Facile synthesis of annulated heterocyclic benzo[kl]acridine derivatives via one-pot N–H/C–H coupling†
Yulian
Zagranyarski‡
ab,
Artem
Skabeev‡
a,
Yingjie
Ma
a,
Klaus
Müllen
ac and
Chen
Li
*a
aMax Planck Institute for Polymer Research, Ackermannweg 10, 55128 Mainz, Germany. E-mail: lichen@mpip-mainz.mpg.de
bFaculty of Chemistry and Pharmacy, Sofia University ‘St. Kliment Ohridski’, 1 James Bourchier Ave., Sofia 1164, Bulgaria
cInstitute of Physical Chemistry, Johannes Gutenberg University Mainz, Duesbergweg 10-14, 55128 Mainz, Germany
First published on 14th September 2016
Abstract
An efficient N–H/C–H one-pot coupling method for the preparation of benzo[kl]acridines has been developed based on palladium-catalyzed domino synthesis. Using commercially available starting materials such as dihalonaphthalenes and diphenylamines, and combining amination with catalysts for C–H activation, up to 95% overall yield can be achieved.
With the development of organic electronics and photonics, nitrogen-containing heteroarene building blocks such as carbazoles, indoles, and imidazoles have been intensively studied for application in organic light-emitting diodes (OLED), organic photovoltaics (OPV), and organic field-effect transistors (OFET).1 In addition, these compounds are used not only as active components, but also as auxiliary layer ingredients, for instance hole/electron transporting/blocking materials. Particularly, many carbazole-based polymers and oligomers exhibit outstanding performance in electronic devices.2
Using 2-azido-1,1′-biphenyl derivatives as starting materials, Smith and Brown established carbazole synthesis by thermal cyclization in 1951.3 Cadogan reported a more practical carbazole forming protocol in 1965 from 2-nitro-1,1′-biphenyl derivatives refluxed in triethylphosphite.4 Later, 2,2′-bisfunctionalized (halogen/pseudohalogen) biphenyls made it possible to obtain N-alkylated/arylated carbazoles under Buchwald–Hartwig reaction conditions.5 However, all three methods are limited due to the additional preparation of biphenyl building blocks. Recent synthetic methods offer a more powerful and elegant domino process that takes advantage of transition metal catalyzed amination between aryl halides and primary/secondary amines as well as intramolecular C–C coupling of aryl halides and C–H bonds.6 Additionally, transition metal-, typically palladium-mediated domino annulations are implemented under mild reaction conditions and provide access to N-alkylated/arylated carbazoles from secondary amines.7
Up to now, six-membered N–H containing neutral aromatic compounds e.g. 7H-benzo[kl]acridines have received little attention. One reason is that the Cadogan reaction is not favorable for the formation of N-doped six-membered rings.8 Moreover, bisfunctional biaryl precursors for the Buchwald–Hartwig N-annulations are not easily obtained.
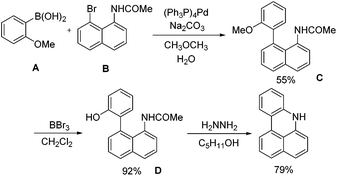
Several recent patents claim N-substituted benzo[kl]acridine derivatives as electroluminescent materials.9 However, the syntheses of those benzo[kl]acridines were rather complicated, including multi-step reactions.10 For example (Scheme 1), the “three-step” synthesis of 7H-benzo[kl]acridine with an overall yield of only 40% actually requires the synthesis of the commercially unavailable starting material, i.e. N-(8-bromonaphthalen-1-yl)acetamide (B).9e
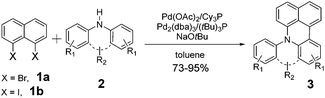
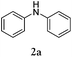
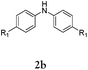
Herein, we describe a novel palladium-catalyzed domino reaction for the preparation of benzo[kl]acridine derivatives. To the best of our knowledge, there is no report on one-pot reactions for their synthesis. Our strategy is to couple commercial dihalonaphthalenes e.g. 1,8-dibromonaphthalene (1a) or 1,8-diiodonaphthalene (1b), with readily available secondary aromatic amines, such as diphenylamines (Table 1).
| Entry | Halide | Aromatic amine | Product | Yieldb [%] |
|---|---|---|---|---|
| a Reaction conditions: 1 (3.5 mmol), 2 (3 mmol), NaOtBu (7 mmol), Pd(OAc)2 (3 mol%), Pd2(dba)3 (3 mol%), Cy3P (7 mol%), (tBu)3P (7 mol%), dry toluene, 90 °C, 10 h. b Yields of isolated product. c R1 is tert-octyl. | ||||
| 1 | 1a |
|
|
91 |
| 2 | 1b | 95 | ||
| 3 | 1a |
|
|
84c |
| 4 | 1b | 95c | ||
| 5 | 1a |
|
|
79 |
| 6 | 1b | 83 | ||
| 7 | 1a |
|
|
85 |
| 8 | 1b | 89 | ||
| 9 | 1a |
|
|
77 |
| 10 | 1b | 81 | ||
| 11 | 1a |
|
|
0 |
| 12 | 1b | 0 | ||
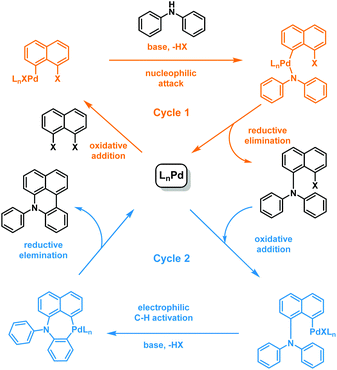
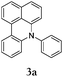
During the course of studies targeting 1,8-bis(diphenylamino)naphthalenes by the reaction between 1a and diphenylamine (2a) using a conventional Buchwald–Hartwig catalyst system, i.e. Pd2(dba)3, (tBu)3P and base NaOtBu, we noted the formation of an unprecedented six-membered ring fusion product 3a (in yield of 25%) where a new C–C bond was formed between the naphthalene and the α-phenyl position of the diphenylamino group. Mass spectral characterization indicated 1-(diphenylamino)naphthalene as a byproduct. Additionally, after 24 hours, the reaction of 1a and 2a catalyzed by a common arylation catalyst, i.e. Pd(OAc)2 with Cy3P, only generated 3a in 50% yield. Therefore, it can be concluded that the amination takes place first followed by C–C coupling. Scheme 2 suggests a possible reaction mechanism similar to that for the one-pot synthesis of carbazoles proposed by Bedford and Cazin.11 The reaction includes two sequential and independent catalytic cycles: (1) aromatic amination in cycle 1, and (2) C–H activation and cyclization.
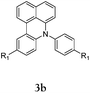
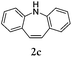
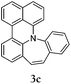
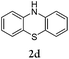
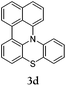
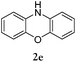
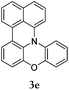
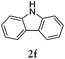
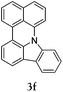
Considering the likely two-step reaction mechanism, we evaluated a mixed catalyst system for both the Buchwald–Hartwig amination and activated C–H coupling conditions, i.e. Pd2(dba)3 and ligand (tBu)3P for amination and Pd(OAc)2 with Cy3P for arylation. In this way, we successfully minimized the dehalogenated byproduct. The reactions were carried out in toluene as a solvent. Six different diphenylamines were evaluated in the reaction, namely diphenylamine (2a), di(p-tert-octylphenyl)amine (2b), 5H-dibenzo[bf]azepine (2c), 10H-phenothiazine (2d), 10H-phenoxazine (2e) and 9H-carbazole (2f). Among these amines, the reactions of diphenylamines 2a and 2b exhibited the best results, especially with 1,8-diiodonapthalene (1b) giving yields as high as 95% (entries 2 and 4). The reaction of the four ortho-linked diphenylamines 2c–2f, however, showed lower yields. Particularly, 3e was not stable and easily decomposed in solution. Moreover there was no 3f formed in the reaction between 2a and 2f, instead the main product was perylene (over 90%), which is the homo-coupling product of 2a. It has been reported that perylene can be synthesized via 1,8-dihalogenated (I or Br) naphthalene in Ni(0)-mediated reactions (yields are up to 88%),12 but we can find no report of the Pd-catalyzed analogue. On the other hand, carbazole substituted naphthalenes have been obtained via amination under either Buchwald–Hartwig13 or Ullmann14 conditions. Therefore, in the cases of entries 11 and 12 there remain some puzzling questions: (1) why were only the starting material carbazole and the homo-coupling product perylene present? (2) Why was no Buchwald–Hartwig amination product observed, i.e. 1-carbzolenaphthalene? And (3) why was the yield of homo-coupling so high? As we continue to investigate the scope of this powerful ring-forming cascade reaction, we will also attempt to answer these questions.
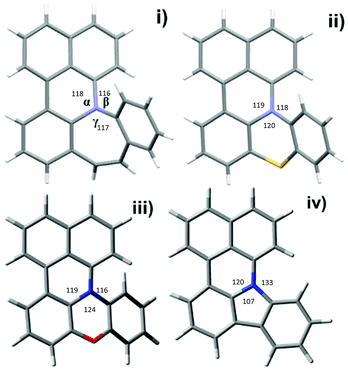
In order to understand the different behaviors of these amines (2c–2f), it is necessary to analyze the molecular structures of 3c–3f. We obtained single crystals of 3c and 3d by slow diffusion of petroleum ether into CH2Cl2 solutions. Furthermore, the molecular geometry of 3c–3f was calculated via density functional theory by Gaussian 09 W software. As shown in Fig. 1, the angles (α, β and γ) between adjacent C–N bonds are nearly the same for the cases of 3c (118°, 116°, 117°) and 3d (119°, 118°, 120°). However, for 3e, the γ angle (124°) is much larger than both α (119°) and β (116°). Nevertheless, the α, β, and γ (120°, 133°, 107) of 3f are largely different. It seems that the uniformity of α, β, and γ (the best will be that α, β, and γ are all close to 120°) is strongly related to both the stability of the compounds and the reaction yields. Among 3c–3f, the yields of 3c and 3d are better than that of 3e, while 3f has never been reported and cannot be synthesized also by this one-pot reaction method.
Conclusions
Herein, we have reported a novel N–H/C–H domino synthesis of benzo[kl]acridine derivatives consisting of an amination and a direct C–H bond arylation starting from readily available anilines and 1,2-dihalonaphthalenes. Remarkably, up to 95% overall yield can be achieved via a one-pot reaction, enabling easy access to useful benzo[kl]acridine derivatives. Additionally, since the stable products 3a, 3c and 3d are potentially suitable for optoelectronic application, we have also checked the optical and electrochemical properties of these compounds (see Fig. S1 and Table S1 in the ESI†). We believe that these compounds and their derivatives will be good candidates for organic optical and electronic devices.Acknowledgements
We gratefully acknowledge financial support from BASF SE. We also thank Prof. Mark Watson (Department of Chemistry, University of Kentucky) for fruitful discussions. K. M. acknowledges support provided by the Johannes Gutenberg-Universität Mainz (JGU) through the Gutenberg Forschungskolleg Fellowship (GFK).Notes and references
- (a) C. Li, M. Liu, N. G. Pschirer, M. Baumgarten and K. Müllen, Chem. Rev., 2010, 110, 6817 CrossRef CAS PubMed; (b) X. Zhao and X. Zhan, Chem. Soc. Rev., 2011, 40, 3728 RSC.
- (a) J. Li, F. Dierschke, J. Wu, A. C. Grimsdale and K. Müllen, J. Mater. Chem., 2006, 16, 96 RSC; (b) W. Huang, F. S. Tang, B. Li, J. H. Su and H. Tian, J. Mater. Chem. C, 2014, 2, 1141 RSC; (c) S. Y. Cai, G. J. Tian, X. Li, J. H. Su and H. Tian, J. Mater. Chem. A, 2013, 1, 11295 RSC; (d) I. McCulloch, M. Heeney, C. Bailey, K. Genevicius, I. MacDonald, M. Shkunov, D. Sparrowe, S. Tierney, R. Wagner, W. Zhang, M. L. Chabinyc, R. J. Kline, M. D. McGehee and M. F. Toney, Nat. Mater., 2006, 5, 328 CrossRef CAS PubMed; (e) A. Nowak-Król, R. Wagener, F. Kraus, A. Mishra, P. Bäuerle and F. Würthner, Org. Chem. Front., 2016, 3, 545 RSC.
- P. A. S. Smith and B. B. Brown, J. Am. Chem. Soc., 1951, 73, 2435 CrossRef CAS.
- (a) J. I. G. Cadogan, M. Carmeron-Wood, R. K. Makie and R. J. G. Searle, J. Chem. Soc., 1965, 4831 RSC; (b) J. I. G. Cadogan, Synthesis, 1969, 11 CrossRef CAS.
- (a) D.-M. Chen, Q. Qin, Z.-B. Sun, Q. Peng and C.-H. Zhao, Chem. Commun., 2014, 50, 782–784 RSC; (b) J. T. Markiewicz, O. Wiest and P. Helquist, J. Org. Chem., 2010, 75, 4887–4890 CrossRef CAS PubMed; (c) T. Kitawaki, Y. Hayashi, A. Ueno and N. Chida, Tetrahedron, 2006, 62, 6792–6801 CrossRef CAS; (d) Q. Shen and J. F. Hartwig, J. Am. Chem. Soc., 2006, 128, 10028–10029 CrossRef CAS PubMed.
- (a) A. K. Yadav, S. Peruncheralathan, H. Ila and H. Junjappa, J. Org. Chem., 2007, 72, 1388 CrossRef CAS PubMed; (b) V. Dhayalan, J. A. Clement, R. Jagan and A. K. Mohanakrishnan, Eur. J. Org. Chem., 2009, 531 CrossRef CAS; (c) R. Y. Tang and J. H. Li, Chem. – Eur. J., 2010, 16, 4733 CrossRef CAS PubMed; (d) F. Jafarpour and H. Hazrati, Adv. Synth. Catal., 2010, 352, 363 CrossRef CAS; (e) T. Nanjo, C. Tsukano and Y. Takemoto, Org. Lett., 2012, 14, 4270 CrossRef CAS PubMed; (f) S. Chitra, N. Paul, S. Muthusubramanian and P. Manisankar, Green Chem., 2011, 13, 2777 RSC; (g) J. R. Domingo, J. A. Saez and S. R. Emamian, Org. Biomol. Chem., 2015, 13, 2034 RSC; (h) R. Y. Huang, P. T. Franke, N. Nicolaus and M. Lautens, Tetrahedron, 2013, 69, 4395 CrossRef CAS.
- (a) S. Maity, S. Pathak and A. Pramanik, Eur. J. Org. Chem., 2014, 4651 CrossRef CAS; (b) L. Ackermann and A. Althammer, Angew. Chem., Int. Ed., 2007, 46, 1627 CrossRef CAS PubMed.
- (a) J. I. G. Cadogan, S. Kulik and M. J. Todd, Chem. Commun., 1968, 736a RSC; (b) J. I. G. Cadogan, Q. Rev. Chem. Soc., 1968, 22, 222 RSC; (c) J. I. G. Cadogan, R. K. Mackie and M. J. Todd, Chem. Commun., 1966, 491a RSC.
- (a) Y. M. Baek, S. M. Kim, C. J. Lee, J. Y. Shin, E. J. Lee, H. C. Park and T. H. Kim, KR20150086071, Doosan Corp., 2015 Search PubMed; (b) K. E. Lee, J. S. Choi, Y. H. An, W. S. Kim, Y. S. Lee, J. D. Lee, D. J. Kim and Y. S. No, KR20140021969, Heesung Material Ltd., 2014 Search PubMed; (c) T. Fujiyama, Y. Toya and M. Nakatsuka, JP2008227088, Mitsui Chemicals Inc., 2008 Search PubMed; (d) D. S. Choi, B. S. Moon, Y. S. Kim, I. Y. So, C. R. Oh, M. S. Lee, S. M. Lee, W. J. Lee, E. J. Jeon and J. Y. Lee, KR20140096002, Samyang Corp., 2014 Search PubMed; (e) X. Ma, W. Li, M. Li and B. Peng, CN103589421, Jilin Optical & Electronic Materials Co. Ltd., 2013 Search PubMed.
- (a) X. Ma, H. Li, M. Chen and W. Li, CN103614133, Jilin Optical & Electronic Materials Co. Ltd., 2013 Search PubMed; (b) S. M. Kim, Y. M. Baek, H. S. Son, J. Y. Shin, H. C. Park and T. H. Kim, WO2013100497, Doosan Corp., 2013 Search PubMed.
- R. B. Bedford and C. S. J. Cazin, Chem. Commun., 2002, 2310 RSC.
- (a) R. A. Bugum, N. Chanda, T. V. V. Ramakrishna and P. R. Sharp, J. Am. Chem. Soc., 2005, 127, 13494 CrossRef PubMed; J. S. Brown and P. R. Sharp, Organometallics, 2003, 22, 3604 Search PubMed.
- S. I. Kho, H. K. Kim, M. K. Kim and H. H. Cho, US20150053942, Samsung Display Co. Ltd., 2015 Search PubMed.
- (a) J. H. Park, Y. K. Kim, E. Y. Lee, E. J. Jeong and S. H. Hwang, US2015171336, Samsung Display Co. Ltd., 2015 Search PubMed; (b) W. J. Yoo, T. Tsukamoto and S. Kobayashi, Org. Lett., 2015, 17, 3640 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available: Full experimental details and characterization data (PDF); crystal structures of 3c and 3d (CIF). CCDC 1494768 and 1494769. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c6qo00371k |
| ‡ These authors contributed equally. |
| This journal is © the Partner Organisations 2016 |