An organocatalytic enantioselective vinylogous Mannich reaction of α,α-dicyanoolefins with isatin N-Boc ketimines†
Yi
Zhu‡
a,
Yao
Li‡
a,
Qingbin
Meng
*b and
Xin
Li
*a
aState Key Laboratory of Elemento-Organic Chemistry, Collaborative Innovation Center of Chemical Science and Engineering, Department of Chemistry, Nankai University, Tianjin 300071, China. E-mail: xin_li@nankai.edu.cn
bState Key Laboratory of Toxicology and Medical Countermeasures, Beijing Institute of Pharmacology and Toxicology, Beijing, 100850, China. E-mail: nankaimqb@sina.com
First published on 29th March 2016
Abstract
The first catalytic enantioselective vinylogous Mannich reaction of α,α-dicyanoolefins with ketimines derived from isatins has been developed. High yields and enantioselectivities were observed for the reaction of various ketimines and α,α-dicyanoolefins using a tertiary butyl substituted cinchona alkaloid type squaramide catalyst.
The asymmetric organocatalytic Mannich reaction is becoming one of the most important protocols for the synthesis of optically pure amine compounds.1 In order to highlight its synthetic efficiency, the Mannich reaction is not only employed in the synthesis of alkaloids,1a,2 but is also used in the synthesis of medicinal compounds, such as rolitetracycline, fluoxetine, tramadol, and tolmetin and azacyclophanes.3 After these accomplishments, the asymmetric vinylogous Mannich reactions,4 which usually deliver extended carbon skeletons and multifunctional products in comparison with those of simple Mannich reactions, have also become a convenient and efficient procedure to realize enantioselective carbon–carbon bond formation. However, most of the vinylogous Mannich reactions are focused on the aldimines.5 Up to now, examples of the enantioselective vinylogous Mannich reaction of ketimines have been very limited.6 The reason for this status is maybe due to the fact that the activity of ketimines is usually lower than that of aldimines. Thus, the discovery of highly reactive ketimines for vinylogous Mannich reactions should arouse wide interest among organic chemists.
The reactions of ketimines derived from isatins, especially for isatin N-Boc ketimines, have attracted great attention in recent years.7 As a result, a number of valuable asymmetric transformations, including enantioselective Mannich, Strecker, Friedel–Crafts, aza-Diels–Alder reaction and homoenolate addition reactions featuring isatin N-Boc ketimines as electrophiles, have been realized.8 The wide application of isatin N-Boc ketimines as substrates is mainly because the reaction of isatin-derived ketimines affords α-amino peroxides having an oxindole backbone, which is an important structural motif in biologically and pharmacologically active compounds.9 Moreover, the high reactivity of isatin N-Boc ketimines, compared to other ketimines, is another unignored reason for their frequent use as electrophiles. On the other hand, α,α-dicyanoolefins have been widely used as readily available and versatile vinylogous synthons in a number of vinylogous conjugate addition reactions.10 It is especially worth mentioning that the vinylogous Mannich reaction of α,α-dicyanoolefins and aldimines had been successfully realized in high stereoselectivity using a bifunctional thiourea catalyst.5a
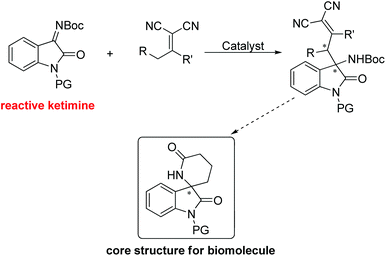
According to the abovementioned consideration, we envisioned that the employment of isatin N-Boc ketimines in the vinylogous Mannich reaction may possibly solve the problem of the low reactivity of ketimine. Herein, we report an enantioselective vinylogous Mannich reaction between isatin N-Boc ketimines and α,α-dicyanoolefins, which provides a new strategy for the synthesis of chiral 3,3′-substituted oxindole type compounds (Scheme 1). It is valuable to note that the obtained product could be easily converted to chiral 3-spirocyclic oxindole type α-lactam, which had the core structure of bioactive molecules.11
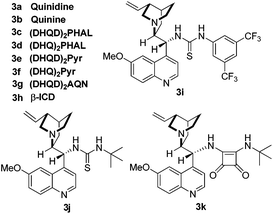
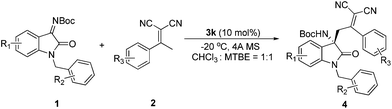
We started the study with eight widely used tertiary-amine catalysts 3a–3h (10 mol%) (Fig. 1) in CHCl3 at room temperature, and performed the reaction between isatin N-Boc ketimine 1a and α,α-dicyanoolefin 2a (Table 1, entries 1–8). As a result, all the catalysts could catalyze the reaction with good yields; however, the enantioselectivities were obtained with different levels, in which bis-cinchona alkali type catalyst 3d gave the best ee of 78% (Table 1, entry 4). This result is obviously not satisfactory. Since hydrogen-bonding was identified to activate imine and cyano groups, three bifunctional tertiary-amine hydrogen-bonding type catalysts 3i–3k (Fig. 1), which were synthesized from quinine, were evaluated in the model reaction. To our delight, the tert-butyl substituted squaramide catalyst 3k exhibited very good reactivity and stereo-induction, which gave the desired product 4a in 88% yield and 88% ee (Table 1, entry 11). The next solvent examination with 3k as the catalyst showed that the initially used CHCl3 was the optimal one (Table 1, entries 11–17). Gratifyingly, lowering the reaction temperature could further lead to an increase of enantioselectivity of the product, and a longer reaction time was required (Table 1, entries 18 and 19). Collectively, the best result with respect to yield and ee value was obtained by performing the reaction at −20 °C with 4 Å molecule sieve in a mixture of CHCl3 and MTBE (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) catalyzed by 10 mol% 3k. Under these conditions, the reaction provided the vinylogous Mannich product 4a in 87% yield and 95% ee (Table 1, entry 20).
1) catalyzed by 10 mol% 3k. Under these conditions, the reaction provided the vinylogous Mannich product 4a in 87% yield and 95% ee (Table 1, entry 20).
| Entry | Cat. | Solvent | Yieldb (%) | eec (%) |
|---|---|---|---|---|
| a The reactions were conducted with 0.12 mmol 1a, 0.1 mmol 2a and catalyst in 1 mL solvent at room temperature for 96 h. b Isolated yields. c Determined by chiral HPLC analysis. d The reaction was performed at 0 °C and the reaction time was extended to 120 h. e The reaction was performed at −20 °C and the reaction time was extended to 120 h. f The reaction was performed at −20 °C with 50 mg 4 Å molecular sieve and the reaction time was extended to 120 h. | ||||
| 1 | 3a | CHCl3 | 85 | 33 |
| 2 | 3b | CHCl3 | 86 | 46 |
| 3 | 3c | CHCl3 | 90 | 69 |
| 4 | 3d | CHCl3 | 83 | 78 |
| 5 | 3e | CHCl3 | 78 | 0 |
| 6 | 3f | CHCl3 | 80 | 5 |
| 7 | 3g | CHCl3 | 76 | 5 |
| 8 | 3h | CHCl3 | 68 | 36 |
| 9 | 3i | CHCl3 | 44 | 20 |
| 10 | 3j | CHCl3 | 63 | 63 |
| 11 | 3k | CHCl3 | 88 | 88 |
| 12 | 3k | Toluene | 92 | 80 |
| 13 | 3k | CH2Cl2 | 70 | 81 |
| 14 | 3k | MTBE | 95 | 79 |
| 15 | 3k | Ethyl acetate | 65 | 71 |
| 16 | 3k | THF | 68 | 75 |
| 17 | 3k | Acetonitrile | 80 | 80 |
| 18d | 3k | CHCl3 | 70 | 93 |
| 19e | 3k | CHCl3 | 65 | 96 |
| 20f | 3k | CHCl3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) MTBE (1 MTBE (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) 1) |
87 | 95 |
With the optimal reaction conditions in hand, we subsequently evaluated the substrate scope of isatin N-Boc ketimine 1 by the reactions with α,α-dicyanoolefin 2a (Table 2). As shown in entries 1–6, whether electron-withdrawing or electron-donating groups at the 5- or 6-position of the oxindole aryl group of the substrate 1 were employed, the reactions proceeded to give the corresponding products 4a–4f in very good yields (73–92%) with good to excellent enantioselectivities (82–95% ee). Further exploration of the reaction scope focused on varying the substituents on the N-protected benzyl group. To our delight, the corresponding vinylogous Mannich products 4g–4i were obtained in high yields and enantioselectivities (Table 2, entries 7–9). Different α,α-dicyanoolefins were next examined. As shown in Table 2, the reactions were shown to work well with a range of α,α-dicyanoolefins bearing either electron-withdrawing or electron-donating groups to give the desired products in very good yields (80–96%) and enantioselectivities (88–96% ee). A more accurate analysis of these results showed that the reactivity and enantioselectivity of the reaction are sensitive to the electronic substitution of the aryl group. Lower yield and ee value were obtained when 1b was applied as the electrophile.
| Entry | R1 | R2 | R3 | Yieldb (%) | eec (%) |
|---|---|---|---|---|---|
a The reactions were conducted with 1 (0.12 mmol), 2 (0.1 mmol) and 10 mol% 3k in a mixture of MTBE and CHCl3 (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, 1 mL) at −20 °C for 72–192 h. The absolute configuration was determined by X-ray analysis of the product 4a.12
b Isolated yields.
c Determined by chiral HPLC analysis. 1, 1 mL) at −20 °C for 72–192 h. The absolute configuration was determined by X-ray analysis of the product 4a.12
b Isolated yields.
c Determined by chiral HPLC analysis.
|
|||||
| 1 | 1a: H | H | 2a: H | 87 | 4a: 95 |
| 2 | 1b: 5-CH3O | H | 2a: H | 80 | 4b: 82 |
| 3 | 1c: 5-CH3 | H | 2a: H | 80 | 4c: 93 |
| 4 | 1d: 5-Cl | H | 2a: H | 73 | 4d: 88 |
| 5 | 1e: 5-Br | H | 2a: H | 88 | 4e: 87 |
| 6 | 1f: 6-Br | H | 2a: H | 92 | 4f: 92 |
| 7 | 1h: H | 4-Cl | 2a: H | 87 | 4g: 95 |
| 8 | 1i: H | 4-Br | 2a: H | 88 | 4h: 95 |
| 9 | 1j: H | 3-F | 2a: H | 83 | 4i: 93 |
| 10 | 1g: H | H | 2b: 4-F | 80 | 4j: 94 |
| 11 | 1a: H | H | 2c: 4-Cl | 90 | 4k: 93 |
| 12 | 1a: H | H | 2d: 4-Br | 87 | 4l: 94 |
| 13 | 1a: H | H | 2e: 4-Me | 83 | 4m: 88 |
| 14 | 1a: H | H | 2f: 3-Cl | 95 | 4n: 96 |
| 15 | 1a: H | H | 2g: 3,4-diCl | 96 | 4o: 95 |
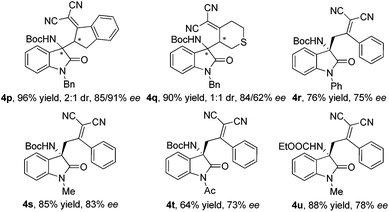
In order to further extend the substrate scope, cyclic α,α-dicyanoolefins were also investigated. As expected, the vinylogous Mannich reactions catalyzed by 3k under the standard conditions occurred smoothly, which afforded the desired products 4p and 4q in very good yields and enantioselectivities. Moreover, three isatin N-Boc ketimines with different N-protecting groups (Ph-, Me- and Ac-) were also tolerated in this vinylogous Mannich strategy (Scheme 2). As a result, the products 4r–4t were obtained in good yields and enantioselectivities. In addition, when the isatin N-CO2Et ketimine was selected as the electrophile, the reaction also proceeded, giving the desired Mannich product 4u in good yield and ee value.
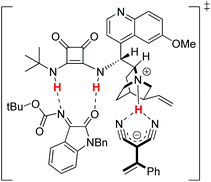
Based on the X-ray crystal structure results and previous mechanism studies, a potential transition-state structure was proposed. As shown in Scheme 3, the tertiary-amine squaramide catalyst serves the dual function of activating both the electrophile and the nucleophile. The tertiary amine of the catalyst deprotonates α,α-dicyanoolefin and, through coordination, holds it in close proximity, while the N–H binding moiety of squaramide activates the isatin N-Boc ketimine through hydrogen bonds. The Re-face attack of the isatin N-Boc ketimine by α,α-dicyanoolefin afforded the desired vinylogous Mannich product.
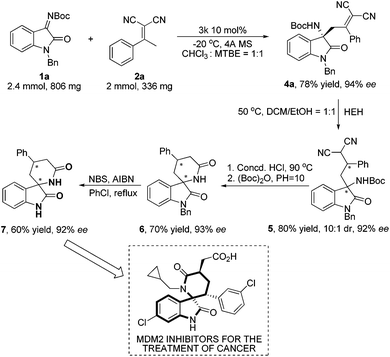
To probe the efficiency of the currently studied asymmetric vinylogous Mannich strategy in preparative synthesis, large scale reactions of 1a and 2a catalyzed by 3k were performed. To our delight, the desired product 4a was obtained with only one percent loss of enantioselectivity (Scheme 4, 94% ee). Further reduction of 4a by Hantzsch ester afforded 5 in almost retentive ee value. After a simple hydrolysis in refluxing concentrated HCl and work up with (Boc)2O in the alkaline environment, we obtained achiral 3-spirocyclic oxindole type α-lactam 6 in 70% yield with 93% ee. After removal of the protecting group (Bn), 7 was obtained in 60% yield with 92% ee, which backbone is a bioactive molecule with anticancer efficacy.11b
Conclusions
In summary, we have developed a highly asymmetric vinylogous Mannich reaction of α,α-dicyanoolefins with isatin N-Boc ketimines catalyzed by a tertiary butyl substituted cinchona alkaloid type squaramide, which worked as a bifunctional catalyst. The reaction scope is substantial, a wide range of isatin N-Boc ketimines as well as α,α-dicyanoolefins were tolerated under the reaction conditions, giving the corresponding Mannich products in high yields (up to 96%) and enantioselectivities (up to 96% ee). Furthermore, the desired product can be easily converted to a valuable 3-spirocyclic oxindole type α-lactam.Acknowledgements
This work was supported by the 973 Program (2012CB821600), NSFC (21390400, 81202465, 81573354 and 21421062), the National Key Technologies R&D Program for New Drugs of China (2012ZX09301003) and the “111” project (B06005) of the Ministry of Education of China.Notes and references
- (a) M. Arend, B. Westermann and N. Risch, Angew. Chem., Int. Ed., 1998, 37, 1044 CrossRef; (b) A. Córdova, Acc. Chem. Res., 2004, 37, 102 CrossRef PubMed; (c) A. Ting and S. E. Schaus, Eur. J. Org. Chem., 2007, 5797 CrossRef CAS; (d) J. M. M. Verkade, L. J. C. van Hemert, P. J. L. M. Quaedflieg and F. P. J. T. Rutjes, Chem. Soc. Rev., 2008, 37, 29 RSC.
- (a) K. F. Maclure and C. H. Heathcock, J. Am. Chem. Soc., 1989, 111, 1530 CrossRef; (b) S. F. Martin, J. Heterocycl. Chem., 1994, 31, 679 CrossRef CAS; (c) S. F. Martin and K. J. Barr, J. Am. Chem. Soc., 1996, 118, 3299 CrossRef CAS; (d) S. F. Martin, Pure Appl. Chem., 1997, 69, 71 CrossRef; (e) F. A. Davis, Y.-L. Zhang and G. Anilkumar, J. Org. Chem., 2003, 68, 8061 CrossRef CAS PubMed; (f) J. C. Lanter, H. Chem, X. Zhang and Z. Sui, Org. Lett., 2005, 7, 5905 CrossRef CAS PubMed; (g) K. Nagata, K. Nishimura, M. Yokoya and T. Itoh, Heterocycles, 2006, 70, 335 CrossRef CAS; (h) X. Chen, J. Chen and J. Zhu, Synthesis, 2006, 4081 CAS; (i) F. A. Davis and M. Santhanaraman, J. Org. Chem., 2006, 71, 4222 CrossRef CAS PubMed; (j) S. G. Pyne, C. W. G. Au, A. S. Davis, I. R. Morgan, T. Ritthiwigrom and A. Yazici, Pure Appl. Chem., 2008, 80, 751 CAS.
- (a) W. J. Gottstein, W. F. Minor and L. C. Cheney, J. Am. Chem. Soc., 1959, 81, 1198 CrossRef CAS; (b) N. Neamati, H.-X. Hong, S. Sunder, G. W. A. Milne and Y. Pommier, Mol. Pharmacol., 1997, 52, 1041 CAS; (c) D. L. Nelson, A. Herbert, S. Bourgoin, J. Glowinski and M. Hamon, Mol. Pharmacol., 1978, 14, 983 CAS; (d) V. E. Frankus, E. Friderichs, S. Kim and G. Osterloh, Arzneim.-Forsch./Drug Res., 1978, 28, 114 Search PubMed; (e) S. Sortino, J. C. Scaiano, G. de Guidi and S. Monti, Photochem. Photobiol., 1999, 70, 549 CAS; (f) R. Quevedo and B. Moreno-Murillo, Tetrahedron Lett, 2009, 50, 936 CrossRef CAS; (g) A. Rivera and R. Quevedo, Tetrahedron Lett., 2004, 45, 8335 CrossRef CAS.
- For reviews, see: (a) S. K. Bur and S. F. Martin, Tetrahedron, 2001, 57, 3221 CrossRef CAS; (b) S. F. Martin, Acc. Chem. Res., 2002, 35, 895 CrossRef CAS PubMed; (c) G. Casiraghi, L. Battistini, C. Curti, G. Rassu and F. Zanardi, Chem. Rev., 2011, 111, 3076 CrossRef CAS PubMed.
- For selected examples, see: (a) T.-Y. Liu, H.-L. Cui, J. Long, B.-J. Li, Y. Wu, L.-S. Ding and Y.-C. Chen, J. Am. Chem. Soc., 2007, 129, 1878 CrossRef CAS PubMed; (b) M. Sickert and C. Schneider, Angew. Chem., Int. Ed., 2008, 47, 3631 CrossRef CAS PubMed; (c) D. S. Giera, M. Sickert and C. Chneider, Org. Lett., 2008, 10, 4259 CrossRef CAS PubMed; (d) M. Sickert, F. Abels, M. Lang, J. Sieler, C. Birkemeyer and C. Schneider, Chem. – Eur. J., 2010, 16, 2806 CrossRef CAS PubMed; (e) Y.-L. Guo, J.-F. Bai, L. Peng, L.-L. Wang, L.-N. Jia, X.-Y. Luo, F. Tian, X.-Y. Xu and L.-X. Wang, J. Org. Chem., 2012, 77, 8338 CrossRef CAS PubMed.
- For recent examples, see: (a) L. C. Wieland, E. M. Vieira, M. L. Snapper and A. H. Hoveyda, J. Am. Chem. Soc., 2009, 131, 570 CrossRef CAS PubMed; (b) M. Hayashi, M. Sano, Y. Funahashi and S. Nakamura, Angew. Chem., Int. Ed., 2013, 52, 5557 CrossRef CAS PubMed; (c) L. Yin, H. Takada, N. Kumagai and M. Shibasaki, Angew. Chem., Int. Ed., 2013, 52, 7310 CrossRef CAS PubMed; (d) Y.-L. Guo, Y. Zhang, L.-W. Qi, F. Tian and L.-X. Wang, RSC Adv., 2014, 4, 27286 RSC; (e) S. Nakamura, R. Yamaji and M. Hayashi, Chem. – Eur. J., 2015, 21, 9615 CrossRef CAS PubMed.
- For reviews, see: (a) J.-S. Yu, F. Zhou, Y.-L. Liu and J. Zhou, Synlett, 2015, 2491 CAS; (b) P. Chauhan and S. S. Chimni, Tetrahedron: Asymmetry, 2013, 24, 343 CrossRef CAS.
- For recent examples, see: (a) Y.-L. Liu, F. Zhou, J.-J. Cao, C.-B. Ji, M. Ding and J. Zhou, Org. Biomol. Chem., 2010, 8, 3847 RSC; (b) W.-J. Yan, D. Wang, J.-C. Feng, P. Li, D.-P. Zhao and R. Wang, Org. Lett., 2012, 14, 2512 CrossRef CAS PubMed; (c) J.-C. Feng, W.-J. Yan, D. Wang, P. Li, Q.-T. Sun and R. Wang, Chem. Commun., 2012, 48, 8003 RSC; (d) Y.-L. Liu and J. Zhou, Chem. Commun., 2013, 49, 4421 RSC; (e) Y.-H. Wang, Z.-Y. Cao, Y.-F. Niu, X.-L. Zhao and J. Zhou, Acta Chim. Sin., 2014, 72, 867 CrossRef CAS; (f) H.-F. Zheng, X.-H. Liu, C.-R. Xu, Y. Xia, L.-L. Lin and X.-M. Feng, Angew. Chem., Int. Ed., 2015, 54, 10958 CrossRef CAS PubMed; (g) J. Xu, C. Mou, T. Zhu, B.-A. Song and Y.-R. Chi, Org. Lett., 2014, 16, 3272 CrossRef CAS PubMed; (h) H.-M. Zhang, Z.-H. Gao and S. Ye, Org. Lett., 2014, 16, 3079 CrossRef CAS PubMed; (i) V. U. B. Rao, A. P. Jadhav, D. Garad and R. P. Singh, Org. Lett., 2014, 16, 648 CrossRef CAS PubMed; (j) Q.-T. Sun, X.-Y. Li, J.-H. Su, L. Zhao, M.-X. Ma, Y.-Y. Zhu, Y.-Y. Zhao, R.-R. Zhu, W.-J. Yan, K.-R. Wang and R. Wang, Adv. Synth. Catal., 2015, 357, 3187 CrossRef CAS; (k) M. Montesinos-Magraner, C. Vila, R. Canton, G. Blay, I. Fernandez, M. C. Munoz and J. R. Pedro, Angew. Chem., Int. Ed., 2015, 54, 6320 CrossRef CAS PubMed.
- For reviews, see: (a) F. Zhou, Y.-L. Liu and J. Zhou, Adv. Synth. Catal., 2010, 352, 1381 CrossRef CAS; (b) L. Hong and R. Wang, Adv. Synth. Catal., 2013, 355, 1023 CrossRef CAS; (c) G. S. Singh and Z. Y. Desta, Chem. Rev., 2012, 112, 6104 CrossRef CAS PubMed; (d) D. Cheng, Y. Ishihara, B. Tan and C. F. Barbas III, ACS Catal., 2014, 4, 743 CrossRef CAS.
- For selected examples, see: (a) J.-W. Xie, L. Yue, D. Xue, X.-L. Ma, Y.-C. Chen, Y. Wu, J. Zhu and J.-G. Deng, Chem. Commun., 2006, 14, 1563 RSC; (b) L. Jiang, H.-T. Zheng, T.-Y. Liu, L. Yue and Y.-C. Chen, Tetrahedron, 2007, 63, 5123 CrossRef CAS; (c) J. Lu, F. Liu and T.-P. Loh, Adv. Synth. Catal., 2008, 350, 1781 CrossRef CAS; (d) J. Lu, W.-J. Zhou, F. Liu and T.-P. Loh, Adv. Synth. Catal., 2008, 350, 1796 CrossRef CAS; (e) X.-F. Xiong, Z.-J. Jia, W. Du, K. Jiang, T.-Y. Liu and Y.-C. Chen, Chem. Commun., 2009, 6994 RSC; (f) J. Peng, X. Huang, H.-L. Cui and Y.-C. Chen, Org. Lett., 2010, 12, 4260 CrossRef CAS PubMed; (g) X.-L. Zhu, W.-J. He, L.-L. Yu, C.-W. Cai, Z.-L. Zuo, D.-B. Qin, Q.-Z. Liu and L.-H. Jing, Adv. Synth. Catal., 2012, 354, 2965 CrossRef CAS; (h) M.-R. Zhang and J.-L. Zhang, Chem. – Eur. J., 2014, 20, 399 CrossRef CAS PubMed.
- (a) G. Hostetler, D. Dunn, B. A. McKenna, K. Kopec and S. Chatterjee, Chem. Biol. Drug Des., 2014, 83, 149 CrossRef CAS PubMed; (b) M. D. Bartberger, A. Gonzalez Buenrostro, H. P. Beck, X.-Q. Chen, R. V. Connors, J. Deignan, J. Duquette, J. Eksterowicz, B. Fisher, and B. M. Fox, et al., Piperidinone derivatives as MDM2 inhibitors and their preparation and use for the treatment of cancer, Patent WO2011153509, 2011 Search PubMed.
- CCDC 1426979 contains the supplementary crystallographic data for compound 4a. For details, also see the ESI.†.
Footnotes |
| † Electronic supplementary information (ESI) available. CCDC 1426979. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6qo00038j |
| ‡ These authors contributed equally to this work. |
| This journal is © the Partner Organisations 2016 |