller, R. Jackstell, R. Jennerjahn and I. Piras, DE, 2012, Chemical Indexing Equivalent to 156:587909; (g) P. J. Baricelli, L. G. Melean, M. Rodriguez, M. dos Santos, M. Rosales and E. Escalante, J. Chem. Chem. Eng., 2013, 7, 299 CAS; (h) S. R. Khan and B. M. Bhanage, Appl. Organomet. Chem., 2013, 27, 313 CrossRef CAS; (i) K. C. B. Oliveira, S. N. Carvalho, M. F. Duarte, E. V. Gusevskaya, E. N. dos Santos, J. E. Karroumi, M. Gouygou and M. Urrutigoity, Appl. Catal., A, 2015, 497, 10 CrossRef CAS. For the only example of a gold catalyzed hydroformylation, see: (j) A. S. K. Hashmi, W. Yang, Y. Yu, M. M. Hansmann, M. Rudolph and F. Rominger, Angew. Chem., Int. Ed., 2013, 52, 1329 CrossRef CAS PubMed; (k) Y. Yu, W. Yang, F. Rominger and A. S. K. Hashmi, Angew. Chem., Int. Ed., 2013, 52, 7586 CrossRef CAS PubMed; (l) Y. Yu, W. Yang, D. Pflästerer and A. S. K. Hashmi, Angew. Chem., Int. Ed., 2014, 53, 1144 CrossRef CAS PubMed; (m) S. Shi, T. Wang, V. Weingand, M. Rudolph and A. S. K. Hashmi, Angew. Chem., Int. Ed., 2014, 53, 1148 CrossRef CAS PubMed.
- For methods other than coupling and hydroformylation, see: (a) J. A. Virgilio and E. Heilweil, Org. Prep. Proced. Int., 1982, 14, 9 CrossRef CAS; (b) M. Hofmann, N. Hampel, T. Kanzian and H. Mayr, Angew. Chem., Int. Ed., 2004, 43, 5402 CrossRef CAS PubMed; (c) G. Rosini, C. Paolucci, F. Boschi, E. Marotta, P. Righi and F. Tozzi, Green Chem., 2012, 12, 1747 RSC; (d) J. Hua, X. Liu, W. Xu, L. An, J. Zhang, K. Wang, M. Wan and F. Zhang, CN 103073403, 2013; (e) V. Beghetto, A. Scrivanti, M. Bertoldini, M. Aversa, A. Zancanaro and U. Matteoli, Synthesis, 2015, 272 CAS.
- N.-A. Harada, T. Nishikata and H. Nagashima, Tetrahedron, 2012, 68, 3243 CrossRef CAS.
- (a) C. C. Price and W. H. Snyder, J. Am. Chem. Soc., 1961, 83, 1773 CrossRef CAS; (b) D. E. Pearson and C. A. Buehler, Chem. Rev., 1974, 74, 45 CrossRef CAS.
- For selected papers on the formation of ion pairs in the Au[I]-catalyzed reactions: (a) P. H.-Y. Cheong, P. Morganelli, M. R. Luzung, K. N. Houk and F. D. Toste, J. Am. Chem. Soc., 2008, 130, 4517 CrossRef CAS PubMed; (b) B. Alcaide, P. Almendros, T. Martínez del Campo, E. Soriano and J. L. Marco-Contelles, Chem. – Eur. J., 2009, 15, 9127 CrossRef CAS PubMed; (c) R.-X. Zhu, D.-J. Zhang, J.-X. Guo, J.-L. Mu, C.-G. Duan and C.-B. Liu, J. Phys. Chem. A, 2010, 114, 4689 CrossRef CAS PubMed; (d) T. J. Brown, A. Sugie, M. G. Dickens and R. A. Widenhoefer, Organometallics, 2010, 29, 4207 CrossRef CAS; (e) O. Nieto Faza and A. R. de Lera, Top. Curr. Chem., 2011, 302, 81 CrossRef; (f) M. Malacria, L. Fensterbank and V. Gandon, Top. Curr. Chem., 2011, 302, 157 CrossRef CAS PubMed; (g) D. V. Vidhani, J. W. Cran, M. E. Krafft and I. V. Alabugin, Org. Biomol. Chem., 2013, 11, 1624 RSC; (h) D. V. Vidhani, J. W. Cran, M. E. Krafft, M. Manoharan and I. V. Alabugin, J. Org. Chem., 2013, 78, 2059 CrossRef CAS PubMed; (i) T. Lauterbach, A. M. Asiri and A. S. K. Hashmi, Adv. Organomet. Chem., 2014, 62, 261 CrossRef CAS; (j) J. Bucher, T. Wurm, K. S. Nalivela, M. Rudolph, F. Rominger and A. S. K. Hashmi, Angew. Chem., Int. Ed., 2014, 53, 3854 CrossRef CAS PubMed; (k) M. Q. Jia and M. Bandini, Acs Catal., 2015, 5, 1638 CrossRef CAS.
- Papers on the general mechanism of [1,3] C → O rearrangement: (a) C. G. Nasveschuk and T. Rovis, Org. Lett., 2005, 7, 2173 CrossRef CAS PubMed; (b) J. D. Frein and T. Rovis, Tetrahedron, 2006, 62, 4573 CrossRef CAS PubMed; (c) C. G. Nasveschuk and T. Rovis, J. Org. Chem., 2008, 73, 612 CrossRef CAS PubMed; (d) S. Hou, X. Li and J. Xu, J. Org. Chem., 2012, 77, 10856 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5qo00397k |
| This journal is © the Partner Organisations 2016 |