α-Regioselective [3 + 2] annulations with Morita–Baylis–Hillman carbonates of isatins and 2-nitro-1,3-enynes†
Guang-Yao
Ran
a,
Pan
Wang
a,
Wei
Du
a and
Ying-Chun
Chen
*ab
aKey Laboratory of Drug-Targeting and Drug Delivery System of the Ministry of Education, West China School of Pharmacy, Sichuan University, Chengdu 610041, China. E-mail: ycchen@scu.edu.cn; Fax: +86 28 85502609
bCollege of Pharmacy, Third Military Medical University, Chongqing 400038, China
First published on 16th May 2016
Abstract
An α-regioselective asymmetric [3 + 2] annulation reaction with Morita–Baylis–Hillman carbonates of isatins and (E)-2-nitro-1,3-enynes catalysed by cinchona-derived amines has been developed, providing spirooxindoles with contiguous chiral centres including adjacent quaternary ones in moderate to good yields with high enantioselectivity and excellent diastereoselectivity.
Nitroolefins are versatile electrophiles which widely serve as Michael acceptors, or as dienophiles, 1,3-dipoles, and heterodienes in a diversity of cycloaddition reactions.1 β-Monosubstituted nitroolefins are usually applied in asymmetric catalytic reactions owing to their ready availability and high reactivity. On the other hand, recent attention has also been paid to the application of more challenging α,β-disubstituted or even α,β,β-trisubstituted nitroolefins, to construct chiral quaternary stereogenic centres. Nevertheless, successful examples with high stereocontrol are still relatively limited.2
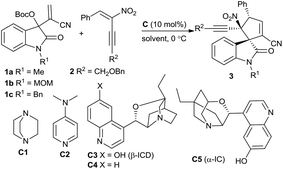
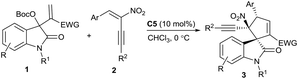
Over the past decade, the in situ generated zwitterionic allylic ylides between Morita–Baylis–Hillman (MBH) derivatives and a tertiary amine or phosphine have been utilised as 1,3-dipoles in an array of [3 + n] annulation reactions, furnishing variously structured carbo- or heterocycles efficiently.3 We successfully used β-monosubstituted nitroolefins in γ-regioselective [3 + 2] annulations with MBH carbonates of isatins under the catalysis of a chiral tertiary amine, providing chiral spirooxindoles incorporating a cyclopentadiene motif due to the easy elimination of a molecule of HNO2.4 Recently, we were able to switch the preferable γ-regioselective [3 + 2] annulations to α-regioselective versions by using activated olefins bearing bulky electron-withdrawing groups.5 Thus, we were interested in the application of the multifunctional α,β-disubstituted nitroolefins, (E)-2-nitro-1,3-enynes, which are readily available6 and have not been explored in asymmetric catalysis.7 Owing to the unique activation effect of the α-ethynyl group,8 their successful assembly with MBH carbonates of isatins might not only switch the previous γ-regioselectivity, but also possibly construct complex spirooxindoles possessing adjacent quaternary chiral centres,9 as outlined in Scheme 1.
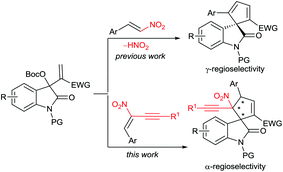 | ||
| Scheme 1 Switchable regioselective [3 + 2] annulations by applying differently substituted nitroolefins. | ||
The initial investigation with commonly used MBH carbonates of isatin and methyl acrylate4 furnished a sluggish and relatively complicated reaction; fortunately, the reaction of MBH carbonate 1a from isatin and acrylonitrile with α,β-disubstituted nitroolefin 2a proceeded very rapidly in CHCl3 at 0 °C catalysed by DABCO C1, giving the desired α-regioselective [3 + 2] annulation product 3a in a moderate yield after only 5 minutes (Table 1, entry 1). Extending the reaction time would diminish the yield due to the generation of a few side products. Nevertheless, DMAP C2 produced a mixture of products (entry 2). To our gratification, a chiral tertiary amine β-isocupreidine10 (β-ICD) C3 efficiently catalysed the reaction, affording 3a in a modest yield with excellent diastereoselectivity and moderate enantioselectivity (entry 3). The enantioselectivity was significantly reduced by using amine C4 without an OH group (entry 4). In addition, α-isocupreine11 (α-IC) C5 delivered product 3a having an opposite configuration with a slightly higher ee value (entry 5). Subsequently, a few solvents were screened under the catalysis of amine C5, while inferior results were generally obtained (entries 6–10).
| Entry | Cat. | 1 | Solvent | t (min) | Yieldb (%) | eec (%) |
|---|---|---|---|---|---|---|
a Unless otherwise noted, reactions were performed with MBH carbonate 1 (0.1 mmol), nitroolefin 2 (0.11 mmol), and amine C (10 mol%) in solvent (1 mL) at 0 °C.
b Isolated yield.
c Determined by chiral HPLC analysis; dr >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by 1H NMR analysis.
d At −20 °C.
e With 5 mol% of C5. 1 by 1H NMR analysis.
d At −20 °C.
e With 5 mol% of C5.
|
||||||
| 1 | C1 | 1a | CHCl3 | 5 | 3a, 68 | — |
| 2 | C2 | 1a | CHCl3 | 30 | — | — |
| 3 | C3 | 1a | CHCl3 | 25 | 3a, 72 | −72 |
| 4 | C4 | 1a | CHCl3 | 25 | 3a, 80 | −30 |
| 5 | C5 | 1a | CHCl3 | 30 | 3a, 74 | 80 |
| 6 | C5 | 1a | DCM | 26 | 3a, 74 | 66 |
| 7 | C5 | 1a | DCE | 540 | 3a, 82 | 80 |
| 8 | C5 | 1a | Toluene | 24 | 3a, 73 | 68 |
| 9 | C5 | 1a | THF | 40 | 3a, 67 | 60 |
| 10 | C5 | 1a | EtOAc | 90 | 3a, 65 | 63 |
| 11d | C5 | 1a | CHCl3 | 60 | 3a, 80 | 78 |
| 12 | C5 | 1b | CHCl3 | 18 | 3b, 71 | 80 |
| 13 | C5 | 1c | CHCl 3 | 25 | 3c , 88 | 87 |
| 14 | C3 | 1c | CHCl 3 | 26 | 3c , 71 | −92 |
| 15e | C5 | 1c | CHCl3 | 75 | 3c, 65 | 86 |
Furthermore, the enantioselectivity could not be improved by conducting the reaction in CHCl3 at lower temperature (entry 11). The same enantioselectivity was attained for product 3b by employing an N-MOM protected MBH carbonate 1b (entry 12); pleasingly, a better ee value was gained for product 3c bearing an N-benzyl group (entry 13), while β-ICD C3 produced 3c even in higher enantioselectivity, albeit in a lower yield (entry 14). Finally, reducing the catalyst loadings (5 mol%) resulted in a lower yield, while the enantioselectivity remained unchanged (entry 15).
Consequently, the scope of MBH carbonates 1 derived from isatins and (E)-2-nitro-1,3-enynes 2 was investigated under the catalysis of α-IC C5 in CHCl3 at 0 °C. In general, the tested reactions exhibited high reactivity, and the results are summarised in Table 2. At first, an array of MBH carbonates 1 bearing either electron-withdrawing or -donating substituents on different positions of the oxindole skeleton were investigated with nitroolefin 2a, generally affording the corresponding α-regioselective spirooxindole products 3d–3i in good yields with high to excellent enantioselectivity (Table 2, entries 2–7). On the other hand, a spectrum of (E)-2-nitro-1,3-enynes 2 with diverse 3-aryl groups were tested in reactions with MBH carbonate 1a as well. Higher ee values were observed for the more electron-rich substrates (entries 8–10). In contrast, moderate enantioselectivity was obtained for nitroolefins with electron-withdrawing substituents (entries 11–14), while the meta-substituted one seemed to have a beneficial effect on the enantiocontrol (entry 12). A high ee value was obtained for the 2-naphthyl-substituted nitroolefin (entry 15); however, very low reactivity was observed for the 2-thienyl-substituted substrate, and no desired product was isolated (entry 16). Moreover, the (E)-2-nitro-1,3-enyne by replacing BnOCH2– with a phenyl group showed lower reactivity, while good data were attained after 2 hours (entry 17). Unfortunately, the 4-alkyl-substituted substrate failed to give the desired product (entry 18).12 Finally, it was found that β-ICD C3 could promote the similar [3 + 2] annulation of a MBH carbonate from isatin and methyl acrylate with nitroolefin 2a in excellent enantioselectivity, though a modest yield was obtained after 48 hours (entry 19). Moreover, a few substrates were investigated catalysed by β-ICD C3, and the desired annulation products with an opposite configuration were obtained in moderate yields but with similar good enantioselectivity to those of α-IC C5 (data in the parentheses).
| Entry | R | Ar | t (min) | Yieldb (%) | eec (%) |
|---|---|---|---|---|---|
a Unless otherwise noted, reactions were performed with MBH carbonate 1 (0.1 mmol), nitroolefin 2 (0.11 mmol), and amine C5 (10 mol%) in CHCl3 (1 mL) at 0 °C; R1 = Bn, EWG = CN, R2 = BnOCH2–.
b Isolated yield.
c Based on chiral HPLC analysis; dr >19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 by 1H NMR analysis.
d R2 = Ph.
e R2 = nPr.
f R1 = Me, EWG = COOMe, with β-ICD C3 for 48 h. 1 by 1H NMR analysis.
d R2 = Ph.
e R2 = nPr.
f R1 = Me, EWG = COOMe, with β-ICD C3 for 48 h.
|
|||||
| 1 | H | Ph | 25 | 3c, 88 (71) | 87 (−92) |
| 2 | 5-Me | Ph | 25 | 3d, 83 | 86 |
| 3 | 5-MeO | Ph | 22 | 3e, 88 | 95 |
| 4 | 5-F | Ph | 9 | 3f, 72 | 86 |
| 5 | 5-Cl | Ph | 7 | 3g, 81 | 84 |
| 6 | 6-Br | Ph | 38 | 3h, 86 | 82 |
| 7 | 7-Cl | Ph | 6 | 3i, 81 (63) | 86 (−84) |
| 8 | H | 4-MeC6H4 | 6 | 3j, 85 | 86 |
| 9 | H | 2-MeOC6H4 | 5 | 3k, 91 (70) | 93 (−94) |
| 10 | H | 4-MeOC6H4 | 13 | 3l, 81 | 85 |
| 11 | H | 4-FC6H4 | 45 | 3m, 79 | 72 |
| 12 | H | 3-ClC6H4 | 13 | 3n, 78 | 87 |
| 13 | H | 4-ClC6H4 | 11 | 3o, 85 | 74 |
| 14 | H | 3-CF3C6H4 | 36 | 3p, 71 | 79 |
| 15 | H | 2-Naphthyl | 26 | 3q, 85 (65) | 90 (−90) |
| 16 | H | 2-Thienyl | 30 | — | — |
| 17d | H | Ph | 120 | 3r, 84 | 88 |
| 18e | H | Ph | 120 | — | — |
| 19f | H | Ph | 48 | 3s, 54 | −98 |
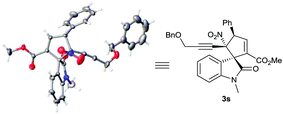
On the other hand, single crystals suitable for X-ray diffraction analysis were obtained from chiral product 3s catalysed by β-ICD C3, which unambiguously determined the α-regioselective annulation pattern and its absolute configuration, as illustrated in Scheme 2. Thus, the absolute configuration of other products catalysed by α-IC C5, which possesses an opposite configuration, could be assigned accordingly.
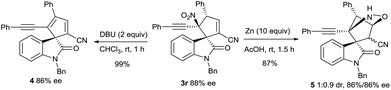
A spirocyclic oxindole 4 incorporating a cyclopentadiene motif was synthesised smoothly from product 3r through the DBU-promoted elimination of HNO2. The attempt to reduce the nitro group of 3r with Zn dust in AcOH generated an NHOH intermediate, from which an intramolecular O-Michael addition occurred to give a bridged heterocycle 5 in a poor diastereomeric ratio (Scheme 3).13
In conclusion, we have developed a highly efficient [3 + 2] annulation reaction of MBH carbonates derived from isatins with (E)-2-nitro-1,3-enynes catalysed by bifunctional tertiary amines from cinchona alkaloids. The reaction exhibited exclusive α-regioselectivity and usually completed in half an hour, constructing densely functionalised spirooxindoles possessing contiguous chiral centres including adjacent quaternary ones in moderate to excellent enantioselectivity. More results in regard to the application of MBH derivatives will be reported in due course.
We are grateful for the financial support from the National Natural Science Foundation of China (21125206, 21572135 and 21321061).
Notes and references
- For selected reviews, see: (a) T. Hashimoto and K. Maruoka, Chem. Rev., 2015, 115, 5366 CrossRef CAS PubMed; (b) M. P. Lalonde, Y. Chen and E. N. Jacobsen, Angew. Chem., Int. Ed., 2006, 45, 6366 CrossRef CAS PubMed; (c) D. M. Flanigan, F. Romanov-Michailidis, N. A. White and T. Rovis, Chem. Rev., 2015, 115, 9307 CrossRef CAS PubMed; (d) S. Lancianesi, A. Palmieri and M. Petrini, Chem. Rev., 2014, 114, 7108 CrossRef CAS PubMed; (e) S. E. Denmark and A. Thorarensen, Chem. Rev., 1996, 96, 137 CrossRef CAS PubMed; (f) A. Z. Halimehjani, I. N. N. Namboothiri and S. E. Hooshmand, RSC Adv., 2014, 4, 48022 RSC; (g) O. M. Berner, L. Tedeschi and D. Enders, Eur. J. Org. Chem., 2002, 1877 CrossRef CAS.
- For selected examples, see: (a) A. Monleón, F. Glaus, S. Vergura and K. A. Jørgensen, Angew. Chem., Int. Ed., 2016, 55, 2478 CrossRef PubMed; (b) J. P. Phelan, E. J. Patel and J. A. Ellman, Angew. Chem., Int. Ed., 2014, 53, 11329 CrossRef CAS PubMed; (c) J.-W. Xie, L.-P. Fan, H. Su, X.-S. Li and D.-C. Xu, Org. Biomol. Chem., 2010, 8, 2117 RSC; (d) Ł. Albrecht, G. Dickmeiss, F. C. Acosta, C. Rodríguez-Escrich, R. L. Davis and K. A. Jørgensen, J. Am. Chem. Soc., 2012, 134, 2543 CrossRef PubMed; (e) X. Hou, H. Ma, Z. Zhang, L. Xie, Z. Qin and B. Fu, Chem. Commun., 2016, 52, 1470 RSC; (f) F.-L. Liu, J.-R. Chen, B. Feng, X.-Q. Hu, L.-H. Ye, L.-Q. Lu and W.-J. Xiao, Org. Biomol. Chem., 2014, 12, 1057 RSC; (g) K. Mori, M. Wakazawa and T. Akiyama, Chem. Sci., 2014, 5, 1799 RSC.
- For selected reviews, see: (a) T.-Y. Liu, M. Xie and Y.-C. Chen, Chem. Soc. Rev., 2012, 41, 4101 RSC; (b) Y. Wei and M. Shi, Chem. Rev., 2013, 113, 6659 CrossRef CAS PubMed; (c) D. Basavaiah, A. J. Rao and T. Satyanarayana, Chem. Rev., 2003, 103, 811 CrossRef CAS PubMed; (d) P. Xie and Y. Huang, Org. Biomol. Chem., 2015, 13, 8578 RSC. For selected examples, see: (e) Y.-L. Liu, X. Wang, Y.-L. Zhao, F. Zhu, X.-P. Zeng, L. Chen, C.-H. Wang, X.-L. Zhao and J. Zhou, Angew. Chem., Int. Ed., 2013, 125, 13980 CrossRef; (f) N.-J. Zhong, F. Wei, Q.-Q. Xuan, L. Liu, D. Wang and Y.-J. Chen, Chem. Commun., 2013, 49, 11071 RSC; (g) J. Peng, X. Huang, L. Jiang, H.-L. Cui and Y.-C. Chen, Org. Lett., 2011, 13, 4584 CrossRef CAS PubMed; (h) F. Wei, H.-Y. Huang, N.-J. Zhong, C.-L. Gu, D. Wang and L. Liu, Org. Lett., 2015, 17, 1688 CrossRef CAS PubMed; (i) R. Chen, S. Xu, L. Wang, Y. Tang and Z. He, Chem. Commun., 2013, 49, 3543 RSC; (j) G. Zhan, M.-L. Shi, Q. He, W. Du and Y.-C. Chen, Org. Lett., 2015, 17, 4750 CrossRef CAS PubMed; (k) Y. Du, J. Feng and X. Lu, Org. Lett., 2005, 7, 1987 CrossRef CAS PubMed; (l) B. Tan, N. R. Candeias and C. F. Barbas III, J. Am. Chem. Soc., 2011, 133, 4672 CrossRef CAS PubMed; (m) F. Zhong, X. Han, Y. Wang and Y. Lu, Angew. Chem., Int. Ed., 2011, 123, 7983 CrossRef; (n) Y. Du, X. Lu and C. Zhang, Angew. Chem., Int. Ed., 2003, 42, 1035 CrossRef CAS PubMed; (o) D. Wang, Y. Wei and M. Shi, Chem. Commun., 2012, 48, 2764 RSC; (p) L. Zhang, H. Liu, G. Qiao, Z. Hou, Y. Liu, Y. Xiao and H. Guo, J. Am. Chem. Soc., 2015, 137, 4316 CrossRef CAS PubMed.
- J. Peng, G.-Y. Ran, W. Du and Y.-C. Chen, Synthesis, 2015, 2538 CAS.
- (a) K.-K. Wang, T. Jin, X. Huang, Q. Ouyang, W. Du and Y.-C. Chen, Org. Lett., 2016, 18, 872 CrossRef CAS PubMed; (b) G. Zhan, M.-L. Shi, Q. He, W.-J. Lin, Q. Ouyang, W. Du and Y.-C. Chen, Angew. Chem., Int. Ed., 2016, 55, 2147 CrossRef CAS PubMed.
- M. Ganesh and I. N. N. Namboothiri, Tetrahedron, 2007, 63, 11973 CrossRef CAS.
- (a) G. Bharathiraja, S. Sakthivel, M. Sengoden and T. Punniyamurthy, Org. Lett., 2013, 15, 4996 CrossRef CAS PubMed; (b) G. Bharathiraja, M. Sengoden, M. Kannan and T. Punniyamurthy, Org. Biomol. Chem., 2015, 13, 2786 RSC.
- S.-J. Min, G. O. Jones, K. N. Houk and S. J. Danishefsky, J. Am. Chem. Soc., 2007, 129, 10078 CrossRef CAS PubMed.
- For selected examples, see: (a) R. Alam, T. Vollgraff, L. Eriksson and K. J. Szabó, J. Am. Chem. Soc., 2015, 137, 11262 CrossRef CAS PubMed; (b) O. D. Engl, S. P. Fritz and H. Wennemers, Angew. Chem., Int. Ed., 2015, 54, 8193 CrossRef CAS PubMed; (c) J.-L. Li, B. Sahoo, C.-G. Daniliuc and F. Glorius, Angew. Chem., Int. Ed., 2014, 53, 10515 CrossRef CAS PubMed; (d) K. Ohmatsu, Y. Ando and T. Ooi, J. Am. Chem. Soc., 2013, 135, 18706 CrossRef CAS PubMed; (e) W.-T. Wei, C.-X. Chen, R.-J. Lu, J.-J. Wang, X.-J. Zhang and M. Yan, Org. Biomol. Chem., 2012, 10, 5245 RSC; (f) B. Tan, N. R. Candeias and C. F. Barbas III, Nat. Chem., 2011, 3, 473 CAS; (g) W.-B. Chen, Z.-J. Wu, J. Hu, L.-F. Cun, X.-M. Zhang and W.-C. Yuan, Org. Lett., 2011, 13, 2472 CrossRef CAS PubMed; (h) F. Pesciaioli, P. Righi, A. Mazzanti, G. Bartoli and G. Bencivenni, Chem. – Eur. J., 2011, 17, 2842 CrossRef CAS PubMed; (i) X. Huang, J. Peng, L. Dong and Y.-C. Chen, Chem. Commun., 2012, 48, 2439 RSC.
- (a) M. Shi and Y.-M. Xu, Angew. Chem., Int. Ed., 2002, 41, 4507 CrossRef CAS; (b) Y. Iwabuchi, M. Nakatani, N. Yokoyama and S. Hatakeyama, J. Am. Chem. Soc., 1999, 121, 10219 CrossRef CAS.
- Y. Nakamoto, F. Urabe, K. Takahashi, J. Ishihara and S. Hatakeyama, Chem. – Eur. J., 2013, 19, 12653 CrossRef CAS PubMed.
- α-Phenyl, vinyl or alkyl substituted nitroalkenes did not undergo the desired [3 + 2] annulation with the MBH carbonate 1c.
- For more details, see the ESI.†.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, structural proof, and CIF file of 3s. CCDC 1470348. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6qo00118a |
| This journal is © the Partner Organisations 2016 |