Iron-catalyzed trifluoromethylation of vinylcyclopropanes: facile synthesis of CF3–containing dihydronaphthalene derivatives†
Zong-Rui
Li
a,
Xing-Xing
Bao
b,
Jian
Sun
a,
Jing
Shen
a,
Dun-Qi
Wu
a,
Yan-Kai
Liu
a,
Qing-Hai
Deng
*a and
Feng
Liu
*b
aThe Education Ministry Key Lab of Resource Chemistry and Shanghai Key Laboratory of Rare Earth Functional Materials, Shanghai Normal University, Shanghai 200234, P. R. China. E-mail: qinghaideng@hotmail.com
bJiangsu Key Laboratory of Translational Research and Therapy for Neuro-Psycho-Diseases and Department of Medicinal Chemistry, College of Pharmaceutical Sciences, Soochow University, 199 Ren-Ai Road, Suzhou, Jiangsu 215123, People's Republic of China. E-mail: fliu2@suda.edu.cn
First published on 23rd May 2016
Abstract
An efficient Fe(II)-catalyzed trifluoromethylation of vinylcyclopropanes was successfully developed. A series of CF3-containing dihydronaphthalene derivatives were prepared in moderate to high yields (up to 96%) under mild reaction conditions using the Togni reagent as the CF3 source.
Introduction
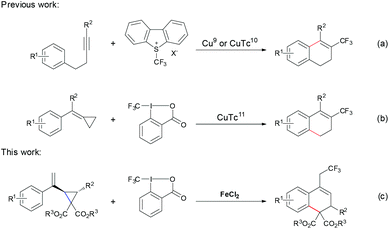
Molecules bearing trifluoromethyl group(s), with remarkable biological activity, high hydrophobicity and metabolic stability, have been widely applied in the pharmaceutical and agrochemical industries.1 Thus, during the past few decades, great effort has been devoted to the development of broadly applicable methods for constructing C–CF3 bonds.2 Among the transition metal catalysts, copper3,4 and palladium5 complexes have been shown to be quite efficient for the construction of the C–CF3 bond. In sharp contrast, iron, which is a widely abundant and environmentally benign transition metal, remains less explored in this area and only a few examples have been reported.6 After Yamakawa6a in 2008 first reported an iron catalyzed trifluoromethylation of uracil, Gillaizeau6b developed an iron catalyzed trifluoromethylation of enamides. Notably, Buchwald's group6c achieved a significant breakthrough by using FeCl2 as the catalyst to realize the trifluoromethylation of potassium vinyltrifluoroborates. Moreover, the groups of Plietker,6d Sodeoka6e and Shi6f demonstrated that iron salts are capable of catalyzing the trifluoromethylation of carbonyl compounds, alkenes and alkylidenecyclopropanes to generate the corresponding trifluoromethylated products. Thus, in terms of sustainability, the discovery and development of iron catalysts for trifluoromethylation are still highly required.Dihydronaphthalenes represent an important type of structural motif in many biologically active molecules and useful building blocks in organic synthesis.7 Consequently, many strategies have been developed for their synthesis.8 However, incorporation of the CF3 group into dihydronaphthalenes has rarely been reported. In 2014, Fu9 and Xiao10 developed copper-catalyzed trifluoromethylation of internal alkynes to generate CF3-containing dihydronaphthalenes, respectively (Scheme 1, eqn (a)). Very recently, Shi reported copper-catalyzed trifluoromethylation of methylenecyclopropanes resulting in CF3-substituted dihydronaphthalenes11 (Scheme 1, eqn (b)). Herein, we report the iron catalyzed trifluoromethylation of vinyl cyclopropanes, which includes the radical initiated ring opening and the subsequent intramolecular cyclization, to generate CF3-containing dihydronaphthalenes with moderate to good yields under mild conditions.
Results and discussion
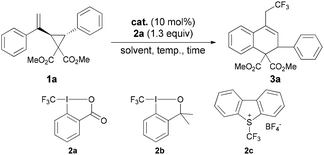
At the outset, we chose the reaction of vinyl cyclopropane 1a catalyzed by CuCl as the model reaction to investigate different kinds of CF3 reagents and found Togni's reagent 2a![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 12 to be the most suitable CF3 source to obtain the desired product 3a with 71% yield (Table 1, entries 1–3). It should be noted that FeCl2 gave the product in higher yield and in a considerably shorter reaction time compared with the Cu catalyst (entry 1 vs. 4). To avoid the interference of the Lewis acid character of FeCl2, other Lewis acids such as AlCl3, Sc(OTf)3, ZnCl2 and CaCl2 were tested to give no conversion (entries 5–8). Next, solvent screening showed that DCE, CH2Cl2 and CHCl3 were suitable for this reaction (entries 4 and 9–14), and CH2Cl2 was identified as the best choice to give 82% yield at 40 °C only in 10 minutes. A comparable result (84% yield in 10 minutes) was obtained in the presence of ultrapure FeCl2, thus suggesting that the catalyst system is iron based (entry 15).13,6b,c However, performing the reaction at room temperature led to a prolonged reaction time to 2.5 hours (entry 16). Reducing the catalyst loading to 5 mol% resulted in a lower yield (entry 17). Based on these optimization experiments, entry 13 was identified as the optimal conditions for the substrate scope investigations.
12 to be the most suitable CF3 source to obtain the desired product 3a with 71% yield (Table 1, entries 1–3). It should be noted that FeCl2 gave the product in higher yield and in a considerably shorter reaction time compared with the Cu catalyst (entry 1 vs. 4). To avoid the interference of the Lewis acid character of FeCl2, other Lewis acids such as AlCl3, Sc(OTf)3, ZnCl2 and CaCl2 were tested to give no conversion (entries 5–8). Next, solvent screening showed that DCE, CH2Cl2 and CHCl3 were suitable for this reaction (entries 4 and 9–14), and CH2Cl2 was identified as the best choice to give 82% yield at 40 °C only in 10 minutes. A comparable result (84% yield in 10 minutes) was obtained in the presence of ultrapure FeCl2, thus suggesting that the catalyst system is iron based (entry 15).13,6b,c However, performing the reaction at room temperature led to a prolonged reaction time to 2.5 hours (entry 16). Reducing the catalyst loading to 5 mol% resulted in a lower yield (entry 17). Based on these optimization experiments, entry 13 was identified as the optimal conditions for the substrate scope investigations.
| Entry | Cat. | Solvent | Temp. (°C) | Time | Yieldb (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 1a (0.30 mmol), 2a (1.3 equiv), catalyst (10 mol%), and solvent (2 mL) were used. b The yields of isolated products. c 2a was replaced by 2b. d 2a was replaced by 2c. e No reaction. f Ultrapure FeCl2 (99.998% based on trace metals, Sigma-Aldrich) was used. g 5 mol% of FeCl2 was used. | |||||
| 1 | CuCl | DCE | 60 | 75 min | 71 |
| 2c | CuCl | DCE | 60 | 15 h | 63 |
| 3d | CuCl | DCE | 60 | 15 h | NRe |
| 4 | FeCl2 | DCE | 60 | 10 min | 80 |
| 5 | AlCl3 | DCE | 60 | 24 h | NRe |
| 6 | Sc(OTf)3 | DCE | 60 | 24 h | NRe |
| 7 | ZnCl2 | DCE | 60 | 24 h | NRe |
| 8 | CaCl2 | DCE | 60 | 24 h | NRe |
| 9 | FeCl2 | DMSO | 60 | 24 h | 10 |
| 10 | FeCl2 | THF | 60 | 24 h | 60 |
| 11 | FeCl2 | MeCN | 60 | 24 h | NRe |
| 12 | FeCl2 | Toluene | 60 | 24 h | 40 |
| 13 | FeCl2 | DCM | 40 | 10 min | 82 |
| 14 | FeCl2 | CHCl3 | 40 | 40 min | 80 |
| 15f | FeCl2 | DCM | 40 | 10 min | 84 |
| 16 | FeCl2 | DCM | 25 | 2.5 h | 79 |
| 17g | FeCl2 | DCM | 40 | 12 h | 50 |
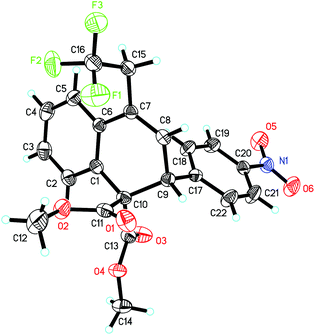
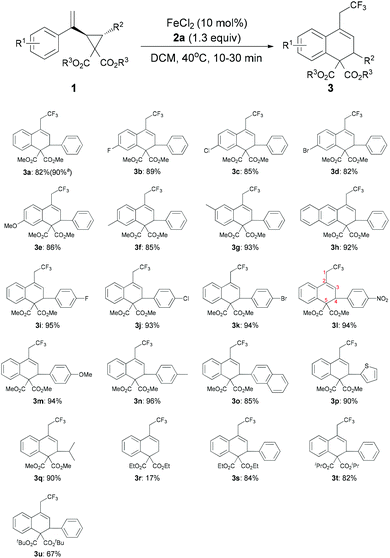
The scope and limitations of substrates were investigated as shown in Table 2. The substituent effect on the 2-phenyl ring (R1) was firstly probed. Both electron-withdrawing and -donating groups at the para-position of the phenyl ring led to the desired products (3b–3f) in good yields (82%–89%). For substrate 1g bearing a methyl group at the meta-position, the desired product 3g could be obtained as the sole product in 93% yield. 1,2-Dihydroanthracene derivative 3h was also obtained in 92% yield. We then screened the general scope of R2 at the cyclopropane ring. As for substrates 1i–1n, in which the R2 groups are a range of para-substituted phenyl groups, the corresponding products 3i–3n were formed in high yields (92–96%). The molecular structure of 3l, established by X-ray diffraction, confirmed the ring opening of the cyclopropane unit, which was carried out between C3 and C5 regioselectively (Fig. 1). Though the reason for the high regioselectivity of the cyclopropane ring opening in this system is unclear, we proposed that FeCl2 may also coordinate with the two ester groups to assist C3–C5 bond breaking.14 Notably, 2-naphthyl (3o), 2-thienyl (3p) and isopropyl (3q) groups were also tested in the reaction and afforded the desired products in high yields. However, substrate 1r (R2 = H) was only converted into the corresponding product 3r in a relatively low yield (17%). In addition, we also investigated the influence of the ester substituent (R3). Upon going from 3s to 3t, the bulkier substituent was found to reduce the reactivity, with the tert-butyl ester being converted to the product 3u in 67% yield. Remarkably, the reaction could be scaled up to gram quantities smoothly and provided 3a in 90% yield.
| a 3.30 mmol of substrate 1a was used. |
|---|
|
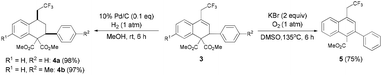
To highlight the synthetic utility of this method, we undertook further transformations of the resulting dihydronaphthalenes (Scheme 2). The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C bond can easily undergo hydrogenation15 to form the corresponding tetrahydronaphthalene products 4 with the cis configuration (determination by using the NOESY spectrum, see the ESI†) in excellent yields. Successive decarboxylation and autoxidation16 of 3a gave the naphthalene 5 in 75% yield.
C bond can easily undergo hydrogenation15 to form the corresponding tetrahydronaphthalene products 4 with the cis configuration (determination by using the NOESY spectrum, see the ESI†) in excellent yields. Successive decarboxylation and autoxidation16 of 3a gave the naphthalene 5 in 75% yield.
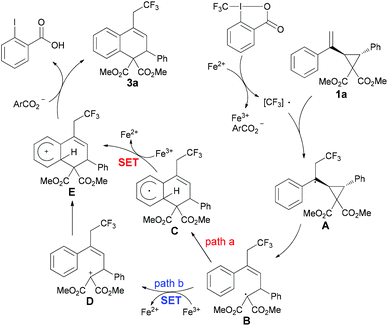
Based on the above results and previous related reports,6b,11,17 a plausible mechanism for this catalytic reaction is proposed in Scheme 3. In the initial step, the Togni reagent 2a reacts with FeCl2 to generate the CF3 radical, followed by a reaction with substrate 1a to give the radical intermediate A, which undergoes a ring-opening process to form the radical intermediate B. Subsequently, there are two possible ways for the formation of cationic intermediate E from B. In path a, radical B undergoes radical addition to obtain intermediate C, which would be oxidized by Fe(III) to afford cation E. An alternative reaction pathway involving the formation of cation D through single-electron oxidation from Fe(III) species, followed by a subsequent Friedel–Crafts reaction also resulting in intermediate E, could not be excluded at the present stage (path b). Finally, E could further undergo deprotonation to furnish the desired product 3a.
Conclusions
In conclusion, we have successfully developed an efficient Fe-catalyzed trifluoromethylation of vinylcyclopropanes and subsequent intramolecular cyclization for the direct synthesis of CF3-containing dihydronaphthalenes under mild conditions. The reaction can be readily scaled up to gram amounts and the products 3 can be converted into the corresponding tetranaphthalenes or naphthalene by further hydrogenation or decarboxylation/oxidation, respectively. Further studies on mechanistic investigations and the applications of this protocol are ongoing in our group.Acknowledgements
Q.-H. Deng is grateful for the generous financial support from the Program of the Professor of Special Appointment (Eastern Scholar) at Shanghai Institutions of Higher Learning, the National Natural Science Foundation of China (no. 21402119), and the Shanghai Rising-Star Program (no. 16QA1403100). F. Liu is grateful for the financial support from the National Natural Science Foundation of China (no. 21302134), the Natural Science Foundation of Jiangsu (no. BK20130338), and the Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD).References
- (a) J. Wang, M. Sánchez-Roselló, J. L. Aceña, C. del Pozo, A. E. Sorochinsky, S. Fustero, V. A. Soloshonok and H. Liu, Chem. Rev., 2014, 114, 2432–2506 CrossRef CAS PubMed; (b) S. Purser, P. R. Moore, S. Swallow and V. Gouverneur, Chem. Soc. Rev., 2008, 37, 320–330 RSC; (c) K. L. Kirk, Org. Process Res. Dev., 2008, 12, 305–321 CrossRef CAS; (d) K. Müller, C. Faeh and F. Diederich, Science, 2007, 317, 1881–1886 CrossRef PubMed; (e) M. Schlosser, Angew. Chem., Int. Ed., 2006, 45, 5432–5446 CrossRef CAS PubMed.
- For selected reviews, see: (a) Y. Li, D. P. Hari, M. V. Vita and J. Waser, Angew. Chem., Int. Ed., 2016, 55, 4436–4454 CrossRef CAS PubMed; (b) X. Yang, T. Wu, R. J. Phipps and F. D. Toste, Chem. Rev., 2015, 115, 826–870 CrossRef CAS PubMed; (c) X.-H. Xu, K. Matsuzaki and N. Shibata, Chem. Rev., 2015, 115, 731–764 CrossRef CAS PubMed; (d) C. Ni, M. Hu and J. Hu, Chem. Rev., 2015, 115, 765–825 CrossRef CAS PubMed; (e) X. Liu, C. Xu, M. Wang and Q. Liu, Chem. Rev., 2015, 115, 683–730 CrossRef CAS PubMed; (f) J. Charpentier, N. Frueh and A. Togni, Chem. Rev., 2015, 115, 650–682 CrossRef CAS PubMed; (g) C. Alonso, E. Martinez de Marigorta, G. Rubiales and F. Palacios, Chem. Rev., 2015, 115, 1847–1935 CrossRef CAS PubMed; (h) H. Egami and M. Sodeoka, Angew. Chem., Int. Ed., 2014, 53, 8294–8308 CrossRef CAS PubMed; (i) L. Chu and F.-L. Qing, Acc. Chem. Res., 2014, 47, 1513–1522 CrossRef CAS PubMed; (j) P. Chen and G. Liu, Synthesis, 2013, 2919–2939 CAS; (k) T. Furuya, A. S. Kamlet and T. Ritter, Nature, 2011, 473, 470–477 CrossRef CAS PubMed; (l) O. A. Tomashenko and V. V. Grushin, Chem. Rev., 2011, 111, 4475–4521 CrossRef CAS PubMed; (m) J.-A. Ma and D. Cahard, Chem. Rev., 2008, 108, PR1–PR43 CrossRef CAS PubMed.
- For selected reviews on copper catalyzed trifluoromethylation, see: (a) X. Liu and X. Wu, Synlett, 2013, 1882–1886 CAS; (b) T. Liu and Q. Shen, Eur. J. Org. Chem., 2012, 6679–6687 CrossRef CAS.
- For selected recent examples of copper catalyzed trifluoromethylation, see: (a) Z. He, P. Tan and J. Hu, Org. Lett., 2016, 18, 72–75 CrossRef CAS PubMed; (b) F. Wang, N. Zhu, P. Chen, J. Ye and G. Liu, Angew. Chem., Int. Ed., 2015, 54, 9356–9360 CrossRef CAS PubMed; (c) L. Huang, S.-C. Zheng, B. Tan and X.-Y. Liu, Chem. – Eur. J., 2015, 21, 6718–6722 CrossRef CAS PubMed; (d) L. Huang, J.-S. Lin, B. Tan and X.-Y. Liu, ACS Catal., 2015, 5, 2826–2831 CrossRef CAS; (e) F. Wang, D. Wang, X. Mu, P. Chen and G. Liu, J. Am. Chem. Soc., 2014, 136, 10202–10205 CrossRef CAS PubMed; (f) F. Wang, X. Qi, Z. Liang, P. Chen and G. Liu, Angew. Chem., Int. Ed., 2014, 53, 1881–1886 CrossRef CAS PubMed; (g) P. Yu, J.-S. Lin, L. Li, S.-C. Zheng, Y.-P. Xiong, L.-J. Zhao, B. Tan and X.-Y. Liu, Angew. Chem., Int. Ed., 2014, 53, 11890–11894 CrossRef CAS PubMed; (h) Y.-P. Xiong, M.-Y. Wu, X.-Y. Zhang, C.-L. Ma, L. Huang, L.-J. Zhao, B. Tan and X.-Y. Liu, Org. Lett., 2014, 16, 1000–1003 CrossRef CAS PubMed; (i) J.-S. Lin, Y.-P. Xiong, C.-L. Ma, L.-J. Zhao, B. Tan and X.-Y. Liu, Chem. – Eur. J., 2014, 20, 1332–1340 CrossRef CAS PubMed; (j) M. Shang, S.-Z. Sun, H.-L. Wang, B. N. Laforteza, H.-X. Dai and J.-Q. Yu, Angew. Chem., Int. Ed., 2014, 53, 10439–10442 CrossRef CAS PubMed; (k) F. Yang, P. Klumphu, Y. M. Liang and B. H. Lipshutz, Chem. Commun., 2014, 50, 936–938 RSC; (l) H. Egami, R. Shimizu, S. Kawamura and M. Sodeoka, Angew. Chem., Int. Ed., 2013, 52, 4000–4003 CrossRef CAS PubMed; (m) H. Egami, R. Shimizu and M. Sodeoka, J. Fluorine Chem., 2013, 152, 51–55 CrossRef CAS; (n) Z.-M. Chen, W. Bai, S.-H. Wang, B.-M. Yang, Y.-Q. Tu and F.-M. Zhang, Angew. Chem., Int. Ed., 2013, 52, 9781–9785 CrossRef CAS PubMed; (o) X. Liu, F. Xiong, X. Huang, L. Xu, P. Li and X. Wu, Angew. Chem., Int. Ed., 2013, 52, 6962–6966 CrossRef CAS PubMed; (p) X.-Y. Jiang and F.-L. Qing, Angew. Chem., Int. Ed., 2013, 52, 14177–14180 CrossRef CAS PubMed; (q) E. Pair, N. Monteiro, D. Bouyssi and O. Baudoin, Angew. Chem., Int. Ed., 2013, 52, 5346–5349 CrossRef CAS PubMed; (r) W. Kong, M. Casimiro, E. Merino and C. Nevado, J. Am. Chem. Soc., 2013, 135, 14480–14483 CrossRef CAS PubMed; (s) L. Chu and F.-L. Qing, Org. Lett., 2012, 14, 2106–2109 CrossRef CAS PubMed; (t) R. Zhu and S. L. Buchwald, J. Am. Chem. Soc., 2012, 134, 12462–12465 CrossRef CAS PubMed; (u) Z. He, T. Luo, M. Hu, Y. Cao and J. Hu, Angew. Chem., Int. Ed., 2012, 51, 3944–3947 CrossRef CAS PubMed; (v) Q.-H. Deng, H. Wadepohl and L. H. Gade, J. Am. Chem. Soc., 2012, 134, 10769–10772 CrossRef CAS PubMed; (w) L. Chu and F.-L. Qing, J. Am. Chem. Soc., 2012, 134, 1298–1304 CrossRef CAS PubMed; (x) A. T. Parsons and S. L. Buchwald, Angew. Chem., Int. Ed., 2011, 50, 9120–9123 CrossRef CAS PubMed; (y) J. Xu, Y. Fu, D.-F. Luo, Y.-Y. Jiang, B. Xiao, Z.-J. Liu, T.-J. Gong and L. Liu, J. Am. Chem. Soc., 2011, 133, 15300–15303 CrossRef CAS PubMed; (z) A. E. Allen and D. W. C. MacMillan, J. Am. Chem. Soc., 2010, 132, 4986–4987 CrossRef CAS PubMed.
- For selected recent examples of palladium catalyzed trifluoromethylation, see: (a) L. A. Smyth, E. M. Phillips, V. S. Chan, J. G. Napolitano, R. Henry and S. Shekhar, J. Org. Chem., 2016, 81, 1285–1294 CrossRef CAS PubMed; (b) K. Natte, R. V. Jagadeesh, L. He, J. Rabeah, J. Chen, C. Taeschler, S. Ellinger, F. Zaragoza, H. Neumann, A. Brueckner and M. Beller, Angew. Chem., Int. Ed., 2016, 55, 2782–2786 CrossRef CAS PubMed; (c) W. Yin, Z. Wang and Y. Huang, Adv. Synth. Catal., 2014, 356, 2998–3006 CrossRef CAS; (d) A. Maleckis and M. S. Sanford, Organometallics, 2014, 33, 2653–2660 CrossRef CAS; (e) A. Jimenez-Aquino, J. A. Vega, A. A. Trabanco and C. Valdes, Adv. Synth. Catal., 2014, 356, 1079–1084 CrossRef CAS; (f) L.-S. Zhang, K. Chen, G. Chen, B.-J. Li, S. Luo, Q.-Y. Guo, J.-B. Wei and Z.-J. Shi, Org. Lett., 2013, 15, 10–13 CrossRef CAS PubMed; (g) X.-G. Zhang, H.-X. Dai, M. Wasa and J.-Q. Yu, J. Am. Chem. Soc., 2012, 134, 11948–11951 CrossRef CAS PubMed; (h) X. Mu, T. Wu, H.-y. Wang, Y.-l. Guo and G. Liu, J. Am. Chem. Soc., 2012, 134, 878–881 CrossRef CAS PubMed; (i) X. Mu, S. Chen, X. Zhen and G. Liu, Chem. – Eur. J., 2011, 17, 6039–6042 CrossRef CAS PubMed; (j) E. J. Cho and S. L. Buchwald, Org. Lett., 2011, 13, 6552–6555 CrossRef CAS PubMed; (k) N. D. Ball, J. B. Gary, Y. Ye and M. S. Sanford, J. Am. Chem. Soc., 2011, 133, 7577–7584 CrossRef CAS PubMed; (l) X. Wang, L. Truesdale and J.-Q. Yu, J. Am. Chem. Soc., 2010, 132, 3648–3649 CrossRef CAS PubMed; (m) E. J. Cho, T. D. Senecal, T. Kinzel, Y. Zhang, D. A. Watson and S. L. Buchwald, Science, 2010, 328, 1679–1681 CrossRef CAS PubMed.
- For the examples of iron catalyzed trifluoromethylation, see: (a) D. Uraguchi, K. Yamamoto, Y. Ohtsuka, K. Tokuhisa and T. Yamakawa, Appl. Catal., A, 2008, 342, 137–143 CrossRef CAS; (b) R. Rey-Rodriguez, P. Retailleau, P. Bonnet and I. Gillaizeau, Chem. – Eur. J., 2015, 21, 3572–3575 CrossRef CAS PubMed; (c) A. T. Parsons, T. D. Senecal and S. L. Buchwald, Angew. Chem., Int. Ed., 2012, 51, 2947–2950 CrossRef CAS PubMed; (d) J. E. M. N. Klein, S. Rommel and B. Plietker, Organometallics, 2014, 33, 5802–5810 CrossRef CAS; (e) H. Egami, R. Shimizu, Y. Usui and M. Sodeoka, Chem. Commun., 2013, 49, 7346–7348 RSC; (f) L.-Z. Yu, Q. Xu, X.-Y. Tang and M. Shi, ACS Catal., 2016, 6, 526–531 CrossRef CAS.
- (a) E. C. N. Nono, P. Mkounga, V. Kuete, K. Marat, P. G. Hultin and A. E. Nkengfack, J. Nat. Prod., 2010, 73, 213–216 CrossRef CAS PubMed; (b) M. Voets, I. Antes, C. Scherer, U. Müller-Vieira, K. Biemel, S. Marchais-Oberwinkler and R. W. Hartmann, J. Med. Chem., 2006, 49, 2222–2231 CrossRef CAS PubMed; (c) M. A. Castro, J. M. Miguel del Corral, M. Gordaliza, P. A. García, M. A. Gómez-Zurita, M. D. García-Grávalos, J. de la Iglesia-Vicente, C. Gajate, F. An, F. Mollinedo and A. San Feliciano, J. Med. Chem., 2004, 47, 1214–1222 CrossRef CAS PubMed; (d) R. S. Ward, Nat. Prod. Rep., 1997, 14, 43–74 RSC; (e) A. R. Pape, K. P. Kaliappan and E. P. Kündig, Chem. Rev., 2000, 100, 2917–2940 CrossRef CAS PubMed.
- For selected examples of the synthesis of dihydronaphthalenes, see: (a) R. Lazaro, R. Roman, D. M. Sedgwick, G. Haufe, P. Barrio and S. Fustero, Org. Lett., 2016, 18, 948–951 CrossRef CAS PubMed; (b) L. Devkota, C.-M. Lin, T. E. Strecker, Y. Wang, J. K. Tidmore, Z. Chen, R. Guddneppanavar, C. J. Jelinek, R. Lopez, L. Liu, E. Hamel, R. P. Mason, D. J. Chaplin, M. L. Trawick and K. G. Pinney, Bioorg. Med. Chem., 2016, 24, 938–956 CrossRef CAS PubMed; (c) H. Qian, W. Zhao, Z. Wang and J. Sun, J. Am. Chem. Soc., 2015, 137, 560–563 CrossRef CAS PubMed; (d) Y. Luan, K. S. Barbato, P. N. Moquist, T. Kodama and S. E. Schaus, J. Am. Chem. Soc., 2015, 137, 3233–3236 CrossRef CAS PubMed; (e) D. Malhotra, L.-P. Liu, M. S. Mashuta and G. B. Hammond, Chem. – Eur. J., 2013, 19, 4043–4050 CrossRef CAS PubMed; (f) X. Li, S. Wang, T. Li, J. Li, H. Li and W. Wang, Org. Lett., 2013, 15, 5634–5637 CrossRef CAS PubMed; (g) Y.-Y. Ye, P.-X. Zhou, J.-Y. Luo, M.-J. Zhong and Y.-M. Liang, Chem. Commun., 2013, 49, 10190–10192 RSC; (h) H. M. L. Davies and Q. Jin, J. Am. Chem. Soc., 2004, 126, 10862–10863 CrossRef CAS PubMed.
- J. Xu, Y.-L. Wang, T.-J. Gong, B. Xiao and Y. Fu, Chem. Commun., 2014, 50, 12915–12918 RSC.
- Y.-L. Ji, J.-H. Lin, J.-C. Xiao and Y.-C. Gu, Org. Chem. Front., 2014, 1, 1280–1284 RSC.
- Z. Z. Zhu, K. Chen, L. Z. Yu, X. Y. Tang and M. Shi, Org. Lett., 2015, 17, 5994–5997 CrossRef CAS PubMed.
- (a) R. Koller, K. Stanek, D. Stolz, R. Aardoom, K. Niedermann and A. Togni, Angew. Chem., Int. Ed., 2009, 48, 4332–4336 CrossRef CAS PubMed; (b) K. Stanek, R. Koller and A. Togni, J. Org. Chem., 2008, 73, 7678–7685 CrossRef CAS PubMed; (c) P. Eisenberger, I. Kieltsch, N. Armanino and A. Togni, Chem. Commun., 2008, 1575–1577 RSC; (d) P. Eisenberger, S. Gischig and A. Togni, Chem. – Eur. J., 2006, 12, 2579–2586 CrossRef CAS PubMed.
- (a) S. L. Buchwald and C. Bolm, Angew. Chem., Int. Ed., 2009, 48, 5586–5587 CrossRef CAS PubMed; (b) I. Thome, A. Nijs and C. Bolm, Chem. Soc. Rev., 2012, 41, 979–987 RSC.
- (a) H. Xu, J.-L. Hu, L. Wang, S. Liao and Y. Tang, J. Am. Chem. Soc., 2015, 137, 8006–8009 CrossRef CAS PubMed; (b) A. T. Parsons, A. G. Smith, A. J. Neel and J. S. Johnson, J. Am. Chem. Soc., 2010, 132, 9688–9692 CrossRef CAS PubMed.
- (a) J. Clayden, M. N. Kenworthy and M. Helliwell, Org. Lett., 2003, 5, 831–834 CrossRef CAS PubMed; (b) F. G. Morin, W. J. Horton, D. M. Grant, R. J. Pugmire and D. K. Dalling, J. Org. Chem., 1985, 50, 3380–3388 CrossRef CAS.
- Y. J. Im, K. Y. Lee, T. H. Kim and J. N. Kim, Tetrahedron Lett., 2002, 43, 4675–4678 CrossRef CAS.
- (a) X. Wang, Y. Ye, S. Zhang, J. Feng, Y. Xu, Y. Zhang and J. Wang, J. Am. Chem. Soc., 2011, 133, 16410–16413 CrossRef CAS PubMed; (b) Y. Li, Y. Lu, G. Qiu and Q. Ding, Org. Lett., 2014, 16, 4240–4243 CrossRef CAS PubMed; (c) A. Prieto, R. Melot, D. Bouyssi and N. Monteiro, Angew. Chem., Int. Ed., 2016, 55, 1885–1889 CrossRef CAS PubMed; (d) A. Prieto, R. Melot, D. Bouyssi and N. Monteiro, ACS Catal., 2016, 6, 1093–1096 CrossRef CAS; (e) Z. Feng, Q. Q. Min, H. Y. Zhao, J. W. Gu and X. Zhang, Angew. Chem., Int. Ed., 2015, 54, 1270–1274 CrossRef CAS PubMed; (f) T. W. Liwosz and S. R. Chemler, Org. Lett., 2013, 15, 3034–3037 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental details, characterization data, NOESY spectra, NMR spectra for new compounds, and CIF file giving crystallographic data for compound 3l. CCDC 1474908. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6qo00166a |
| This journal is © the Partner Organisations 2016 |