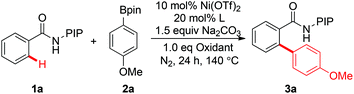Nickel(II)-catalyzed direct arylation of aryl C–H bonds with aryl-boron reagents directed by a removable bidentate auxiliary†
Bin
Liu
,
Zhuo-Zhuo
Zhang
,
Xin
Li
and
Bing-Feng
Shi
*
Department of Chemistry, Zhejiang University, Hangzhou 310027, China. E-mail: bfshi@zju.edu.cn
First published on 23rd May 2016
Abstract
Ni(II)-catalyzed C–H arylation using Ar2I+X− or ArX as the arylating reagent via oxidative addition has been well investigated. However, the analogous cross-coupling of C–H bonds with organoboron reagents via transmetallation remains a great challenge. Here we report the first Ni(II)-catalyzed direct C–H arylation with arylboronic acid esters assisted by a removable bidentate auxiliary. This procedure is scalable and compatible with a wide range of functional groups, providing a complementary protocol for the synthesis of biaryl compounds.
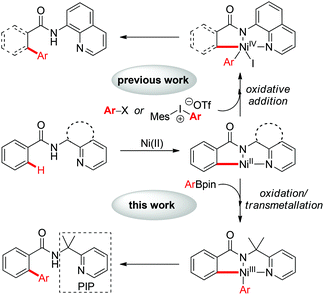
Over the past few decades, transition-metal catalyzed arylation of C(sp2)–H bonds has emerged as an attractive and complementary strategy to the traditional cross-coupling reactions for the synthesis of a range of functionalized biaryl compounds, in the fields of pharmaceutical, agrochemical and materials science.1 Meanwhile, the use of first-row transition metals such as nickel in the direct functionalization of unactivated C–H bonds has attracted much attention, owing to its relatively low-cost, abundancy and versatile reactivity.2 The seminal work by Chatani and co-workers showed the Ni(II)-catalyzed alkylation of unactivated C(sp2)–H bonds with alkyl iodides by the use of an 8-aminoquinoline bidentate directing group.3a Shortly after this, several Ni(II)-catalyzed C–H functionalization reactions were reported by Chatani,3 Ge,4 Ackermann,5 You,6 and others.7,8 The combination of nickel(II) catalysts and bidentate directing groups has shown superior reactivity in nickel-catalyzed auxiliary-assisted functionalization of unactivated C–H bonds. In this context, Ni(II)-catalyzed C–H arylation using ArX or diaryliodonium salts as the arylating reagent involving a Ni(II)/Ni(IV) catalytic cycle via oxidative addition has been well investigated (Scheme 1).3c,d However, Ni-catalyzed C–H arylation using organometallic reagents via transmetallation remained a huge challenge and has not been reported.9 Here we reported the first example of nickel-catalyzed C–H arylation with organoboron reagents directed by a removable PIP (2-pyridinylisopropyl) bidenate directing group. A putative Ni(II)/Ni(III) catalytic cycle via transmetallation was proposed. This reaction provides a complementary protocol for the synthesis of biaryl compounds.
Inspired by the seminal work of Daugulis and co-workers on the use of a bidentate, monoanionic auxiliary in the direct functionalization of C–H bonds,10,11 our group has developed a removable PIP bidentate auxiliary,12 which has shown to be effective in Ni-catalyzed direct functionalization of unactivated C–H bonds.8 As part of our ongoing research on base-metal catalyzed C–H functionalization with the assistance of a PIP auxiliary,8,13 we embarked on the synthesis of biaryl compounds via Ni(II)-catalyzed C–H arylation with arylboronic acid pinacol esters. A preliminary experiment was conducted on the coupling of benzamide 1a and phenylboronic acid pinacol ester 2a in the presence of catalytic Ni(OTf)2, Ag3PO4, Na2CO3 and PPh3 at 140 °C (Table 1). Encouragingly, the ortho-arylated product 3a was isolated in 85% yield (entry 1). After screening a number of phosphorus ligands, we found that PCy3, dppp and BINAP resulted in reduced yields (entries 2–4). The yield could be improved slightly when P(o-MeOC6H4)3 was used as a ligand (entry 5, 89%). The yield was reduced to 49% when Ag2CO3 was used as an oxidant (entry 6). Control experiments revealed that both Ag3PO4 and P(o-MeOC6H4)3 were crucial for the success of this reaction, since no desired product 3a was observed in the absence of Ag3PO4 or P(o-MeOC6H4)3 (entries 7 and 8). Finally, toluene was found to be the best solvent after the screening of different solvents (entries, 5, 9 and 10). The attempt to use arylboronic acid as the coupling partner gave only a trace amount of the desired product.
| Entry | Oxidant | Ligand | Solvent | Yieldb (%) |
|---|---|---|---|---|
a Reaction conditions: 1a (0.1 mmol), 2a (0.15 mmol), Ni(OTf)2 (10 mol%), Ag3PO4 (1.0 equiv.), ligand (20 mol%), in toluene (1.5 mL) at 140 °C for 24 h under N2.
b ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1H NMR yields using 1,3,5-trimethoxybenzene as the internal standard.
c Isolated yield. 1H NMR yields using 1,3,5-trimethoxybenzene as the internal standard.
c Isolated yield.
|
||||
| 1 | Ag3PO4 | PPh3 | Toluene | 85%c |
| 2 | Ag3PO4 | PCy3 | Toluene | 40% |
| 3 | Ag3PO4 | dppp | Toluene | 38% |
| 4 | Ag3PO4 | BINAP | Toluene | 72% |
| 5 | Ag 3 PO 4 | P(o-MeOC 6 H 4 ) 3 | Toluene | 89% |
| 6 | Ag2CO3 | P(o-MeOC6H4)3 | Toluene | 49%c |
| 7 | — | P(o-MeOC6H4)3 | Toluene | 0% |
| 8 | Ag3PO4 | — | Toluene | 0% |
| 9 | Ag3PO4 | P(o-MeOC6H4)3 | CH3CN | 0% |
| 10 | Ag3PO4 | P(o-MeOC6H4)3 | DMSO | 20% |
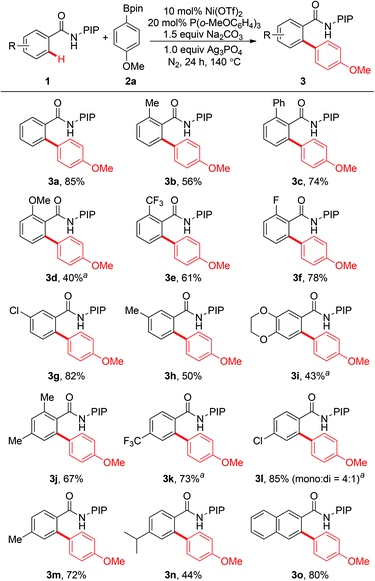
The scope of this reaction with respect to the substituted benzamides was examined under the optimized conditions. Benzamides bearing both electron-donating and electron-withdrawing groups were tolerated to give the desired biaryl products (Scheme 2). Benzamides with methyl, phenyl, methoxy, trifluoromethyl, and fluoro groups at the ortho-position gave the corresponding biaryl products in moderate to good yields (3b–3f). Electron-donating groups, such as methoxy and 1,4-dioxin, could be tolerated, and moderate yields were obtained (3d and 3i). This reaction was compatible with halogen atoms such as fluoro and chloro, which might be synthetically useful for further elaboration (3f and 3l). It was worth noting that meta-substituted benzamides exhibited excellent regioselectivities to give the biaryl products selectively at the less sterically hindered position (3g–3i). Moreover, arylation of 2-naphthamide also occurred at the less sterically hindered position to afford the corresponding product in 80% yield (3o). Benzamides with methyl and isopropyl groups at the para-position gave monoarylated products 3m and 3n exclusively; while p-chlorobenzamide 1l gave a mixture of mono- and diarylated products (3l, 85%, mono![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) di = 4
di = 4![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1).
1).
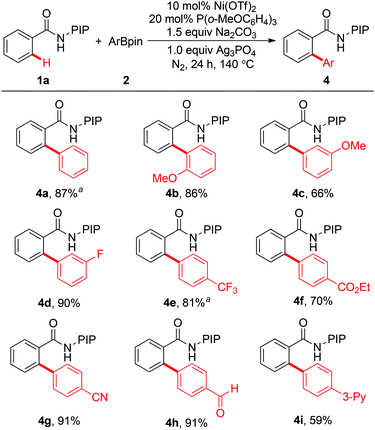
A variety of arylboronates were investigated as coupling partners using 1a as the substrate (Scheme 3). Generally, a wide range of arylboron reagents participate in the arylation reaction efficiently. Electron-withdrawing groups such as fluoro, trifluoromethyl, cyano, formyl, and ethyoxycarbonyl are compatible with the protocol, providing the coupling products in 70–91% yields (4d–4h). In particular, the formyl group was well tolerated, which could be used as a versatile handle for further transformations. Arylboronates bearing electron-donating groups in the aromatic ring also reacted effectively with 1a to give the desired biaryl products in good yields (4b and 4c). It is worth noting that arylboronates with a pyridyl group could also work, albeit in moderate yield (4i, 59%).
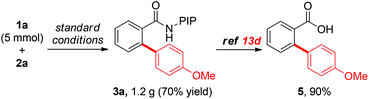
To evaluate the efficiency and practicality of this reaction further, a 5-mmol scale reaction was conducted using 1a as the substrate and the reaction proceeded smoothly to furnish product 3a in good yield (Scheme 4, 70% yield, 1.2 g of 3a). Moreover, the PIP directing group could be easily removed using a previously reported protocol.13d
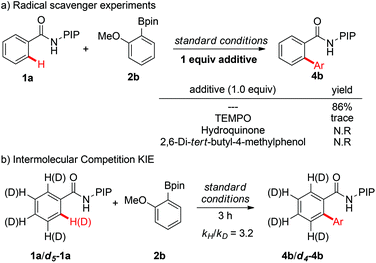
To gain more insight into the mechanism of the C–H arylation reactions, a series of experiments were conducted (Scheme 5). First, the addition of 1 equivalent of radical inhibitors, such as TEMPO, hydroquinone, or 2,6-di-tert-butyl-4-methylphenol, inhibited the reaction completely, which indicated that a single-electron transfer (SET) mechanism might be involved in the reaction (see the ESI† for details). Next, the intermolecular kinetic isotope effect (KIE) was determined to be 3.2, indicating that C–H bond cleavage might be the rate-determining step of the catalytic cycle.
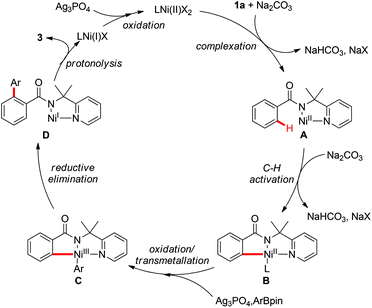
On the basis of these observations and earlier precedents,3,9i,14 a putative reaction pathway was proposed as shown in Scheme 6. First, the coordination of amide 1a to the Ni(II) species followed by C–H cleavage gives the Ni(II)–aryl complex B, which is oxidized to the Ni(III) species followed by transmetallation to generate Ni(III) intermediate C. Subsequent reductive elimination of the intermediate C followed by protonolysis delivers the final arylated product 3 with the formation of a Ni(I) species. The catalytic cycle is closed by the reoxidation of Ni(I) to Ni(II) by Ag3PO4.
Conclusions
In conclusion, we have developed a new catalytic system for Ni(II)-catalyzed C–H arylation of benzamides with the assistance of a removable bidentate auxiliary. This transformation represents the first example of organoboron reagents applied to nickel-catalyzed C–H arylation reactions through an otherwise difficult transmetallation pathway. This finding might pave the way for developing nickel-catalyzed C–H arylation with organometallic reagents through Ni(II)/Ni(III) catalysis.Acknowledgements
Financial support from the National Basic Research Program of China (2015CB856600), the NSFC (21572201, 21422206, 21272206) and the Fundamental Research Funds for the Central Universities (2015XZZX004-03) is gratefully acknowledged.Notes and references
- For selected reviews on transition-metal-catalyzed C–H arylation, see: (a) L. Ackermann, J. Org. Chem., 2014, 79, 8948 CrossRef PubMed; (b) F. Shibahara and T. Murai, Asian J. Org. Chem., 2013, 2, 624 CrossRef; (c) P. Arockiam, B. C. Bruneau and P. H. Dixneuf, Chem. Rev., 2012, 112, 5879 CrossRef PubMed; (d) J. Yamaguchi, A. D. Yamaguchi and K. Itami, Angew. Chem., Int. Ed., 2012, 51, 8960 CrossRef PubMed; (e) C. Liu, H. Zhang, W. Shi and A. Lei, Chem. Rev., 2011, 111, 1780 CrossRef PubMed; (f) T. W. Lyons and M. S. Sanford, Chem. Rev., 2010, 110, 1147 CrossRef PubMed; (g) L. Ackermann, R. Vicente and A. Kapdi, Angew. Chem., Int. Ed., 2009, 48, 9792 CrossRef PubMed; (h) O. Daugulis, H.-Q. Do and D. Shabashov, Acc. Chem. Res., 2009, 42, 1074 CrossRef PubMed; (i) X. Chen, K. M. Engle, D.-H. Wang and J.-Q. Yu, Angew. Chem., Int. Ed., 2009, 48, 5094 CrossRef PubMed; (j) B. Li, S. Yang and Z. Shi, Synlett, 2008, 949 Search PubMed; (k) I. V. Seregin and V. Gevorgyan, Chem. Soc. Rev., 2007, 36, 1173 RSC.
- For selected reviews, see: (a) B.-B. Zhan, B. Liu, F. Hu and B.-F. Shi, Chin. Sci. Bull., 2015, 60, 2907 CrossRef; (b) L. C. M. Castro and N. Chatani, Chem. Lett., 2015, 44, 410 CrossRef; (c) J. Yamaguchi, K. Muto and K. Itami, Eur. J. Org. Chem., 2013, 19 CrossRef.
- (a) Y. Aihara and N. Chatani, J. Am. Chem. Soc., 2013, 135, 5308 CrossRef PubMed; (b) Y. Aihara and N. Chatani, J. Am. Chem. Soc., 2014, 136, 898 CrossRef PubMed; (c) M. Iyanaga, Y. Aihara and N. Chatani, J. Org. Chem., 2014, 79, 11933 CrossRef PubMed; (d) A. Yokota, Y. Aihara and N. Chatani, J. Org. Chem., 2014, 79, 11922 CrossRef PubMed; (e) Y. Aihara, M. Tobisu, Y. Fukumoto and N. Chatani, J. Am. Chem. Soc., 2014, 136, 15509 CrossRef PubMed; (f) A. Yokota and N. Chatani, Chem. Lett., 2015, 44, 902 CrossRef; (g) Y. Aihara, J. Wuelbern and N. Chatani, Bull. Chem. Soc. Jpn., 2015, 88, 438 CrossRef; (h) L. C. M. Castro, A. Obata, Y. Aihara and N. Chatani, Chem. – Eur. J., 2016, 22, 1362 CrossRef PubMed; (i) T. Uemura, M. Yamaguchi and N. Chatani, Angew. Chem., Int. Ed., 2016, 55, 3162 CrossRef PubMed; (j) T. Kubo and N. Chatani, Org. Lett., 2016, 18, 1698 CrossRef PubMed.
- (a) X. Wu, Y. Zhao and H. Ge, J. Am. Chem. Soc., 2014, 136, 1789 CrossRef PubMed; (b) X. Wu, Y. Zhao and H. Ge, Chem. – Eur. J., 2014, 20, 9530 CrossRef PubMed; (c) X. Wu, Y. Zhao and H. Ge, J. Am. Chem. Soc., 2015, 137, 4924 CrossRef PubMed.
- (a) W. Song, S. Lackner and L. Ackermann, Angew. Chem., Int. Ed., 2014, 53, 2477 CrossRef PubMed; (b) Z. Ruan, S. Lacker and L. Ackermann, Angew. Chem., Int. Ed., 2016, 55, 3153 CrossRef PubMed.
- (a) M. Li, J. Dong, X. Huang, K. Li, Q. Wu, F. Song and J. You, Chem. Commun., 2014, 50, 3944 RSC; (b) M. Li, Y. Yang, D. Zhou, D. Wan and J. You, Org. Lett., 2015, 17, 2546 CrossRef CAS PubMed.
- (a) X. Cong, Y. Li, Y. Wei and X. Zeng, Org. Lett., 2014, 16, 3926 CrossRef CAS PubMed; (b) C. Liu, W. Yu, J. Yao, B. Wang, Z. Liu and Y. Zhang, Org. Lett., 2015, 17, 1340 CrossRef PubMed; (c) C. Lin, D. Li, B. Wang, J. Yao and Y. Zhang, Org. Lett., 2015, 17, 1328 CrossRef CAS PubMed; (d) X. Wang, L. Zhu, S. Chen, X. Xu, C.-T. Au and R. Qiu, Org. Lett., 2015, 17, 5228 CrossRef CAS PubMed; (e) X. Wang, C. Yan, V. P. Reddy, L. Zhu, X. Xu and S.-F. Yin, Org. Lett., 2015, 17, 1970 CrossRef CAS PubMed; (f) X. Ye, J. L. Petersen and X. Shi, Chem. Commun., 2015, 51, 7863 RSC; (g) V. P. Reddy, R. Qiu, T. Iwasakia and N. Kambe, Org. Biomol. Chem., 2015, 13, 6803 RSC; (h) J. Yi, L. Yang, C. Xia and F. Li, J. Org. Chem., 2015, 80, 6213 CrossRef CAS PubMed; (i) S. Maity, S. Agasti, A. M. Earsad, A. Hazra and D. Maiti, Chem. – Eur. J., 2015, 21, 11320 CrossRef CAS PubMed; (j) Q. Yan, Z. Chen, W. Yu, H. Yin, Z. Liu and Y. Zhang, Org. Lett., 2015, 17, 2482 CrossRef CAS PubMed; (k) V. G. Landge, C. H. Shewale, G. Jaiswal, M. K. Sahoo, S. P. Midya and E. Balaraman, Catal. Sci. Technol., 2016, 6, 1946 RSC.
- (a) S.-Y. Yan, Y.-J. Liu, B. Liu, Y.-H. Liu and B.-F. Shi, Chem. Commun., 2015, 51, 4069 RSC; (b) Y.-J. Liu, Y.-H. Liu, S.-Y. Yan and B.-F. Shi, Chem. Commun., 2015, 51, 6388 RSC; (c) S.-Y. Yan, Y.-J. Liu, B. Liu, Y.-H. Liu, Z.-Z. Zhang and B.-F. Shi, Chem. Commun., 2015, 51, 7341 RSC; (d) Y.-J. Liu, Z.-Z. Zhang, S.-Y. Yan, Y.-H. Liu and B.-F. Shi, Chem. Commun., 2015, 51, 7899 RSC; (e) Y.-H. Liu, Y.-J. Liu, S.-Y. Yan and B.-F. Shi, Chem. Commun., 2015, 51, 11650 RSC; (f) B.-B. Zhan, Y.-H. Liu, F. Hu and B.-F. Shi, Chem. Commun., 2016, 52, 4934 RSC.
- For selected examples of C–H arylation with organoboron reagents, see: (a) F. Kakiuchi, S. Kan, K. Igi, N. Chatani and S. Murai, J. Am. Chem. Soc., 2003, 125, 1698 CrossRef CAS PubMed; (b) X. Chen, C. E. Goodhue and J.-Q. Yu, J. Am. Chem. Soc., 2006, 128, 12634 CrossRef CAS PubMed; (c) D.-H. Wang, M. Wasa, R. Giri and J.-Q. Yu, J. Am. Chem. Soc., 2008, 130, 7190 CrossRef CAS PubMed; (d) J. Karthikeyan, R. Haridharan and C.-H. Cheng, Angew. Chem., Int. Ed., 2012, 51, 12343 CrossRef CAS PubMed; (e) T. Nishikata, A. R. Abela, S. Huang and B. H. Lipshutz, J. Am. Chem. Soc., 2010, 132, 4978 CrossRef CAS PubMed; (f) T. Vogler and A. Studer, Org. Lett., 2008, 10, 129 CrossRef CAS PubMed; (g) F. Kakiuchi, Y. Matsuura, S. Kan and N. Chatani, J. Am. Chem. Soc., 2005, 127, 5936 CrossRef CAS PubMed; (h) H. Li, W. Wei, Y. Xu, C. Zhang and X. Wan, Chem. Commun., 2011, 47, 1497 RSC; (i) M. Shang, S.-Z. Sun, H.-X. Dai and J.-Q. Yu, Org. Lett., 2014, 16, 5666 CrossRef CAS PubMed.
- (a) V. G. Zaitsev, D. Shabashov and O. Daugulis, J. Am. Chem. Soc., 2005, 127, 13154 CrossRef CAS PubMed; (b) D. Shabashov and O. Daugulis, J. Am. Chem. Soc., 2010, 132, 3965 CrossRef CAS PubMed.
- For representative reviews, see: (a) G. Rouquet and N. Chatani, Angew. Chem., Int. Ed., 2013, 52, 11726 CrossRef CAS PubMed; (b) M. Corbet and F. De Campo, Angew. Chem., Int. Ed., 2013, 52, 9896 CrossRef CAS PubMed; (c) B. Zhang, H.-X. Guan, B. Liu and B.-F. Shi, Chin. J. Org. Chem., 2014, 34, 1487 CrossRef CAS; (d) O. Daugulis, J. Roane and L. D. Tran, Acc. Chem. Res., 2015, 48, 1063 CrossRef PubMed; (e) R. K. Rit, R. Yadav, K. Ghosh and A. K. Sahoo, Tetrahedron, 2015, 71, 4450 CrossRef CAS.
- (a) F.-J. Chen, S. Zhao, F. Hu, K. Chen, Q. Zhang, S.-Q. Zhang and B.-F. Shi, Chem. Sci., 2013, 4, 4187 RSC; (b) Q. Zhang, K. Chen, W.-H. Rao, Y.-J. Zhang, F.-J. Chen and B.-F. Shi, Angew. Chem., Int. Ed., 2013, 52, 13588 CrossRef CAS PubMed.
- (a) X. Li, Y.-H. Liu, W.-J. Gu, B. Li, F.-J. Chen and B.-F. Shi, Org. Lett., 2014, 16, 3904 CrossRef CAS PubMed; (b) Y.-J. Liu, Y.-H. Liu, X.-S. Yin, W.-J. Gu and B.-F. Shi, Chem. – Eur. J., 2015, 21, 205 CrossRef CAS PubMed; (c) X.-S. Yin, Y.-C. Li, J. Yuan, W.-J. Gu and B.-F. Shi, Org. Chem. Front., 2015, 2, 119 RSC; (d) S. Zhao, Y.-J. Liu, S.-Y. Yan, F.-J. Chen, Z.-Z. Zhang and B.-F. Shi, Org. Lett., 2015, 17, 3338 CrossRef CAS PubMed.
- H. Tang, B. Zhou, X.-R. Huang, C. Wang, J. Yao and H. Chen, ACS Catal., 2014, 4, 649 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c6qo00149a |
| This journal is © the Partner Organisations 2016 |