Mechanism, catalysis and predictions of 1,3,2-diazaphospholenes: theoretical insight into highly polarized P–X bonds†
Liu Leo Liu*a,
Yile Wua,
Peng Chena,
Chinglin Chanc,
Ji Xud,
Jun Zhu*b and
Yufen Zhao*a
aDepartment of Chemistry and Chemical Biology, College of Chemistry and Chemical Engineering, Key Laboratory for Chemical Biology of Fujian Province, China. E-mail: chemliu@stu.xmu.edu.cn; yfzhao@xmu.edu.cn
bState Key Laboratory of Physical Chemistry of Solid Surfaces and Fujian Provincial Key Laboratory of Theoretical and Computational Chemistry, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen 361005, Fujian, China. E-mail: jun.zhu@xmu.edu.cn
cDepartment of Chemistry, National Tsing Hua University, Hsinchu 30013, Taiwan
dMaterials Science and Engineering Program, University of California, San Diego, La Jolla, California 92093, USA
First published on 9th February 2016
Abstract
Density functional theory (DFT) calculations were carried out to investigate the hydridic character of several main group hydrides. A P-hydrido-1,3,2-diazaphospholene 1f with two π-electron donor amino groups on the heterocyclic skeleton framework performs as a strong hydride donor owing to the significant n(N)–σ*(P–H) hyperconjugation. The natural bond orbital analysis reveals that high π-electron delocalization exists in both 1f and the corresponding stable phosphenium Ef+. In addition, 1f is calculated to have a similar catalytic ability for the hydroboration of acetone with pinacolborane, compared to 1e. Thus, a variety of organic substrates activated by 1f are explored, including ketone, imine, isocyanate, CO2, diazene, alkene, alkyne and epoxide. The results show that the highly polarized and electron-deficient bonds such as C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O π bonds are readily activated, whereas 1f seems difficult to react with electron-rich unsaturated bonds of propene and propyne. More importantly, 1,3,2-diazaphospholene-based compounds, featuring an extremely polarized P–X bond (X = C
O π bonds are readily activated, whereas 1f seems difficult to react with electron-rich unsaturated bonds of propene and propyne. More importantly, 1,3,2-diazaphospholene-based compounds, featuring an extremely polarized P–X bond (X = C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CMe, NMe2, PMe2 and SMe), are predicted to have a useful catalytic ability. The preliminary computational results suggest that these P–X compounds could catalyze the silylamination, silylphosphination and silylsulfenylation of acetone with TMSNMe2, TMSPMe2 and TMSSMe, respectively. The products are silylethers, which are equivalent to the corresponding alcohols since they easily undergo hydrolysis. Our computational study opens a new avenue to the design of novel main group organocatalysts.
CMe, NMe2, PMe2 and SMe), are predicted to have a useful catalytic ability. The preliminary computational results suggest that these P–X compounds could catalyze the silylamination, silylphosphination and silylsulfenylation of acetone with TMSNMe2, TMSPMe2 and TMSSMe, respectively. The products are silylethers, which are equivalent to the corresponding alcohols since they easily undergo hydrolysis. Our computational study opens a new avenue to the design of novel main group organocatalysts.
Introduction
The last three decades have witnessed spectacular development of the chemistry of heavier main-group elements.1 Syntheses and isolations of heavier homologues of ethylene,2 acetylene,2 carbene,3 ketone,4 and benzene5 have been successfully achieved by various research groups. Not only do the compounds of heavier main group elements have different electronic properties or molecular structures from their lighter congeners, but also the chemical behavior of these compounds resembles that of transition-metal complexes. For example, some of these heavier compounds showed intriguing reactivity for activation of small molecules, including carbon monoxide,6 carbon dioxide,7 dihydrogen,8 ammonia7a,8c,9 and molecules featuring C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C,10,11 C
C,10,11 C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) C,7a N
C,7a N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N11 and C–H7a,12 bonds, which provides a desirable alternative to transition-metal catalysis.13 Particularly, recent progress in reducing organic substrates into valuable products by main group element mimicking of typical transition metal catalysts has attracted increasing attention due to its reduced environmental impact and lower cost.13c For instance, Hill and co-workers developed a Mg-catalyzed hydroboration of carbonyl compounds14a and imines14b with pinacolborane.
N11 and C–H7a,12 bonds, which provides a desirable alternative to transition-metal catalysis.13 Particularly, recent progress in reducing organic substrates into valuable products by main group element mimicking of typical transition metal catalysts has attracted increasing attention due to its reduced environmental impact and lower cost.13c For instance, Hill and co-workers developed a Mg-catalyzed hydroboration of carbonyl compounds14a and imines14b with pinacolborane.
Since the observation of strong n(N)–σ*(P–Cl) hyperconjugation in P-chloro-1,3,2-diazaphospholenes by Gudat and co-workers,15 both experimental and theoretical studies have revealed that the corresponding P-hydrido-1,3,2-diazaphospholenes 1 contain an umpolung P–H bond (Fig. 1), which showed a hydridic character.16 Gudat demonstrated that the reaction of 1 with stoichiometric amounts of benzaldehyde gave benzyloxyphosphine.16a,b,17 Following this, Kinjo, Hirao and co-workers showed that benzyloxyphosphine can further react with pinacolborane to afford PhCH2OBpin and regenerate 1,18a which posed an interesting question as to whether 1 can be possibly used as an organocatalyst. Indeed, very recently, 1-catalyzed hydroboration18a of carbonyl compounds, as well as the hydrogenation18b of the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond with NH3BH3 and N-formylation18c of amines, have been reported.
N bond with NH3BH3 and N-formylation18c of amines, have been reported.
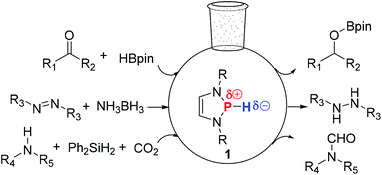 | ||
Fig. 1 P-Hydrido-1,3,2-diazaphospholenes 1 catalyzed hydroboration of carbonyl compounds, hydrogenation of the N![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) N bond and N-formylation of amines. N bond and N-formylation of amines. | ||
Inspired by the interesting results, we performed DFT calculations to investigate the hydridic character of several E–H bonds (E = Mg, Al, Ge and P). We found that the amino groups bonded to the unsaturated carbons of 1f (Table 1) can further stabilize the corresponding phosphenium cation by means of hyperconjugative delocalization and thus 1f is a better hydride donor. Furthermore, our calculations revealed that 1f has a similar catalytic ability for the hydroboration of acetone compared to 1e. Activation of a small molecule is one of the crucial reactions of transition-metal compounds, which usually acts as an essential step of a catalytic cycle. Therefore, a computational prediction was carried out to probe the small molecule (ketone, imine, isocyanate, CO2, diazene, alkene, alkyne and epoxide) activation ability of 1f. More importantly, the catalytic ability of a series of P–X (X = C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CMe, NMe2, PMe2 and SMe) compounds for the functionalization of acetone was investigated, which provides hints for further development of 1,3,2-diazaphospholene-based organocatalysis.
CMe, NMe2, PMe2 and SMe) compounds for the functionalization of acetone was investigated, which provides hints for further development of 1,3,2-diazaphospholene-based organocatalysis.
| Entry | EH 1 | NBO chargesa of EH | ΔHb |
|---|---|---|---|
| a NBO charges are given in atomic units. b Energies are given in kcal mol−1. | |||
| 1 | 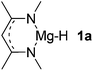 |
Mg (1.56) H (−0.69) | 125.3 |
| 2 | 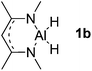 |
Al (1.32) H (−0.40) | 113.4 |
| 3 | 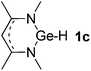 |
Ge (0.76) H (−0.28) | 100.1 |
| 4 | 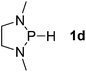 |
P (0.93) H (−0.15) | 117.8 |
| 5 | 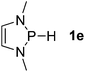 |
P (0.93) H (−0.16) | 102.5 |
| 6 | 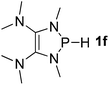 |
P (0.94) H (−0.16) | 93.3 |
| 7 | 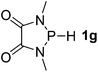 |
P (0.96) H (−0.08) | 160.3 |
| 8 | 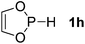 |
P (1.13) H (−0.14) | 156.9 |
Results and discussion
Exploration of hydridic character
Main group hydridic compounds have emerged in the past decade as organocatalysts.13c,19 The strong hydridic character of the well-defined β-diketiminato magnesium 1a,14,19a,20 aluminium 1b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19b and germanium 1c
19b and germanium 1c![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 19c (Table 1, entries 1–3) was demonstrated experimentally by various research groups. In this work, we first probed the hydridic character of various E–H bonds (E = Mg, Al, Ge and P) by means of the relative heterolytic bond dissociation (HBD) energies and natural bond orbital (NBO) analysis at the M06-2X/TZVP level of theory. The relative HBD energies were calculated from the reactions between BEt3 and E–H compounds (Table 1).21 The NBO charges of 1 show that the E–H bonds are polarized at Eδ+–Hδ−. For main group metal hydrides (entries 1–3), the Mg atom of 1a is the most positively charged owing to its considerably lower electronegativity (1.31) compared to those of Al (1.61) and Ge (2.01).22 However, the E–H HBD energy of 1a (125.3 kcal mol−1) is higher than those of 1b (113.4 kcal mol−1) and 1c (100.1 kcal mol−1).
19c (Table 1, entries 1–3) was demonstrated experimentally by various research groups. In this work, we first probed the hydridic character of various E–H bonds (E = Mg, Al, Ge and P) by means of the relative heterolytic bond dissociation (HBD) energies and natural bond orbital (NBO) analysis at the M06-2X/TZVP level of theory. The relative HBD energies were calculated from the reactions between BEt3 and E–H compounds (Table 1).21 The NBO charges of 1 show that the E–H bonds are polarized at Eδ+–Hδ−. For main group metal hydrides (entries 1–3), the Mg atom of 1a is the most positively charged owing to its considerably lower electronegativity (1.31) compared to those of Al (1.61) and Ge (2.01).22 However, the E–H HBD energy of 1a (125.3 kcal mol−1) is higher than those of 1b (113.4 kcal mol−1) and 1c (100.1 kcal mol−1).
Next, a P-hydrido-1,3,2-diazaphospholidine 1d (entry 4), several P-hydrido-1,3,2-diazaphospholenes with different substituents (1e–1g, entries 5–7), and a 1,3,2-dioxaphosphole 1h (entry 8) were explored. The lone pair of electrons of P-bonded amino or alkoxyl groups could donate into the P–H σ* orbital with concurrent increasing charge density at the hydrogen atom, which can enhance the hydridic character of the P–H bond.16 However, the higher electronegativity22 of an oxygen atom (3.44) relative to a nitrogen atom (3.04) prevents the above interaction and results in a higher P–H HBD energy (156.9 kcal mol−1) of 1h than that (102.5 kcal mol−1) of 1e (entries 5 and 8).
Although the NBO charges of 1d and 1e are similar (entries 4 and 5), the P–H HBD energy of 1e is lower than that (117.8 kcal mol−1) of 1d. We reason that the saturated carbons of the heterocyclic skeleton in 1d undoubtedly prevent the residual π-electron delocalization of the C2N2 fragment (σ* aromaticity)15,16 and thus strengthen the P–H bond.
It is important to note that Lewis acidic23 phospheniums E+ are formed with heterolytic cleavage of the P–H bond. The stability of E+ plays a crucial role in the dissociation process. We assumed that incorporation of functional groups on the heterocyclic skeleton would constitute a rational strategy to stabilize or destabilize E+. Indeed, modification of the electronic donor properties by two dimethylamino groups of 1f on the skeleton leads to a weakening of the P–H bond (93.3 kcal mol−1, entry 6) relative to 1e, whereas two strong electron-withdrawing carbonyl groups of 1g strengthen the P–H bond (160.3 kcal mol−1, entry 7). In brief summary, 1f is evaluated to be the best hydride donor, even better than main group metal hydrides.
To gain more insight into the strong hydridic character of 1f, NBO analysis was employed for both 1f and the corresponding Ef+. Fig. 2 depicts the 3D structures and selected Wiberg bond index (WBIs) of 1f (a) and Ef+ (b). As expected, 1f has a distorted ring (sum of the N1 angles: 341.1°) with a pyramidal geometry around the phosphorus center, and Ef+ contains a planar ring (sum of the N1 angles: 359.8°). In Ef+, the computed nucleus independent chemical shift NICS(0)zz and NICS(1)zz values are −2.9 and −21.8 ppm, respectively.24 These data are a clear indication that the five-membered ring of Ef+ is 6π-electron aromatic. The π electron delocalization over the ring of Ef+ results in the shortening of the P–N and C–N bonds and lengthening of the C1–C2 bond compared to those of 1f. In addition, WBIs show that the bond orders of C1–N1 and C1–C2 in 1f are 1.01 and 1.68, indicating single and double bond characters, respectively, while they are averaged to be 1.19 and 1.34 in Ef+. Note that the geometry of the ring in 1f has transformed from distorted to planar during the process of heterolytic cleavage of the P–H bond. In the meanwhile, the aromaticity of the ring was gained, which could facilitate the process.
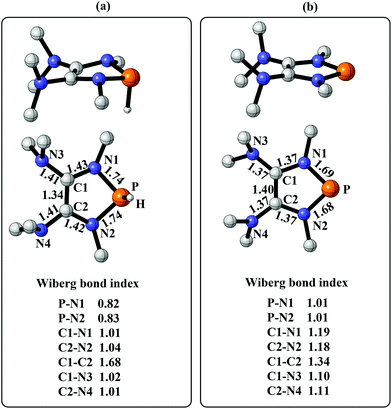 | ||
| Fig. 2 (a) The 3D structure and selected WBIs of 1f. (b) The 3D structure and selected WBIs of Ef+. The bond lengths are given in angstroms. | ||
We next turned our attention to finding out how the amino groups promote the heterolytic P–H bond dissociation of 1f. The selected natural localized molecular orbitals (NLMOs) of 1f are illustrated in Fig. 3, displaying the highly delocalized π bond contributions from the lone pairs of electrons of the nitrogen atoms in 1f. As shown in Fig. 3a, two dimethyl amino groups push their electrons into the C–C π* orbital of 1f, which makes the two P-bonded amino groups stronger electron donors by means of lone pair repulsion. The lone pairs of the P-bonded nitrogen atoms are significantly delocalized over the CNP fragments. Direct consequences of this delocalization in 1f are an increase of the charge density at the hydrogen atom of the P–H bond. Indeed, the second-order perturbation theory of the NBO method manifests two hyperconjugative delocalizations from the p-type N lone pairs to the P–H σ* antibonding orbital with donor–acceptor stabilization energies of 8.0 and 8.3 kcal mol−1 (Fig. 3b).
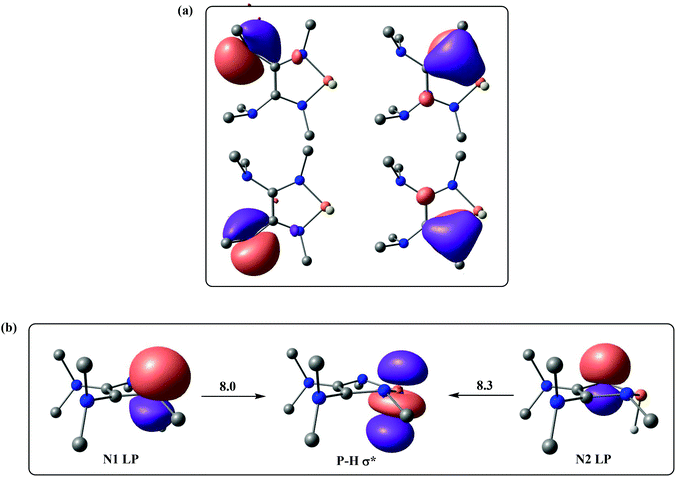 | ||
| Fig. 3 (a) Selected NLMOs of 1f. (b) Selected NBOs of 1f for the second-order perturbation theory analysis (energies are given in kcal mol−1). | ||
Also, high π-electron delocalization is found in Ef+ (Fig. 4). The selected NLMOs of Ef+ reveal that the lone pairs of the P-bonded amino groups not only delocalize onto the phosphorus, but also onto the unsaturated carbons (Fig. 4a). Interestingly, the second-order perturbation theory analysis suggests strong hyperconjugative delocalizations from the p-type N lone pairs of the dimethyl amino groups to the C–N π* orbitals (Fig. 4b). The strong donor–acceptor interactions between the lone pair of phosphorus and the C–N π* orbitals are also suggested by the stabilization energy of approximately 58.0 kcal mol−1. This high π-electron delocalization in Ef+, coupled with the aromaticity, results in stabilizing the electron-deficient phosphorus center and promoting the heterolytic P–H bond cleavage.
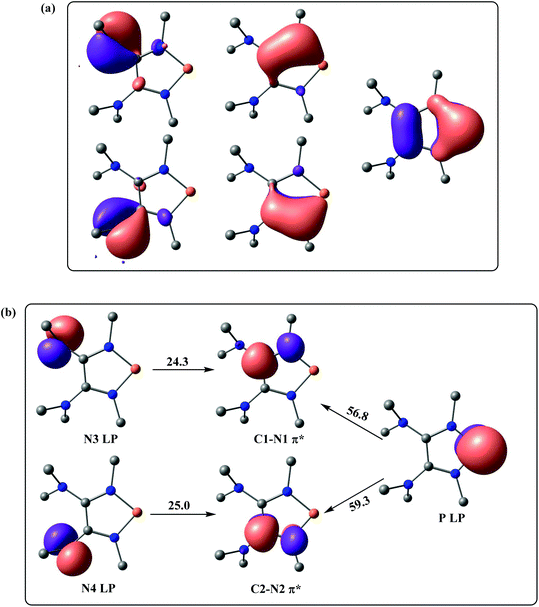 | ||
| Fig. 4 (a) Selected NLMOs of Ef+. (b) Selected NBOs of Ef+ for the second-order perturbation theory analysis (energies are given in kcal mol−1). | ||
Investigation of the catalytic ability
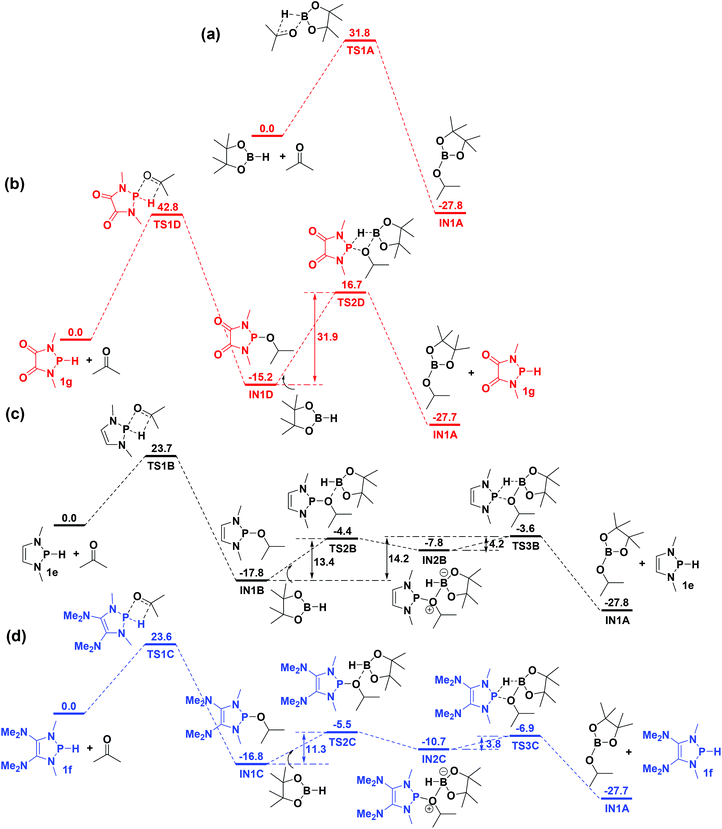
Kinjo, Hirao and co-workers reported the 1-catalyzed hydroboration of carbonyl compounds (Fig. 1), and the mechanism study revealed that the process involved a stepwise σ-bond metathesis, including P–H cleavage/P–O formation, B–O formation, and B–H cleavage/P–H formation.18a Our calculation results are consistent with their findings (Fig. 5c). For comparison, the catalyst-free hydroboration of acetone was also researched and it revealed a relatively high energy barrier of 31.8 kcal mol−1 (Fig. 5a). Expectedly, the less hydridic character of the P–H bond in 1g leads to a significantly high barrier (42.8 kcal mol−1) in the P–H cleavage/P–O formation step (Fig. 5b). To our delight, 1f is predicted to have a similar catalytic ability for the hydroboration of acetone relative to 1e (Fig. 5c and d). For instance, the energy barriers of 1e (23.7 kcal mol−1) and 1f (23.6 kcal mol−1) are similar in the P–H cleavage/P–O formation step (rate-determining step). Further, in the B–O formation and B–H cleavage/P–H formation steps, the energy barrier of 1f is 11.3 kcal mol−1, which is 2.9 and 20.6 kcal mol−1 lower than those of 1e (14.2 kcal mol−1) and 1h (31.9 kcal mol−1), respectively.Activation of organic substrates
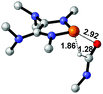
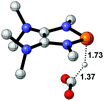
Functionalization of unsaturated bonds has been a continuous research interest of chemists. Experimentally, 1,3,2-diazaphospholenes 1 have been reported to activate some unsaturated organic substrates such as benzaldehyde16,18a and CO2.18c Encouraged by our achievements in theoretical predictions,25 activation of several organic substrates by 1f is evaluated computationally, involving ketone, imine, isocyanate, CO2, diazene, alkene, alkyne and epoxide (Table 2). We find that the substrates S, featuring a highly polarized bond, are readily activated by 1f. For example, the NBO atomic-charge distributions (C = 0.57 a.u. and O = −0.52 a.u.) on the C–O bond in acetone are much more polarized than those (C = 0.29 a.u. and N = −0.42 a.u.) on the C–N bond in N-methylpropan-2-imine, resulting in a lower energy barrier (23.6 kcal mol−1) of acetone activation (entries 1 and 2). The more polarized C–O bonds in isocyanatomethane and CO2 lead to much lower activation energies (relative to acetone, entries 1, 3 and 4).| Entry | Sa | 3D structureb of TS | ΔGTSc | Pa | ΔGpc |
|---|---|---|---|---|---|
| a NBO charges are given in atomic units at the M06-2X/TZVP//B3LYP/6-31G(d) level of theory. b Bond lengths are given in angstroms. c Energies are given in kcal mol−1. | |||||
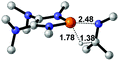
| 1 | 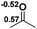 |
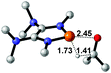 |
23.6 | 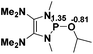 |
−16.8 |
| 2 | 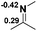 |
 |
32.1 | 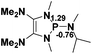 |
−19.3 |
| 3 | 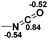 |
 |
8.5 | 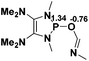 |
−12.3 |
| 4 | 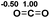 |
 |
9.4 | 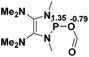 |
−7.6 |
| 5 | 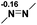 |
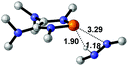 |
29.7 | 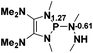 |
−20.0 |
| 6 | 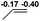 |
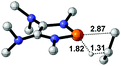 |
38.3 | 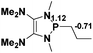 |
−23.7 |
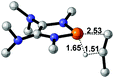
| 7 | 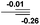 |
 |
36.5 | 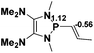 |
−29.1 |
| 8 | 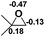 |
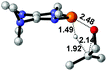 |
49.5 | 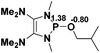 |
−45.2 |
NBO analysis suggests that dimethyldiazene, propene, and propyne are electron-rich unsaturated substrates (entries 5–7), which appear to be less susceptible to 1f with activation energies of 29.7, 38.3 and 36.5 kcal mol−1, respectively. Lastly, we investigated the ring opening of 2,2-dimethyloxirane mediated by 1f (entry 8). The high activation barrier (49.5 kcal mol−1) indicates that the process is impossible to take place.
It is interesting to note that the NBO charges of the products P in Table 2 show that the phosphorus atoms are highly positively charged, whereas the P-bonded atoms (O, N and C) carry more negative charges compared to the corresponding starting materials (i.e. acetone and CO2), which could be mainly attributed to the significant hyperconjugation of the five-membered ring as discussed above. Further, Gudat and co-workers showed that P-phosphino-1,3,2-diazaphospholene, which contains a polarized P–P bond, could stoichiometrically react with electron-poor alkenes and alkynes, affording the corresponding phosphinyl-phosphination products.17,26 We were wondering whether these 1,3,2-diazaphospholene-based compounds, featuring extremely polarized P–X bonds (X = O, N or C), can be used as organocatalysts.
Catalytic ability of P–X compounds
The model P–X compounds (X = C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CMe, NMe2, PMe2 and SMe) 2 were chosen to explore the catalytic ability (Fig. 6). As expected, all of the P–X bonds of 2 are found to be highly polarized and weak with partial single bond character (WBIs of P–X bonds are less than 1.0). We therefore assumed that the polarization would bring the desired reactivity.
CMe, NMe2, PMe2 and SMe) 2 were chosen to explore the catalytic ability (Fig. 6). As expected, all of the P–X bonds of 2 are found to be highly polarized and weak with partial single bond character (WBIs of P–X bonds are less than 1.0). We therefore assumed that the polarization would bring the desired reactivity.
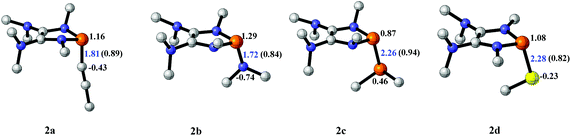 | ||
Fig. 6 3D structures of P–X (X = C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CMe, NMe2, PMe2 and SMe) compounds. NBO charges are given in atomic units in black. Bond lengths are given in angstroms in blue. WBIs are given in parentheses. CMe, NMe2, PMe2 and SMe) compounds. NBO charges are given in atomic units in black. Bond lengths are given in angstroms in blue. WBIs are given in parentheses. | ||
Catalytic functionalization of carbonyl compounds is a fundamental research topic for chemists. Gratifyingly, the energies of the transition states TS4 involving C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O bond activation of acetone by 2a, 2b, 2c, and 2d are computed to be 30.4, 19.2, 23.7, and 25.2 kcal mol−1 (Fig. 7), respectively, which are much lower than those of the direct addition of the corresponding HX [X = C
O bond activation of acetone by 2a, 2b, 2c, and 2d are computed to be 30.4, 19.2, 23.7, and 25.2 kcal mol−1 (Fig. 7), respectively, which are much lower than those of the direct addition of the corresponding HX [X = C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CMe (65.5 kcal mol−1), NMe2 (39.7 kcal mol−1), PMe2 (49.4 kcal mol−1) and SMe (43.2 kcal mol−1)] to acetone. However, the reversible processes are identified for 2b and 2d as the reverse barrier being equal or less compared to the forward barrier (Fig. 7b and d).
CMe (65.5 kcal mol−1), NMe2 (39.7 kcal mol−1), PMe2 (49.4 kcal mol−1) and SMe (43.2 kcal mol−1)] to acetone. However, the reversible processes are identified for 2b and 2d as the reverse barrier being equal or less compared to the forward barrier (Fig. 7b and d).
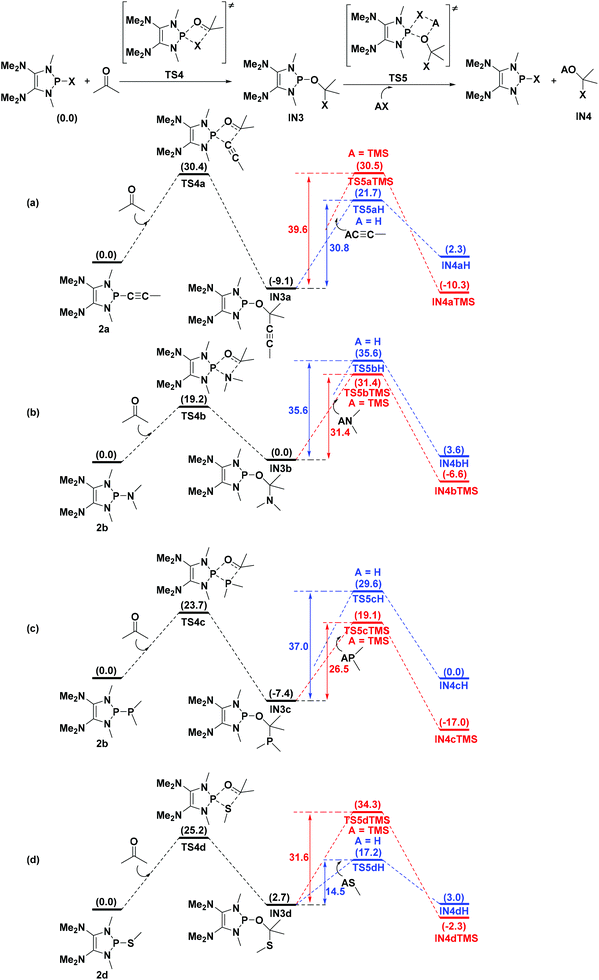 | ||
Fig. 7 Theoretical predictions of the catalysis ability of P–X [X = C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CMe (a), NMe2 (b), PMe2 (c) and SMe (d)] compounds. Energies are given in kcal mol−1. CMe (a), NMe2 (b), PMe2 (c) and SMe (d)] compounds. Energies are given in kcal mol−1. | ||
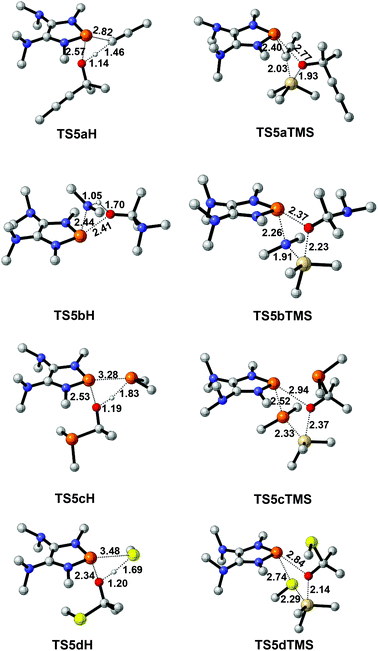
To design a catalytic cycle, we need to regenerate catalysts from IN3 (Fig. 7). Several AX (A = H or TMS, TMS = trimethylsilyl, X = C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CMe, NMe2, PMe2 or SMe) compounds are tested to react with IN3, which would lead to the P–O cleavage/P–X formation via four-membered ring transition states TS5 (Fig. 8).
CMe, NMe2, PMe2 or SMe) compounds are tested to react with IN3, which would lead to the P–O cleavage/P–X formation via four-membered ring transition states TS5 (Fig. 8).
For 2a (Fig. 7a), the result shows that while the activation energy (30.8 kcal mol−1) of HC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CMe with IN3a is much lower than that (39.6 kcal mol−1) of TMSC
CMe with IN3a is much lower than that (39.6 kcal mol−1) of TMSC![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CMe, the formation of IN4aH is endergonic by 2.3 kcal mol−1. These data suggest that 2a might be reluctant to catalyze the hydroalkynation of acetone.
CMe, the formation of IN4aH is endergonic by 2.3 kcal mol−1. These data suggest that 2a might be reluctant to catalyze the hydroalkynation of acetone.
For 2b and 2c (Fig. 7b and c), the relatively high barriers of hydroamination (35.6 kcal mol−1) and hydrophosphination (37.0 kcal mol−1) indicate that IN3c and IN3d might be inert to HNMe2 and HPMe2. However, the silylamination and silylphosphination (rate-determining steps) of acetone with TMSNMe2 or TMSPMe2 seem facile to take place since the processes are not energy demanding (31.4 kcal mol−1 for TMSNMe2 and 26.5 kcal mol−1 for TMSPMe2) and exergonic, especially at elevated temperatures.27
Lastly (Fig. 7d), although the reaction of IN3d with HSMe (14.5 kcal mol−1) seems much easier than that (31.6 kcal mol−1) of IN3d with TMSSMe, the energy of the product IN4dH is 3.0 kcal mol−1, which is higher than that (2.7 kcal mol−1) of IN3d, indicating that the hydrosulfenylation is endothermic. However, the silylsulfenylation (rate-determining step, 31.6 kcal mol−1) of acetone with TMSSMe appears to be possible at elevated temperatures.
Conclusions
The hydridic characters of eight main group hydrides have been investigated computationally. Among them, 1f is the best hydride donor with the aid of two π-electron donor amino groups. NLMOs and the second-order perturbation theory of the NBO method performed on 1f and the corresponding stable phosphenium Ef+ show strong π-electron delocalization. The lone pairs of electrons of the P-bonded amino groups donate into the P–H σ* orbital in 1f. This significant hyperconjugation could increase the electron density at the H atom of the P–H bond, enhancing the hydridic character. The mechanism study of the hydroboration of acetone confirms the high catalytic ability of 1f compared to 1e and 1h. Moreover, several organic substrates activated by 1f were explored. The highly polarized and electron-deficient bonds such as C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O π bonds in CO2 are readily activated, whereas it seems difficult to react with electron-rich unsaturated bonds of propene and propyne by 1f. Finally, the catalytic ability of the P–X (X = C
O π bonds in CO2 are readily activated, whereas it seems difficult to react with electron-rich unsaturated bonds of propene and propyne by 1f. Finally, the catalytic ability of the P–X (X = C![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) CMe, NMe2, PMe2 and SMe) compounds 2 has been probed. To our delight, the silylamination, silylphosphination and silylsulfenylation of acetone with TMSNMe2, TMSPMe2 and TMSSMe, respectively, seem highly possible to take place because the processes are not energy demanding with activation energies of 31.4 kcal mol−1 (TMSNMe2), 26.5 kcal mol−1 (TMSPMe2), and 31.6 kcal mol−1 (TMSSMe). The products are silylethers, which are equivalent to the corresponding alcohols since they easily undergo hydrolysis. Our findings provide hints for further development of main group organocatalysts.
CMe, NMe2, PMe2 and SMe) compounds 2 has been probed. To our delight, the silylamination, silylphosphination and silylsulfenylation of acetone with TMSNMe2, TMSPMe2 and TMSSMe, respectively, seem highly possible to take place because the processes are not energy demanding with activation energies of 31.4 kcal mol−1 (TMSNMe2), 26.5 kcal mol−1 (TMSPMe2), and 31.6 kcal mol−1 (TMSSMe). The products are silylethers, which are equivalent to the corresponding alcohols since they easily undergo hydrolysis. Our findings provide hints for further development of main group organocatalysts.
Computational details
Calculations were performed with the Gaussian 09 package.28 Natural bond orbital (NBO) calculations were carried out using the NBO 5.9 program.29 The M06-2X30 functional with the TZVP31 basis set was used for the study of hydridic characters of various E–H bonds (Table 1) and NBO analysis of 1f and Ef+ (Fig. 2–4). The nucleus independent chemical shift (NICS)32 of E+ was computed at the M06-2X/6-311++G(2d,p)//M06-2X/TZVP level of theory in the gas-phase. When the environments at points 1 angstrom above and below the ring centers are not equivalent, the averaged values are used for NICS(1)zz.For the mechanistic studies (Fig. 5–8 and Table 2), geometry optimizations were carried out with the B3LYP functional33 and the 6-31G(d)34 basis set was used for all the atoms. Frequency calculations at the same level of theory were performed to identify the number of imaginary frequencies (zero for local minimum and one for transition states) and provide the thermal corrections of the Gibbs free energy. Dispersion correction calculations using the corresponding B3LYP-D functional were performed with the DFT-D3 program of Grimme.35 The transition states were subjected to intrinsic reaction coordinate (IRC)36 calculations to determine two corresponding minima.
The M06-2X functional was used for the solution-phase, single-point energy calculations (Fig. 5–8 and Table 2), because this method was recently established as an excellent functional for describing reaction energetics of main group systems.37 The gas-phase geometry was used for all the solution phase calculations. A similar treatment was also used in many recent computational studies.38 A larger basis set, that is, 6-311++G(2d,p)39 was used. The default self-consistent reaction field (SCRF) polarizable continuum model (PCM) was used with acetonitrile,40 while Bondi radii41 were chosen as the atomic radii to define the molecular cavity. The Gibbs energy corrections from the frequency calculations and dispersion corrections were added to the single-point energies to obtain the Gibbs free energies in solution. All the solution-phase free energies reported in this paper correspond to the reference state of 1 mol L−1, 298 K. The optimized structures were visualized by the CYLview program42 or Chemcraft.43
Acknowledgements
We acknowledge financial support from the Chinese National Natural Science Foundation (Grants 21375113, 21232005, 21103142, 21573179, and 21133007), the National Basic Research Program of China (Grants 2012CB821600, 2013CB910700 and 2011CB808504), the Program for New Century Excellent Talents in University (NCET-13-0511), the Fundamental Research Funds for the Central Universities (Grant 2012121021), and the Program for Changjiang Scholars and Innovative Research Team in University (Grant IRT1263). Thanks are also given to Dr D. A. Ruiz from UCSD for the valuable discussion.Notes and references
- For reviews, see: (a) P. P. Power, Nature, 2010, 463, 171–177 CrossRef CAS PubMed; (b) R. Gleiter and D. B. Werz, Chem. Rev., 2010, 110, 4447–4488 CrossRef CAS PubMed; (c) M. A. Malik, M. Afzaal and P. O'Brien, Chem. Rev., 2010, 110, 4417–4446 CrossRef CAS PubMed.
- For a review, see: R. C. Fischer and P. P. Power, Chem. Rev., 2010, 110, 3877–3923 CrossRef CAS PubMed.
- For a review, see: Y. Mizuhata, T. Sasamori and N. Tokitoh, Chem. Rev., 2009, 109, 3479–3511 CrossRef CAS PubMed.
- L. Li, T. Fukawa, T. Matsuo, D. Hashizume, H. Fueno, K. Tanaka and K. Tamao, Nat. Chem., 2012, 4, 361–365 CrossRef CAS PubMed.
- (a) K. Wakita, N. Tokitoh, R. Okazaki and S. Nagase, Angew. Chem., Int. Ed., 2000, 39, 634–636 CrossRef CAS; (b) N. Nakata, N. Takeda and N. Tokitoh, J. Am. Chem. Soc., 2002, 124, 6914–6920 CrossRef CAS PubMed.
- X. Wang, Z. Zhu, Y. Peng, H. Lei, J. C. Fettinger and P. P. Power, J. Am. Chem. Soc., 2009, 131, 6912–6913 CrossRef CAS PubMed.
- (a) S. Yao, Y. Xiong and M. Driess, Organometallics, 2011, 30, 1748–1767 CrossRef CAS; (b) J. Li, M. Hermann, G. Frenking and C. Jones, Angew. Chem., Int. Ed., 2012, 51, 8611–8614 CrossRef CAS PubMed.
- (a) G. H. Spikes, J. C. Fettinger and P. P. Power, J. Am. Chem. Soc., 2005, 127, 12232–12233 CrossRef CAS PubMed; (b) Y. Peng, M. Brynda, B. D. Ellis, J. C. Fettinger, E. Rivard and P. P. Power, Chem. Commun., 2008, 6042–6044 RSC; (c) Y. Peng, J.-D. Guo, B. D. Ellis, Z. Zhu, J. C. Fettinger, S. Nagase and P. P. Power, J. Am. Chem. Soc., 2009, 131, 16272–16282 CrossRef CAS PubMed; (d) J. Li, C. Schenk, C. Goedecke, G. Frenking and C. Jones, J. Am. Chem. Soc., 2011, 133, 18622–18625 CrossRef CAS PubMed.
- (a) S. M. McCarthy, Y.-C. Lin, D. Devarajan, J. W. Chang, H. P. Yennawar, R. M. Rioux, D. H. Ess and A. T. Radosevich, J. Am. Chem. Soc., 2014, 136, 4640–4650 CrossRef CAS PubMed; (b) J. Cui, Y. Li, R. Ganguly, A. Inthirarajah, H. Hirao and R. Kinjo, J. Am. Chem. Soc., 2014, 136, 16764–16767 CrossRef CAS PubMed.
- (a) R. Kinjo, M. Ichinohe, A. Sekiguchi, N. Takagi, M. Sumimoto and S. Nagase, J. Am. Chem. Soc., 2007, 129, 7766–7767 CrossRef CAS PubMed; (b) Y. Peng, B. D. Ellis, X. Wang, J. C. Fettinger and P. P. Power, Science, 2009, 325, 1668–1670 CrossRef CAS PubMed.
- C. Cui, M. M. Olmstead, J. C. Fettinger, G. H. Spikes and P. P. Power, J. Am. Chem. Soc., 2005, 127, 17530–17541 CrossRef CAS PubMed.
- (a) O. T. Summerscales, J. C. Fettinger and P. P. Power, J. Am. Chem. Soc., 2011, 133, 11960–11963 CrossRef CAS PubMed; (b) O. T. Summerscales, C. A. Caputo, C. E. Knapp, J. C. Fettinger and P. P. Power, J. Am. Chem. Soc., 2012, 134, 14595–14603 CrossRef CAS PubMed; (c) Z. D. Brown, P. Vasko, J. C. Fettinger, H. M. Tuononen and P. P. Power, J. Am. Chem. Soc., 2012, 134, 4045–4048 CrossRef CAS PubMed.
- For reviews, see: (a) R. L. Melen, Chem. Soc. Rev. 10.1039/C5CS00521C; (b) E. M. Leitao, T. Jurca and I. Manners, Nat. Chem., 2013, 5, 817–829 CrossRef CAS PubMed; (c) K. Revunova and G. I. Nikonov, Dalton Trans., 2015, 44, 840–866 RSC; (d) C. C. Chong and R. Kinjo, ACS Catal., 2015, 5, 3238–3259 CrossRef CAS.
- (a) M. Arrowsmith, T. J. Hadlington, M. S. Hill and G. Kociok-Kohn, Chem. Commun., 2012, 48, 4567–4569 RSC; (b) M. Arrowsmith, M. S. Hill and G. Kociok-Köhn, Chem. – Eur. J., 2013, 19, 2776–2783 CrossRef CAS PubMed.
- D. Gudat, A. Haghverdi, H. Hupfer and M. Nieger, Chem. – Eur. J., 2000, 6, 3414–3425 CrossRef CAS.
- (a) D. Gudat, A. Haghverdi and M. Nieger, Angew. Chem., Int. Ed., 2000, 39, 3084–3086 CrossRef CAS; (b) S. Burck, D. Gudat, M. Nieger and W.-W. Du Mont, J. Am. Chem. Soc., 2006, 128, 3946–3955 CrossRef CAS PubMed.
- D. Gudat, Acc. Chem. Res., 2010, 43, 1307–1316 CrossRef CAS PubMed.
- (a) C. C. Chong, H. Hirao and R. Kinjo, Angew. Chem., Int. Ed., 2015, 54, 190–194 CrossRef CAS PubMed; (b) C. C. Chong, H. Hirao and R. Kinjo, Angew. Chem., Int. Ed., 2014, 53, 3342–3346 CrossRef CAS PubMed; (c) C. C. Chong and R. Kinjo, Angew. Chem., Int. Ed., 2015, 54, 12116–12120 CrossRef CAS PubMed.
- For selected recent publications, see: (a) D. J. Liptrot, M. S. Hill, M. F. Mahon and A. S. S. Wilson, Angew. Chem., Int. Ed., 2015, 54, 13362–13365 CrossRef CAS PubMed; (b) Z. Yang, M. Zhong, X. Ma, S. De, C. Anusha, P. Parameswaran and H. W. Roesky, Angew. Chem., Int. Ed., 2015, 54, 10225–10229 CrossRef CAS PubMed; (c) T. J. Hadlington, M. Hermann, G. Frenking and C. Jones, J. Am. Chem. Soc., 2014, 136, 3028–3031 CrossRef CAS PubMed.
- For selected recent publications, see: (a) C. Weetman, M. S. Hill and M. F. Mahon, Chem. Commun., 2015, 51, 14477–14480 RSC; (b) D. J. Liptrot, M. Arrowsmith, A. L. Colebatch, T. J. Hadlington, M. S. Hill, G. Kociok-Köhn and M. F. Mahon, Angew. Chem., Int. Ed., 2015, 54, 15280–15283 CrossRef CAS PubMed.
- M. T. Mock, R. G. Potter, D. M. Camaioni, J. Li, W. G. Dougherty, W. S. Kassel, B. Twamley and D. L. DuBois, J. Am. Chem. Soc., 2009, 131, 14454–14465 CrossRef CAS PubMed.
- A. L. Allred, J. Inorg. Nucl. Chem., 1961, 17, 215–221 CrossRef CAS.
- (a) J. M. Slattery and S. Hussein, Dalton Trans., 2012, 41, 1808–1815 RSC; (b) L. Liu, D. A. Ruiz, F. Dahcheh and G. Bertrand, Chem. Commun., 2015, 51, 12732–12735 RSC.
- The NICS values of the E+ derivatives listed in Table 1 are shown in Table S1 in the ESI.†.
- (a) L. Liu, H. Yuan, T. Fu, T. Wang, X. Gao, Z. Zeng, J. Zhu and Y. Zhao, J. Org. Chem., 2014, 79, 80–87 CrossRef CAS PubMed; (b) T. Wang, S. Sang, L. Liu, H. Qiao, Y. Gao and Y. Zhao, J. Org. Chem., 2014, 79, 608–617 CrossRef CAS PubMed; (c) L. Liu, Y. Wu, Z. Wang, J. Zhu and Y. Zhao, J. Org. Chem., 2014, 79, 6816–6822 CrossRef CAS PubMed; (d) L. Liu, C. Chan, J. Zhu, C.-H. Cheng and Y. Zhao, J. Org. Chem., 2015, 80, 8790–8795 CrossRef CAS PubMed; For experimental realization of ref. 25d, see: (e) D. Wu, R. Ganguly, Y. Li, S. N. Hoo, H. Hirao and R. Kinjo, Chem. Sci., 2015, 6, 7150–7155 RSC; (f) K. An and J. Zhu, Organometallics, 2014, 33, 7141–7146 CrossRef CAS; (g) Y. Huang and J. Zhu, Chem. Asian J., 2015, 10, 405–410 CrossRef CAS PubMed.
- (a) S. Burck, D. Gudat and M. Nieger, Angew. Chem., Int. Ed., 2004, 43, 4801–4804 CrossRef CAS PubMed; (b) I. Hajdók, F. Lissner, M. Nieger, S. Strobel and D. Gudat, Organometallics, 2009, 28, 1644–1651 CrossRef.
- We estimate the barrier value from the Eyring equation, i.e., k = (kBT/h) exp(−ΔG‡/RT), where k is the rate constant, kB is the Boltzmann's constant, h is the Planck's constant, ΔG‡ is the activation free energy, R is the gas constant, and T is the temperature. Assuming that the concentration of each reactant is 1 mol L−1 and the second-order rate constant k of a reaction whose half-life is 24 h is 1.2 × 10−5 L mol−1 s−1, we obtain ΔG‡ as 31.4 kcal mol−1 at 110 °C.
- M. J. Frisch, G. W. Trucks, H. B. Schlegel, G. E. Scuseria, M. A. Robb, J. R. Cheeseman, G. Scalmani, V. Barone, B. Mennucci, G. A. Petersson, H. Nakatsuji, M. Caricato, X. Li, H. P. Hratchian, A. F. Izmaylov, J. Bloino, G. Zheng, J. L. Sonnenberg, M. Hada, M. Ehara, K. Toyota, R. Fukuda, J. Hasegawa, M. Ishida, T. Nakajima, Y. Honda, O. Kitao, H. Nakai, T. Vreven, J. A. Montgomery Jr., J. E. Peralta, F. Ogliaro, M. Bearpark, J. J. Heyd, E. Brothers, K. N. Kudin, V. N. Staroverov, T. Keith, R. Kobayashi, J. Normand, K. Raghavachari, A. Rendell, J. C. Burant, S. S. Iyengar, J. Tomasi, M. Cossi, N. Rega, J. M. Millam, M. Klene, J. E. Knox, J. B. Cross, V. Bakken, C. Adamo, J. Jaramillo, R. Gomperts, R. E. Stratmann, O. Yazyev, A. J. Austin, R. Cammi, C. Pomelli, J. W. Ochterski, R. L. Martin, K. Morokuma, V. G. Zakrzewski, G. A. Voth, P. Salvador, J. J. Dannenberg, S. Dapprich, A. D. Daniels, O. Farkas, J. B. Foresman, J. V. Ortiz, J. Cioslowski and D. J. Fox, Gaussian 09, Revision B.01, Gaussian, Inc., Wallingford CT, 2010 Search PubMed.
- E. D. Glendening, J. K. Badenhoop, A. E. Reed, J. E. Carpenter, J. A. Bohmann, C. M. Morales and F. Weinhold, NBO 5.9, Theoretical Chemistry Institute, University of Wisconsin, Madison, WI, 2009; http://www.chem.wisc.edu/~nbo5.
- Y. Zhao and D. Truhlar, Theor. Chem. Acc., 2008, 120, 215–241 CrossRef CAS.
- A. Schäfer, C. Huber and R. Ahlrichs, J. Chem. Phys., 1994, 100, 5829–5835 CrossRef.
- P. V. R. Schleyer, C. Maerker, A. Dransfeld, H. Jiao and N. J. R. V. E. Hommes, J. Am. Chem. Soc., 1996, 118, 6317–6318 CrossRef CAS.
- (a) C. Lee, W. Yang and R. G. Parr, Phys. Rev. B: Condens. Matter, 1988, 37, 785–789 CrossRef CAS; (b) P. J. Stephens, F. J. Devlin, C. F. Chabalowski and M. J. Frisch, J. Phys. Chem., 1994, 98, 11623–11627 CrossRef CAS.
- (a) R. Ditchfield, W. J. Hehre and J. A. Pople, J. Chem. Phys., 1971, 54, 724–728 CrossRef CAS; (b) W. J. Hehre, R. Ditchfield and J. A. Pople, J. Chem. Phys., 1972, 56, 2257–2261 CrossRef CAS; (c) P. C. Hariharan and J. A. Pople, Theor. Chim. Acta, 1973, 28, 213–222 CrossRef CAS; (d) J. D. Dill and J. A. Pople, J. Chem. Phys., 1975, 62, 2921–2923 CrossRef CAS; (e) M. M. Francl, W. J. Pietro, W. J. Hehre, J. S. Binkley, M. S. Gordon, D. J. DeFrees and J. A. Pople, J. Chem. Phys., 1982, 77, 3654–3665 CrossRef CAS.
- (a) S. Grimme, J. Antony, S. Ehrlich and H. Krieg, J. Chem. Phys., 2010, 132, 154104 CrossRef PubMed; (b) S. Grimme, J. Comput. Chem., 2006, 27, 1787–1799 CrossRef CAS PubMed; (c) S. Grimme, J. Comput. Chem., 2004, 25, 1463–1473 CrossRef CAS PubMed.
- (a) K. Fukui, J. Phys. Chem., 1970, 74, 4161–4163 CrossRef CAS; (b) K. Fukui, Acc. Chem. Res., 1981, 14, 363–368 CrossRef CAS.
- (a) Y. Zhao and D. Truhlar, Theor. Chem. Acc., 2008, 120, 215–241 CrossRef CAS; (b) Y. Zhao and D. G. Truhlar, J. Chem. Theory Comput., 2008, 4, 1849–1868 CrossRef CAS PubMed; (c) Y. Wu, L. Liu, J. Su, K. Yan, J. Zhu and Y. Zhao, Chem. Commun., 2016, 52, 1582–1585 RSC.
- For selected recent publications, see: (a) L. Liu, J. Zhu and Y. Zhao, Chem. Commun., 2014, 50, 11347–11349 RSC; (b) M. N. Grayson, M. J. Krische and K. N. Houk, J. Am. Chem. Soc., 2015, 137, 8838–8850 CrossRef CAS PubMed; (c) G. Lu, C. Fang, T. Xu, G. Dong and P. Liu, J. Am. Chem. Soc., 2015, 137, 8274–8283 CrossRef CAS PubMed; (d) Y. Yang and P. Liu, ACS Catal., 2015, 5, 2944–2951 CrossRef CAS; (e) L. Liu, Y. Wu, T. Wang, X. Gao, J. Zhu and Y. Zhao, J. Org. Chem., 2014, 79, 5074–5081 CrossRef CAS PubMed.
- (a) R. Krishan, J. S. Binkley, R. Seeger and J. A. Pople, J. Chem. Phys., 1980, 72, 650–654 CrossRef; (b) A. D. McLean and G. S. Chandler, J. Chem. Phys., 1980, 72, 5639–5648 CrossRef CAS; (c) T. Clark, J. Chandrasekhar, G. W. Spitznagel and P. V. R. Schleyer, J. Comput. Chem., 1983, 4, 294–301 CrossRef CAS.
- Experimentally, all of the 1,3,2-diazaphospholene-catalyzed reactions were performed in acetonitrile. See ref. 18 for details.
- A. Bondi, J. Phys. Chem., 1964, 68, 441–451 CrossRef CAS.
- C. Y. Legault, CYLview, 1.0b, Université de Sherbrooke, Sherbrooke, Québec, Canada, 2009; http://www.cylview.org Search PubMed.
- G. A. Andrienko, ChemCraft, http://www.chemcraftprog.com.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6qo00002a |
| This journal is © the Partner Organisations 2016 |