Effect of double tailed cationic surfactants on the physicochemical behavior of hybrid vesicles†
Suraj Koiralaa,
Biplab Royb,
Pritam Guhac,
Ravi Bhattaraia,
Manish Sapkotaa,
Prasant Nahakb,
Gourab Karmakarb,
Amit Kumar Mandalc,
Anoop Kumard and
Amiya Kumar Panda*be
aDepartment of Pharmaceutics, Himalayan Pharmacy Institute, Majhitar, Rangpo, East Sikkim, 737136, India
bDepartment of Chemistry, University of North Bengal, Darjeeling-734013, West Bengal, India. E-mail: akpanda1@yahoo.com
cDepartment of Microbiology, Vidyasagar University, Midnapore-721102, West Bengal, India
dDepartment of Biotechnology, University of North Bengal, Darjeeling-734013, West Bengal, India
eDepartment of Chemistry and Chemical Technology, Vidyasagar University, Midnapore-721102, West Bengal, India
First published on 26th January 2016
Abstract
Hybrid vesicles, prepared from soy phosphatidylcholine (SPC), ion pair amphiphile (IPA), cholesterol (CHOL) and dihexadecyldimethylammmonium bromide (DHDAB), were investigated to assess their potential as novel drug delivery systems for indomethacin (IMC), a nonsteroidal anti inflammatory drug (NSAID). The size, polydispersity index and zeta potential values of the vesicles were monitored with respect to time using dynamic light scattering (DLS) measurements which confirmed the profound effect of both DHDAB and the drug. Incorporation of DHDAB, although resulting in the size enhancement of the liposome, however, enhanced vesicle stability induced by electrostatic repulsion. Both conventional and freeze-fractured transmission electron microscopic (TEM) studies revealed the spherical morphology of the vesicles. FTIR studies confirmed perceptible interaction among the drug and the lipid–surfactant mixtures. Thermal behavior of the vesicles was assessed by means of differential scanning calorimetric (DSC) studies in order to understand the interaction between the incorporated NSAID and lipids. The state of polarity of IMC and another fluorescent molecular probe (7-hydroxycoumarin) were examined via absorption and emission spectroscopy respectively. Results on the fluorescence anisotropy studies helped in understanding the head group packing of the amphiphiles in the vesicles. An in vitro release study of the NSAID from vesicles revealed that incorporation of DHDAB promoted the release of the drug. Among different proposed models, the Korsmeyer–Peppas model was found to be the best one and the release mechanism was predominantly Fickian diffusion. The formulations, in the absence and presence of IMC showed no cytotoxicity in healthy human blood cell lymphocyte as well as in the human breast adenocarcinoma cell line (MCF 7). The aforementioned studies provided deeper insight into the interaction pattern of DHDAB with hybrid vesicles thereby exploring the usefulness of such systems as effective drug carriers.
1. Introduction
Colloidal nanocarriers have evolved as promising vehicles in drug delivery systems.1 Due to their high surface area to volume ratio, they exhibit superior pharmacokinetics and bio-distribution of drugs which ultimately increase the therapeutic activity and reduce drug toxicity.2 Among the different colloidal systems, liposome, a spherical vesicle made up of cholesterol and phospholipids,3 has evolved as a potential carrier of bioactive compounds for its biocompatibility issue. Liposome was first discovered by the late Alec Bangham in 1965.3–7 Later on, Gregory Gregoriadis4 developed the concept that liposome can be used to deliver drugs; since then it has been comprehensively studied as vehicles for drug, vaccine, gene, etc.8 The drugs, entrapped inside a liposome, have completely different biodistribution than the free drug molecules; which leads to increase in therapeutic outcome of drugs.3,4,9 Furthermore, liposome surface can deliberately be modified by adhering suitable ligands, thus making it capable to bind to specific site inside the body without causing damage to other cells.10 Although a large number of liposome formulations are approved by FDA and are available in market,11 however, formulating drug entrapped liposome is equally difficult task due to its stability and leakage problem. Stability of liposomes can be improved by modifying its surface using different polymers.12 Phospholipids, used as the main component, are biocompatible in one hand but equally prone to degradation on the other hand imparting instability of liposome based formulations inside the body.13 Therefore, studies on liposomes on the basis of both in vivo and in vitro stability are warranted in order to develop effective drug delivery systems. Stability of liposome depends largely on its composition. For example, saturated phospholipids increase the rigidity of liposome,3 whereas cholesterol controls the rigidity of the liposome as well as in vivo stability.14 There are two ways to increase the in vitro stability of liposomes, viz., electrostatic and electrosteric. Incorporation of charge in liposome forms electro statically stable liposome.13Quest for new stable drug delivery systems have led to the development of different vesicles during last few decades. Vesicles made up of ion pair amphiphiles (IPA), also known as catanosomes, have extensively been studied as potential carrier for the drugs.15–19 Due to the simplicity in preparation, cheaper production cost and enhanced stability, IPA can be used as novel vesicle for drug delivery.16,18,20,21 Similarly, vesicles made from a mixture of cationic–zwitterionic surfactants show potential application in gene therapy.22 Although vesicles made up of such surfactants are easy to formulate, however they exhibit substantial toxicities, especially to cellular systems. Recently it has been found that toxicity of catanosomes (vesicle prepared by using cationic surfactants) can be reduced by adding phosphatidylcholine to it.23 Such hybrid vesicles, made up of zwitterionic lipid and cationic surfactant, not only can reduce toxicity but also have increased stability. Sobral et al.24 characterized the vesicle made up of dioctadecyldimethylammonium bromide (DODAB) and dipalmitoylphosphatidyl choline (DPPC) and found that at 50 mol% DODAB, most stable vesicle was formed. Chang et al.25 studied the stability of vesicle made up of zwitterionic lipid distearoylphosphatidylcholine (DSPC) mixed with dihexadecyldimethylammonium bromide (DHDAB) and found that physical stability increased while increasing DHDAB concentration from 10 to 50 mol%. Similarly, Zhang et al.26 reported that addition of cationic surfactant to zwitterionic phospholipids increased the stability of vesicle by electrostatic stitching in gel phase of the phospholipids. Moreover, effective gene transfer, cytotoxicity and stability of vesicle are reported to depend on the composition of vesicles.19 Thus, investigation on the effect of double tailed cationic surfactant as well as ion pair amphiphiles (prepared by stoichiometric mixing of two oppositely charged surfactants in water) on the different physicochemical properties of liposomes or other synthetic vesicle formulations are considered to be worth investigating considering their potential application in targeted drug and gene delivery. The potential of such liposomes are not only limited on the drug delivery systems, but also in the fundamental understanding viewpoint as model biological membranes.7 Liposomes are used to study interaction of drug with membrane which can provide significant information on different pharmacokinetic properties of drugs.27,28 Likewise, membrane fusion, molecular recognition, cell adhesion, membrane trafficking and other membrane related properties can be studied by using liposome as model systems.29
Indomethacin (IMC) is a non-steroidal anti-inflammatory drug (NSAID).30,31 It is COX inhibitor and used to treat inflammation, fever and pain. The most common side effect of IMC is gastric irritation and gastrointestinal perforation.32 Regardless of its potent anti-inflammatory and analgesic effect, its use is limited due to its severe side effects. Recently it was found that there occurred decrease in GI perforation when the NSAID is administered along with phospholipids.33 Moreover, due to its toxicity, NSAIDs can alternatively be used topically for diseases like arthritis and ankylosing spondylitis. Surfactant based vesicles have recently been used as vehicle for topical delivery. Among different surfactants, cationic and anionic surfactant are more detrimental to stratum corneum than nonionic surfactants and allow greater amount of drug flow inside the body.34,35 Similarly, unsaturated lecithin can be used as penetration enhancer due to its ability to increase fluidity of stratum corneum.6 However, the choice of the surfactant should be rational so that it does not cause significant harm to the body. Thus studies on the vesicle containing mixture of surfactant with optimum property for penetration of stratum corneum with reduced toxicity are gaining significant interest day by day.
In the present study, novel vesicles composed of soy phosphatidylcholine (SPC), cholesterol (CHOL), ion pair amphiphile (IPA) and dihexadecyldimethylammonium bromide (DHDAB) were formulated. Effect of DHDAB on vesicle size, polydispersity index and zeta potential were evaluated by dynamic light scattering. Thermotropic behavior of different vesicles were assessed by differential scanning calorimetry (DSC). State of polarity and anisotropy of the palisade layer were assessed by using 7-hydroxycoumarin (7-HC) as molecular probe. To gain further knowledge on the effect of IMC on the prepared vesicle, the NSAID was incorporated into the vesicle and similar studies were made. In vitro drug release kinetics studies were carried out whereby the release constant was determined by using different models. Finally the effect of the different liposomal formulations were investigated on human cell lines (both normal and cancerous) in order to assess its cytotoxicity. Such a comprehensive set of studies are expected to develop potential drug delivery systems with novel substitutes of the conventional phospholipids.
2. Experimental section
2.1. Materials
Hexadecyltrimethylammonium bromide (HTAB, CTAB), sodium dodecyl sulfate (SDS), dihexadecyldimethylammonium bromide (DHDAB), cholesterol (CHOL) and 7-hydroxy coumarin (7-HC) were purchased from Sigma-Aldrich Chemicals Pvt. Ltd. (USA). Indomethacin, IMC was a generous gift from Florid Laboratories. Pvt. Ltd. (Kathmandu, Nepal). Soy phosphatidylcholine (L-α-phosphatidylcholine) was the product of Calbiochem, Germany. AR grade disodium hydrogen phosphate (Na2HPO4·2H2O), sodium hydrogen phosphate (NaH2PO4·2H2O), sodium chloride (NaCl), HPLC grade chloroform and methanol were obtained from Merck Specialities Pvt. Ltd, India. All the chemicals were stated to be >99.5% pure and were used without further purification. Double distilled water was used during entire work.2.2. Methods
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v). Required volumes of the ingredients from the stock solution were transferred into a round bottom flask. Thin film was generated using a rotary evaporator and was further kept in vacuum overnight to remove all traces of organic solvent. The resultant thin film was hydrated with phosphate buffer pH 7.4 and agitated in rotary evaporator for 1 h at 70 °C to form multilamellar vesicles. Multilamellar vesicle was frozen, thawed and sonicated (in ultrasound bath) for four consecutive cycles in order to obtain monodispersed unilamellar vesicle.23,37 It was filtered through 0.45 micron cellulose nitrate membrane to remove dust particles prior to each measurement. IMC loaded liposome was prepared by co-drying the drug with lipid at the time of thin film formation.38
1, v/v). Required volumes of the ingredients from the stock solution were transferred into a round bottom flask. Thin film was generated using a rotary evaporator and was further kept in vacuum overnight to remove all traces of organic solvent. The resultant thin film was hydrated with phosphate buffer pH 7.4 and agitated in rotary evaporator for 1 h at 70 °C to form multilamellar vesicles. Multilamellar vesicle was frozen, thawed and sonicated (in ultrasound bath) for four consecutive cycles in order to obtain monodispersed unilamellar vesicle.23,37 It was filtered through 0.45 micron cellulose nitrate membrane to remove dust particles prior to each measurement. IMC loaded liposome was prepared by co-drying the drug with lipid at the time of thin film formation.382.3. Instrumentation
In case FF-TEM measurement has been adopted to prevent the drying process that involve in case of normal TEM. FR-7000A (Hitachi High Technologies Ltd., Japan) was used for the studies. In this case, the sample was frozen in liquid propane at −150 °C. Platinum–carbon was used to make the replica of the samples by evaporation technique.23
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5 w/w ratio. Sample was then kept inside the press to form KBr disc. 100 scans of pure IMC, lipid mixture and IMC with lipid were done at 2 cm−1 resolution to obtain spectra from 4000–400 cm−1.
5 w/w ratio. Sample was then kept inside the press to form KBr disc. 100 scans of pure IMC, lipid mixture and IMC with lipid were done at 2 cm−1 resolution to obtain spectra from 4000–400 cm−1.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 100. Emission spectra were recorded in the range 350–600 nm by exciting the probe at 330 nm. Fluorescence anisotropy value (r) was determined by the following equation:43,44
100. Emission spectra were recorded in the range 350–600 nm by exciting the probe at 330 nm. Fluorescence anisotropy value (r) was determined by the following equation:43,44
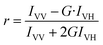 | (1) |
where, IVV is the parallel polarized and IVH is the perpendicular polarized fluorescence intensities, G = IHV/IHH is the monochromator grating correction factor. Felix GX software was used to calculate anisotropy value.
2.3.7.1. Selection of human subjects for collection of lymphocytes. Healthy human subjects (n = 3) were chosen to collect the blood sample for separation of lymphocytes. The subjects who were enrolled in this study were form same geographical area and are all asymptomatic and none of them had abnormal on physical examinations and routine laboratory tests. They received no medication, including anti-oxidant supplementation. The rejection criteria for subjects included not only individuals with acute infections or chronic diseases, but also the healthy individuals undergoing supplementation with antioxidants. Subjects gave written consent. The study protocol was in accordance with the declaration of Helsinki, and was approved by the Ethical Committee of Vidyasagar University.45 Blood samples were collected from these healthy human volunteers by vein-puncture in 5 mL heparin coated Vacutainers satisfying the method of Hudson and Hay.46 Five milliliters of blood were diluted with phosphate buffered saline (PBS) (1
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and layered onto Histopaque 1077 (Sigma) by using a Pasteur pipette and centrifuged at 400 × g (1500 rpm) for 40 min at room temperature and the peripheral blood mononuclear cells (PBMCs) were collected as per the previously described method.45
1) and layered onto Histopaque 1077 (Sigma) by using a Pasteur pipette and centrifuged at 400 × g (1500 rpm) for 40 min at room temperature and the peripheral blood mononuclear cells (PBMCs) were collected as per the previously described method.45After the treatment schedule with drug 1–3, the PBMCs (2 × 105 cells in each set) were washed with PBS (1×) for three times using centrifugation (2200 rpm for 3 min per wash) and were subjected to quantitative estimation for cytotoxicity by a non-radioactive, colorimetric assay systems using tetrazolium salt, 3-[4,5-dimethylthiazol-2-yl]-2,5-diphenil-tetrazolium bromide (MTT).45,46 The percentage of proliferation was calculated by using the following equation:
| %proliferation = [ODsample − ODcontrol] × 100/ODcontrol | (2) |
From the cytotoxicity assay the IC50 values of thee drugs against PBMCs were calculated by non-linear regression equation.
2.3.7.2. Cytotoxicity studies on human breast adenocarcinoma cell line. In another set of experiments, the human breast adenocarcinoma cell line (MCF 7) was obtained from National Centre for Cell Science (NCCS), Pune, India and were cultured in DMEM (Dulbecco's Modified Eagle Medium) supplemented with 10% fetal bovine serum (FBS), 100 units per mL penicillin, 100 mg mL−1 streptomycin, 0.14% sodium bicarbonate and 0.1 mM sodium pyruvate. The cell line was maintained in CO2 incubator (N-Biotech) at 37 °C in a 5% CO2 atmosphere with 95% humidity. Briefly, the cells were seeded in 96-well plates at a density of 5 × 103 cells per well in 200 μL culture medium. Following 24 h incubation and attachment, the cells were treated with higher (0.1 mM) and lower (0.0025 mM) concentrations of vesicles (without and with double tailed cationic surfactant and IMC) on the basis of previous experiment and similar concentration of diluents (PBS) for 24 h. After treatment, media was replaced with MTT solution (10 μL of 5 mg mL−1 per well) prepared in PBS and incubated further for 3 h at 37 °C in a humidified incubator with 5% CO2. Then 50 μL of isopropanol was added to the each well and plates were gently shaken for 1 min and absorbance was taken at 595 nm by micro titer plate reader (Bio-Rad). The percentage of cytotoxicity was calculated as (Y − X)/Y × 100, where Y is the mean optical density of control (PBS treated cells) and X is the mean optical density of treated cells with vesicles. The all experiments were repeated three times independently. Results were presented as mean ± SD of triplicates from three independent experiments.47
All the experiments, except the differential scanning calorimetric studies, were performed at ambient controlled temperature. An average of three sets of experiments was reported.
3. Results and discussion
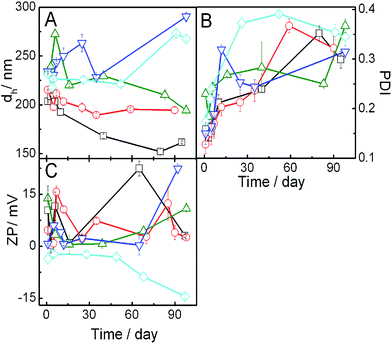
3.1. DLS studies
Dynamic light scattering study is an effective tool for measuring the size, PDI and zeta potential which subsequently can shed light on the physicochemical stability of liposomes. Hydrodynamic diameter of the vesicles with different compositions (SPC, IPA and DHDAB along with 30 mol% cholesterol) in the absence and presence of IMC were measured and analyzed. Asymmetric unimodal size distribution for most of the formulations indicate heterogeneity in the size of the formulation (Fig. S1, ESI† section). Size of pure SPC vesicles were in the range of 100–600 nm with maximum intensity at around 200 nm. Mild shift in the higher size range was noticed for hybrid (SPC + IPA + DHDAB + CHOL) vesicles. Furthermore, incorporation of IMC resulted in the broadening of size distribution curve (indicating heterogeneity in size) along with mild increase in size of the vesicles. Variation in the size of vesicles with time for different combinations have been shown in Fig. 1 and S2† (panel A and B). Size of the pure SPC vesicles was found to be ∼200 nm; results were found to be comparable with previously published reports.23 Upon the progressive addition of IPA, size of the vesicles increased which was due to the chain mismatch between IPA and SPC. Owing to the fact that IPA possesses shorter hydrocarbon chains than the SPC, thus IPA could not intercalate effectively between SPC molecules. This ultimately results in the deformation of the systematic lipid packing order as well as lowering of the extent of hydrophobic interaction among hydrocarbon chains. Subsequently, there occurs an increase in the size of vesicle.48 Additionally, it is conceivable that the head group of zwitterionic amphiphiles, herein the IPA, have larger cross-sectional area. Consequently, the packing density of hydrophobic region will be affected; thus there would be lateral expansion of the lipids resulting in the size enhancement.49Effect of cationic double chain surfactant (DHDAB) on the size of the vesicle was found to be concentration dependent. Addition of DHDAB to SPC + IPA vesicle resulted in the reduction of vesicle hydrodynamic diameter at lower concentration (2 mol%). Such result could be rationalized on the basis of strong head group electrostatic attraction between zwitterionic SPC and cationic charged surfactant as reported earlier.24,25 However, at higher amount of added DHDAB (up to 10 mol%) there occurred an over all increase in the electropositivity of vesicles as also evidence from the enhancement of positive zeta potential values, to be shown and explained later. As a consequence of enhance charge, electrostatic repulsion among the head groups of DHDAB would cause an increasing size.24,49 Among all the combinations, hybrid vesicles containing 5 mol% DHDAB was found to be most stable in terms of size for approximately 120 days. Further studies, therefore, were carried out with 5 mol% DHDAB in combination with SPC, IPA and CHOL. Size of the vesicle dispersions containing 10 mol% IPA did not follow the same trend as other systems which was due to different packing density of hydrophobic chain region in presence of different IPA proportion.
Vesicles containing IMC was found to be stable up to 90 days. A remarkable decrease in size of the SPC/IPA vesicles without DHDAB was noted upon incorporation of the NSAID. Due to the amphiphilic nature of IMC, it has a tendency to self-aggregate and intercalate within the palisade layer of bilayer membranes leading to the disruption and a dis-solubilization of the vesicular components.50 On the contrary, mild increase in the size of vesicle containing DHDAB was observed upon the addition of IMC. This can be ascribed to the fact that IMC (pKa = 4.5) adheres to the surface of vesicle through electrostatic interaction with the positively charged surfactant so that it does not self-aggregate; thus the lipidic organization of the vesicles was not perturbed.
Polydispersity index (PDI) is another parameter that provides useful information on the heterogeneity of the dispersions. PDI values with respect to time of different vesicles are presented in Fig. 1 (panel B) and S2† (panel C and D). PDI values of all the systems were below 0.4 indicating its fairly monodisperse nature. Increase in PDI values with time indicate the formation of the systems heterogeneity due to the formation of differently sized particles through the process of aggregation. PDI value of drug-loaded vesicles containing drug remained constant up to 90 days suggesting substantial stability provided by the drug.
Zeta potential of vesicle provides information regarding the nature and magnitude of surface charge which is another valuable marker to assess its physical stability. Representative zeta potential values of vesicles at different time have been demonstrated in Fig. 1 (panel C) and S2† (panel E and F). Zeta potential value of pure SPC vesicle in PBS solution (pH 7.4) was moderately negative which was due to the presence of negatively charged phosphate groups in the head groups of the zwitterionic phospholipids.49,51,52 Zeta potential values were reverted to mildly positive side upon the progressive addition of DHDAB due to the obvious reason (DHDAB being positively charged, should impart positive zeta potential).25 A little amount of DHDAB was sufficient to reverse the mild negative charge of vesicles. Zeta potential again changed to negative values upon drug incorporation. Indomethacin, being weakly acidic in nature with pKa value of 4.5, ionizes almost completely at pH 7.4 and thus neutralizes the positive charge of vesicle. Although the zeta potential values of the vesicles were less than that required for optimum stability (−30 mV), however, it was sufficient to maintain the stability of vesicle as established from the uniform size of vesicles up to 120 days indicating non-occurrence of agglomeration of particles.53 Lower magnitude of the zeta potential values were due to the presence of electrolytes (10 mM buffer solution). Presence of salt in buffer decreases the size of electrical double layer such that the exact zeta potential is suppressed.8,54
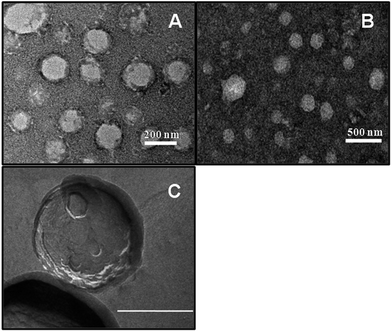
3.2. Morphological studies
Morphology of the vesicles was investigated by normal TEM as well as freeze-fractured TEM (FF-TEM) measurements in order to substantiate the existence of bilayer structure in the vesicles. Normal electron micrograph of hybrid vesicles, SPC + IPA (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, M/M, panel A) and the same with 5 mol% DHDAB (9
1, M/M, panel A) and the same with 5 mol% DHDAB (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 + 5% DHDAB, M/M, panel B) as well as FF-TEM micrograph of 9
1 + 5% DHDAB, M/M, panel B) as well as FF-TEM micrograph of 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 SPC + IPA (9
1 SPC + IPA (9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, M/M, panel C) have been shown in Fig. 2 as representatives. The images of the vesicle provide evidence for the presence of nanometer sized spherical aggregates. Sizes of the vesicles were found to be in the range of approximately 200–250 nm.
1, M/M, panel C) have been shown in Fig. 2 as representatives. The images of the vesicle provide evidence for the presence of nanometer sized spherical aggregates. Sizes of the vesicles were found to be in the range of approximately 200–250 nm.
Nominal numbers of agglomerated vesicles were visible through microscopic studies that suggest substantial stability of vesicle dispersions. It is worthwhile to mention that the size of the vesicles, as obtained from the conventional TEM studies, could not be correlated with the DLS data. However, one can have a very preliminary idea about the shape of the vesicles. Also the existence of the bilayer in such entities could be visualised, although they appeared to be diffused. The differences in size between the TEM and DLS studies were due to the differences in techniques adopted in sample preparation and analysis. There would occur a shrinkage in the vesicle size during drying process for sample preparation in TEM studies unlike the DLS studies done in solution. In case fo FF-TEM studies (panel C) the intact morphology was retained and thus were comparable with the DLS data. Besides, the existence of bilayer was also more distinct in such study.
3.3. FTIR studies
FT-IR was used to study the interaction between the bulk lipid and drug in a physically mixed state. FT-IR is an effective tool to carry out interaction of phospholipids and exogenous substance. We carried out the FT-IR studies on the physical mixture of the components. Simply, the lipids, IPA, surfactant and the drugs were dissolved in chloroform + methanol (3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
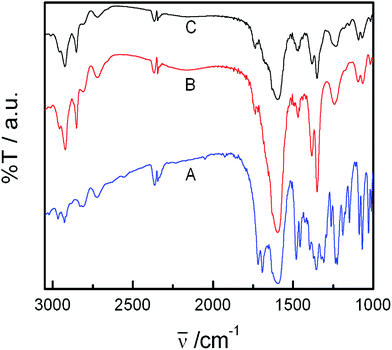
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, v/v) then dried to remove solvent. Finally conventional sample preparation procedure was followed in performing the FT-IR studies. Such studies could help in understanding the nature of the interaction. In case of hydrophobic interaction between the drug and lipids, one could expect changes in the frequency of the hydrophobic components. On the other hand in case of the interaction between the drug and lipid head group, frequency of the hydrocarbon chains would not be affected. NSAID–lipid interaction studies were made by FT-IR for the bulk lipid–NSAID mixtures. FT-IR spectra of pure IMC, lipid mixture in the presence and absence of IMC over the range 400–4000 cm−1 are displayed in Fig. 3.
1, v/v) then dried to remove solvent. Finally conventional sample preparation procedure was followed in performing the FT-IR studies. Such studies could help in understanding the nature of the interaction. In case of hydrophobic interaction between the drug and lipids, one could expect changes in the frequency of the hydrophobic components. On the other hand in case of the interaction between the drug and lipid head group, frequency of the hydrocarbon chains would not be affected. NSAID–lipid interaction studies were made by FT-IR for the bulk lipid–NSAID mixtures. FT-IR spectra of pure IMC, lipid mixture in the presence and absence of IMC over the range 400–4000 cm−1 are displayed in Fig. 3.
Pure IMC shows characteristic bands in the region of 1600 to 1750 cm−1. It was found that the drugs were bound to the palisade layer of vesicles as only the representative vibrational frequency of the head group of the lipidic components were changed upon the addition of IMC. Specifically, peaks appeared for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibrations at 1692 cm−1 (H-bonding to benzoyl C
O stretching vibrations at 1692 cm−1 (H-bonding to benzoyl C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) and 1719 cm−1 (cyclic COOH bonding in the dimer) which corresponds to the gamma (γ) form of IMC. Additional peaks appeared at 2926 and 2850 cm−1 for asymmetric aromatic C–O stretching and at 1087 cm−1 for symmetric aromatic O–H stretching.55 However, in case of FT-IR spectra of IMC embedded in the lipid mixture, significant shift for C
O) and 1719 cm−1 (cyclic COOH bonding in the dimer) which corresponds to the gamma (γ) form of IMC. Additional peaks appeared at 2926 and 2850 cm−1 for asymmetric aromatic C–O stretching and at 1087 cm−1 for symmetric aromatic O–H stretching.55 However, in case of FT-IR spectra of IMC embedded in the lipid mixture, significant shift for C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O stretching vibration (cyclic COOH bonding) to 1740 cm−1 occurred that indicates the formation of α-isoform of indomethacin.56,57 Intensity of the rest of the peaks decreased due to complete mixing of drug with the lipids. The resulting observation confirmed the possibility of significant interaction between drug and the lipids.
O stretching vibration (cyclic COOH bonding) to 1740 cm−1 occurred that indicates the formation of α-isoform of indomethacin.56,57 Intensity of the rest of the peaks decreased due to complete mixing of drug with the lipids. The resulting observation confirmed the possibility of significant interaction between drug and the lipids.
3.4. DSC studies
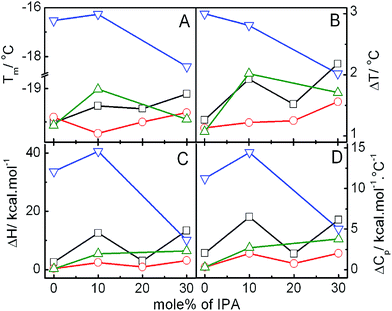
DSC is an effective tool to study the impact of exogenous moieties on the thermodynamic parameters, viz., main phase transition temperature (Tm), peak width (ΔT), enthalpy change (ΔH), heat capacity change (ΔCp), etc., associated with the chain melting of membrane bilayer. Fig. 4 and S3† graphically represent the combined effects of IPA, DHDAB and IMC on the phase transition profile of SPC vesicle. As clearly evident from Fig. 4, DSC thermogram of the SPC vesicle displayed two endothermic events in the temperature range of −20 to −19 °C (peak a) and 3 to 6 °C (peak c) respectively along with the appearance of another event in the temperature range 0 to 3 °C (event b). Peak ‘a’ represents the main phase transition temperature (Tm) of lipid bilayer due to melting of acyl chains of SPC. A progressive upshift in Tm was observed with the addition of IPA and DHDAB. However, a typical exothermic peak appeared in the temperature range of −16 to −18 °C upon the incorporation of IMC to hybrid vesicle containing 5 mol% DHDAB. The chain melting endothermic peak of SPC bilayer was completely overshadowed by exothermic one at the same temperature through the addition of the drug. Such phenomenon can be explained in terms of aggregation of drug on the surface of vesicle. The event ‘b’ around 0 °C for SPC vesicle is the consequence of the heat release from the hydrated water around the head groups of phospholipids and surfactants. Event “b” might be the results of hydration.23,58 Hydration of the amphiphiles leads to strong interaction between water and polar head group region. The energy gained to form a strong hydrogen bond is supplied by energy released from chain melting of soy phosphatidylcholine.51 The extent of hydration was nominal in case of pure SPC vesicle which increased with the addition of IPA and DHDAB; however, opposite trend was observed for the drug loaded hybrid vesicles. Distortion of lipid packing, driven by IPA and DHDAB, allowed water molecules to penetrate and deposit on the bilayer surface thereby enhancing the level of hydration. The second endotherm (peak ‘c’) is due to the absorption of heat in an event of disorganization of water over layer around the vesicles.23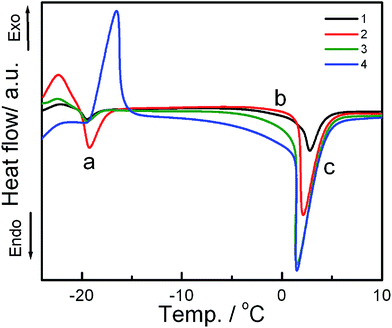 | ||
Fig. 4 DSC thermograms of the vesicles of varying composition (in presence of 30 mol% cholesterol). Systems: 1, SPC; 2, SPC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPA 9 IPA 9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 (M/M); 3, 2 + 5% DHDAB and 4, 3 + IMC. Scan rate: 2 °C min−1. 1 (M/M); 3, 2 + 5% DHDAB and 4, 3 + IMC. Scan rate: 2 °C min−1. | ||
Variation in the thermodynamic parameters, viz., Tm, ΔT, ΔH and ΔCp for the main endothermic transition (marked as ‘a’) with increasing amount of IPA in hybrid vesicles of SPC + IPA + CHOL in the absence and presence of DHDAB and IMC has been graphically presented in Fig. 5. Tm of pure soy phosphatidylcholine vesicle, which appeared at −19.5 °C, was moderately shifted to higher temperature upon the addition of IPA. Increase in ΔT value was also observed with the inclusion of IPA which is due to the chain mismatch between IPA and SPC acyl chain. Thus, IPA evokes disruption of the packing of acyl chain along with the decrease in the cooperativity for SPC transition temperature. However, destabilization caused by the IPA is overpowered by the decrease in head group repulsion among SPC and IPA. This leads to the enhanced stability of vesicle which is also evident from the increase in the values of ΔH and ΔCp.
Addition of double tailed cationic surfactant (DHDAB) moderately decreased the Tm, ΔT, ΔH and ΔCp. DHDAB consists of positively charged head group with a pair of hexadecyl hydrocarbon chain, thus mimicking SPC, a zwitterion molecule, in terms of the chain length. Due to opposite charge and similarity in acyl chain, it cooperatively intercalates along SPC molecule owing to the electrostatic interaction and favourable orientation of lipid chains. Incorporation of drug into pure SPC vesicle leads to insignificant change in the Tm and ΔT values but decreased the enthalpy by 30%. Such observation suggests the location of drug on the surface of vesicle. Constant ΔT value further confirms about the location of indomethacin.59
Interaction between IMC and the bulk amphiphile mixture was also assessed through the DSC study. DSC thermogram of the lipid mixture with and without drug was scanned in the range of 120–180 °C at a scan rate of 10 °C min−1 as shown in ESI section (Fig. S5†). Pure γ-form of IMC showed main transition peak at 160.15 °C.60 Addition of IMC to lipids led to the shift in the main transition to 153.98 °C with a simultaneous decrease in enthalpy change. Decrease in Tm along with enthalpy change is indicative of the formation of α-isoform.60 Thus the DSC studies further support the interaction of drug with the lipid head groups (as established from FTIR spectroscopic studies).
3.5. UV-vis absorption spectroscopic studies
In an attempt to assess the location of drug in the vesicles, absorption spectroscopic studies were carried out for IMC in solvents of different polarity as well as in the prepared vesicles. Fig. S7 (ESI†) represents the absorption spectra of indomethacin in the vesicles of different compositions. The plot of absorption maximum (λmax) of IMC vs. dielectric constant of different solvents is also shown in the ESI (Fig. S6†). A red shift in the absorption maxima (λmax) was noticed with increasing polarity of the medium. λmax of IMC in pure SPC vesicle appeared at 320 nm. Shift in the λmax of the drug was insignificant with the addition of IPA and DHDAB. Polarity of the surrounding environment of the drug inside the bilayer medium does not change as IPA and DHDAB do not involve in any interaction with IMC, leaving the IMC in its original position and this is one of the novel observation. The λmax of drug in different vesicles was in-line with that in the solvent of high polarity. Results indicated that the drug resides in the region near head group of vesicle as reported by Yang et al.61 in their previous study of drug location by second derivative. Spectroscopic studies thus further supports the DSC observation on the location of the drug at the palisade layer.3.6. Steady state fluorescence spectroscopic studies
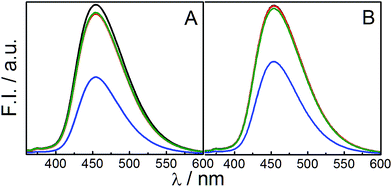
Steady state fluorescence spectroscopic study, using 7-hydroxycoumarin (7-HC), was performed to determine the state of polarity of probe on the palisade layer of vesicles. 7-HC is a solvatochromic probe that resides in the palisade region of membranes and provides information regarding the packing of head groups in vesicle.23 Emission spectra of 7-HC in the vesicles of different compositions are shown in Fig. 6. Fluorescence intensity of 7-HC in pure SPC vesicle was found to be the lowest. Addition of both the IPA and DHDB enhanced the intensity with marginal shifts in the emission maxima (λem). Enhancement of fluorescence intensity could be rationalized on the basis of increased hydration of head group upon IPA/DHDAB incorporation and subsequent enhancement in the orderliness of the surrounding environment of 7-HC residing on the palisade layer of vesicles. It has been reported that on increasing solvent polarity, intensity of 7-HC increases with minimum change in wavelength.62 Thus, it was confirmed that the polarity of the head groups increased with the incorporation of DHDAB or IPA in the SPC vesicle. Again both the graphs are quite similar, but vesicles with 5% DHDAB put on a view that 7-HC is well located into the palisade layer as 10, 20 and 30% IPA does not alter the intensity of the spectra (Fig. 6B). It could be attributed that the only the addition of DHDAB affect the head group region by imparting charge. Consequently 7-HC having being oxygen moiety fells the polar environment in the palisade layer.Fluorescence anisotropy serves as an essential tool to determine the microviscosity of the environment surrounding the probe. The anisotropy study with 7-hydroxycoumarin as a probe confers information related to the head group packing of bilayer due to its tendency to remain in the palisade layer of vesicles. Variation in the fluorescence anisotropy value of 7-HC loaded in SPC/IPA vesicles in the absence and presence of 5 mol% DHDAB with respect to different amount of IPA are represented in Fig. 7. Effect of IPA to SPC vesicle was insignificant in terms of fluorescence anisotropy values. IPA, being an uncharged molecule alike SPC, does not significantly interact with the head group of SPC. With the addition of DHDAB to pure SPC vesicle without and with 10 mol% IPA, increase in the anisotropy value was detected due to significant electrostatic attraction among head groups of SPC and DHDAB. Strong hydration in the presence of DHDAB resulted in the formation of structured hydrated layer over the amphiphile head groups. In contrast, the anisotropy value decreased significantly in the SPC vesicle with DHDAB containing 20 and 30 mol% IPA. Similar declining trend of anisotropy value in the system comprising IPA was reported by Marcelino et al.49 Increased amount of IPA generates a large gap between two oppositely charged lipids leading to electrostatic hindrance.63 Furthermore, mild negative charge, rendered by SPC, was masked by IPA. As a consequence, the head group packing becomes less organized resulting in the comfortable movement of probe in the palisade layer.
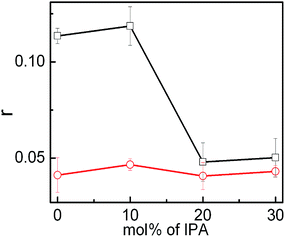 | ||
| Fig. 7 Variation in the fluorescence anisotropy of 7-HC with the mol% of IPA in the vesicles of SPC + IPA + 30 mol% cholesterol with (□) and without (^) DHDAB. Temperature: 25 °C. | ||
3.7. In vitro drug release studies
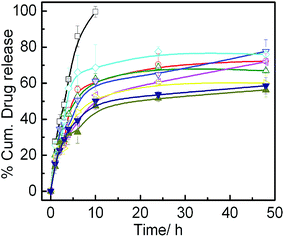
In vitro drug release profile of IMC loaded in different vesicles as well as in an aqueous solution over the period of 48 h is presented in Fig. 8. Biphasic release of IMC was observed for all the formulations. Initial burst release over the period of 5 h was the result of the drug embedded on the outer leaflet of the bilayer as well as the free drug, available in the continuous medium. However, slow and sustained release was due to the drug enclosed inside the vesicle. SPC vesicle exhibited initial burst release up to 50% of total drug at the end of 10 h which is consistent with the result reported by Srinath et al.38 A steady state was maintained up to 48 h. System with higher amount of IPA exhibited negligible difference in the release profile. In case of vesicle containing DHDAB, initial burst release, along with the overall drug release rate, was higher than SPC vesicles with and without IPA. Such phenomenon was due to the adsorption of substantial amount of indomethacin, due to its anionic nature, onto the surface of positively charged liposome. Comparatively, the SPC/IPA vesicles, devoid of DHDAB, showed higher tendency to withhold drug molecule in its bilayer rather than on the palisade layer due to the presence of neutral head groups that explains slower release in such systems.To gain further insights on the release kinetics and mechanism, release data were fitted in four different mathematical release models. Drug release kinetics was studied using DDsolver software. The equations of the respective models are presented below:64
| Higuchi model: F = kH·t0.5 | (3) |
| Korsemeyer–Peppas model: F = kK·tn | (4) |
| Hixson–Crowell model: F = 100[1 − (1 − kHCt)3] | (5) |
| First order model: F = 100[1 − (1 − ek1t)] | (6) |
Different associated parameters of the release process in case of all the models are summarized in Table 1. The regression coefficient (r2) values were used as an indicator in selecting suitable release model. The model having r2 value nearly equal to 1 was considered as the best model fit. In vitro release data were best fitted to Korsmeyer–Peppas model. The release exponent ‘n’ derived from Korsmeyer–Peppas model ranged between 0.203–0.344 for all the vesicles. Since the ‘n’ value is less than 0.450, the predominant release mechanism of IMC from all the formulations is Fickian diffusion.
| Formulations | First order | Higuchi | Hixson–Crowell | Korsmeyer–Peppas | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| k1/h−1 | r2 | kH /h−0.5 | r2 | kHC/mg h−1/3 | r2 | kKP/h−n | n | r2 | ||
SPC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPA IPA |
7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
0.11 | 0.952 | 13.88 | 0.887 | 0.03 | 0.922 | 25.61 | 0.29 | 0.962 |
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
0.12 | 0.940 | 13.79 | 0.858 | 0.03 | 0.890 | 30.84 | 0.23 | 0.952 | |
9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.10 | 0.949 | 13.98 | 0.923 | 0.03 | 0.925 | 26.36 | 0.29 | 0.970 | |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
0.16 | 0.955 | 15.55 | 0.832 | 0.03 | 0.883 | 29.34 | 0.20 | 0.976 | |
SPC![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) IPA + 5% DHDAB IPA + 5% DHDAB |
7![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 3 3 |
0.06 | 0.959 | 12.39 | 0.961 | 0.02 | 0.949 | 19.94 | 0.34 | 0.984 |
8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2 2 |
0.06 | 0.906 | 11.72 | 0.900 | 0.02 | 0.906 | 22.81 | 0.28 | 0.957 | |
9![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1 |
0.04 | 0.867 | 10.61 | 0.911 | 0.01 | 0.831 | 21.32 | 0.27 | 0.972 | |
10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0 0 |
0.05 | 0.870 | 13.88 | 0.907 | 0.01 | 0.839 | 22.15 | 0.27 | 0.969 | |
3.8. Cytotoxicity studies
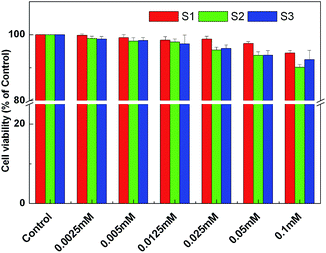
The percentage of cell viability with reference to the control (without the vesicles and/or IMC) are shown in Fig. 9.The cytotoxicity results obtained from MTT assay clearly demonstrate that the all three combinations were completely non-toxic towards healthy cells up to the concentration of 0.1 mM (as used for other physicochemical studies). The nontoxicity of bioactive compound is the most important requirement for therapeutic application. The IC50 value of drug 1–3 drugs were found to be 0.782, 0.652 and 0.692 mM respectively, as obtained by dose response non-linear regression equation.
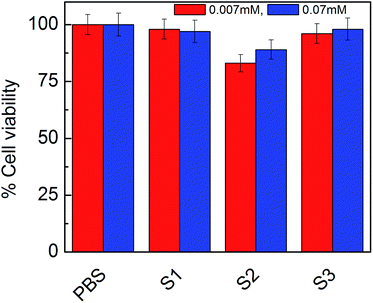
The cytotoxicity results obtained from MTT assay increased our curiosity to determine the effect of diluents on cells, so, we performed cell viability assay on human adenocarcinoma cell with different doses of vesicles. Results are shown in Fig. 10.
As was expected, the vesicles were non-toxic in respect to PBS. Diluents should not affect the releasing of drugs as well as not be toxic in presence of vesicles. So, we had considered PBS treated cells as 100 percent and compare it with vesicles dose. As the result indicate S1, S2 and S3 all were almost non-toxic and did not affect cell viability. It support our previous results and might be consider as good drug delivery system in the presence of PBS and did not affect ionic strength of solution to make it toxic. Thus the formulations could be considered safe in terms of drug delivery system.
4. Conclusion
Effect of double tailed cationic surfactant dihexadecyldimethylammonium bromide (DHDAB) on the vesicles comprising different mol% of soy phosphatidylcholine (SPC) and ion pair amphiphile (IPA), using indomethacin as model drug, were studied by using different techniques. Variation in the size of the SPC/IPA hybrid vesicles was non-systematic and dependent on DHDAB concentration. The system with 5 mol% DHDAB was the most stable one, thus selected for further evaluation with drug. An alteration in the zeta potential values was evidenced with respect to the composition and drug concentration. Spherical morphology of the vesicles was established through TEM studies. Both FTIR and DSC studies verified significant interaction between the drug and lipid–surfactant mixtures. DSC studies, by means of different phase transition parameters, revealed that the extent of crystallinity of the vesicles was greatly influenced by IPA, DHDAB, however, not that significantly by the drug. Polarity study, by means of absorption spectroscopy confirms the location of drug on the palisade layer of vesicles. Fluorescence anisotropy values, using 7-HC as a probe, demonstrated that the influence of DHDAB on the head group packing of hybrid vesicles depended on the concentration of IPA. Faster drug release rate was evidenced with the vesicular systems containing DHDAB due to the adsorption of drug on the surface owing to the electrostatic attraction between oppositely charged molecules. The release profile followed Korsmeyer–Peppas model with Fickian diffusion mechanism. The above set of studies concludes that the search for alternative stable molecules to phospholipids can be quenched with the use of bio-membrane mimicking surfactants like DHDAB and IPA in the preparation of stable vesicles directed for the drug delivery purpose. The vesicles did not exhibit any cytotoxicity in normal human blood lymphocyte as well as on human breast adenocarcinoma cell line (MCF 7), which clearly put forward the vesicles as potent drug delivery systems.Acknowledgements
The research fund provided by Council of Scientific & Industrial Research (Project No.-01(2533)/11/EMR-II), government of India, New Delhi is sincerely acknowledged. S. K. acknowledges Department of Chemistry, University of North Bengal, Darjeeling for providing laboratory facilities. Authors sincerely appreciate the FF-Tem studies generously performed by Prof. K. Torigoe and Takeshi Misono, Department of pure and Applied Chemistry, Tokyo University of Science, Japan.Notes and references
- S. Das and A. Chaudhury, AAPS PharmSciTech, 2011, 12, 62–76 CrossRef CAS PubMed.
- B. Mishra, B. B. Patel and S. Tiwari, Nanomedicine, 2010, 6, 9–24 CrossRef CAS PubMed.
- A. Akbarzadeh, R. Rezaei-Sadabady, S. Davaran, S. W. Joo, N. Zarghami, Y. Hanifehpour, M. Samiei, M. Kouhi and K. Nejati-Koshki, Nanoscale Res. Lett., 2013, 8, 102 CrossRef PubMed.
- T. M. Allen and P. R. Cullis, Adv. Drug Delivery Rev., 2013, 65, 36–48 CrossRef CAS PubMed.
- G. Barratt, Cell. Mol. Life Sci., 2003, 60, 21–37 CrossRef CAS PubMed.
- M. Nasr, S. Mansour, N. D. Mortada and A. El Shamy, AAPS PharmSciTech, 2008, 9, 154–162 CrossRef CAS PubMed.
- G. Sessa and G. Weissmann, J. Lipid Res., 1968, 9, 310–318 CAS.
- J. Sabın, G. Prieto, J. Ruso, R. Hidalgo-Alvarez and F. Sarmiento, Eur. Phys. J. E: Soft Matter Biol. Phys., 2006, 20, 401–408 CrossRef PubMed.
- M. Beija, R. Salvayre, N. Lauth-de Viguerie and J.-D. Marty, Trends Biotechnol., 2012, 30, 485–496 CrossRef CAS PubMed.
- B. r. Goldenbogen, N. Brodersen, A. Gramatica, M. Loew, J. r. Liebscher, A. Herrmann, H. Egger, B. Budde and A. Arbuzova, Langmuir, 2011, 27, 10820–10829 CrossRef CAS PubMed.
- D. Zucker, D. Marcus, Y. Barenholz and A. Goldblum, J. Controlled Release, 2009, 139, 73–80 CrossRef CAS PubMed.
- C. Tan, J. Xue, K. Eric, B. Feng, X. Zhang and S. Xia, J. Agric. Food Chem., 2013, 61, 6901–6910 CrossRef CAS PubMed.
- B. Heurtault, P. Saulnier, B. Pech, J.-E. Proust and J.-P. Benoit, Biomaterials, 2003, 24, 4283–4300 CrossRef CAS PubMed.
- L. Tseng, H. Liang, T. Chung, Y. Huang and D. Liu, J. Med. Biol. Eng., 2007, 27, 29 Search PubMed.
- T. Bramer, N. Dew and K. Edsman, J. Pharm. Pharmacol., 2007, 59, 1319–1334 CrossRef CAS PubMed.
- K. R. Rosholm, A. Arouri, P. L. Hansen, A. González-Pérez and O. G. Mouritsen, Langmuir, 2012, 28, 2773–2781 CrossRef CAS PubMed.
- A.-T. Kuo and C.-H. Chang, Langmuir, 2013, 30, 55–62 CrossRef PubMed.
- K. Maiti, S. Bhattacharya, S. Moulik and A. Panda, J. Chem. Sci., 2010, 122, 867–879 CrossRef CAS.
- M. Rosa, N. Penacho, S. Simoes, M. C. Lima, B. Lindman and M. G. Miguel, Mol. Membr. Biol., 2008, 25, 23–34 CrossRef CAS PubMed.
- E. Kaler, A. Murthy, B. Rodriguez and J. Zasadzinski, Science, 1989, 245, 1371–1374 CAS.
- A. Khan and E. Marques, Catanionic Surfactants, in Specialist Surfactants, Blackie Academic and Professional, London, 1997 Search PubMed.
- E. C. Mbamala, A. Fahr and S. May, Langmuir, 2006, 22, 5129–5136 CrossRef CAS PubMed.
- P. Guha, B. Roy, G. Karmakar, P. Nahak, S. Koirala, M. Sapkota, T. Misono, K. Torigoe and A. K. Panda, J. Phys. Chem. B, 2015, 119, 4251–4262 CrossRef CAS PubMed.
- C. N. Sobral, M. A. Soto and A. M. Carmona-Ribeiro, Chem. Phys. Lipids, 2008, 152, 38–45 CrossRef CAS PubMed.
- C.-H. Chang, C.-H. Liang, Y.-Y. Hsieh and T.-H. Chou, Colloids Surf., B, 2012, 116, 2455–2463 CAS.
- L. Zhang, T. A. Spurlin, A. A. Gewirth and S. Granick, J. Phys. Chem. B, 2006, 110, 33–35 CrossRef CAS PubMed.
- S. Mahajan and R. K. Mahajan, Adv. Colloid Interface Sci., 2013, 199, 1–14 CrossRef PubMed.
- V. Sreekanth and A. Bajaj, J. Phys. Chem. B, 2013, 117, 2123–2133 CrossRef CAS PubMed.
- C. Pereira-Leite, C. Nunes and S. Reis, Prog. Lipid Res., 2013, 52, 571–584 CrossRef CAS PubMed.
- A. Abirami, S. M. Halith, K. K. Pillai and C. Anbalagan, Int. J. Pharm. Pharm. Sci., 2014, 6, 247–250 CAS.
- S. M. Zaki and E. A. Mohamed, Arch. Med. Sci., 2014, 10, 381–388, DOI:10.5114/aoms.2012.28807.
- L. Lichtenberger, J. Romero and E. Dial, Br. J. Pharmacol., 2009, 157, 252–257 CrossRef CAS PubMed.
- M. B. Boggara, A. Faraone and R. Krishnamoorti, J. Phys. Chem. B, 2010, 114, 8061–8066 CrossRef CAS PubMed.
- R. B. Walker and E. W. Smith, Adv. Drug Delivery Rev., 1996, 18, 295–301 CrossRef CAS.
- A. C. Williams and B. W. Barry, Adv. Drug Delivery Rev., 2012, 64, 128–137 CrossRef.
- M. Deleu, J. Lorent, L. Lins, R. Brasseur, N. Braun, K. El Kirat, T. Nylander, Y. F. Dufrêne and M.-P. Mingeot-Leclercq, Biochim. Biophys. Acta, 2013, 1828, 801–815 CrossRef CAS PubMed.
- B. Roy, A. K. Panda, S. Parimi, I. Ametov, T. Barnes and C. A. Prestidge, J. Oleo Sci., 2014, 63, 1185–1193 CrossRef CAS PubMed.
- P. Srinath, S. Vyas and P. V. Diwan, Drug Dev. Ind. Pharm., 2000, 26, 313–321 CrossRef CAS PubMed.
- D. B. Vieira, L. F. Pacheco and A. M. Carmona-Ribeiro, J. Colloid Interface Sci., 2006, 293, 240–247 CrossRef CAS PubMed.
- B. Ruozi, D. Belletti, A. Tombesi, G. Tosi, L. Bondioli, F. Forni and M. A. Vandelli, Int. J. Nanomed., 2011, 6, 557 CrossRef CAS PubMed.
- I. Kyrikou, S. Hadjikakou, D. Kovala-Demertzi, K. Viras and T. Mavromoustakos, Chem. Phys. Lipids, 2004, 132, 157–169 CrossRef CAS PubMed.
- M. M. Moreno, P. Garidel, M. Suwalsky, J. Howe and K. Brandenburg, Biochim. Biophys. Acta, 2009, 1788, 1296–1303 CrossRef CAS PubMed.
- M. Companyó, A. Iborra, J. Villaverde, P. Martínez and A. Morros, Biochim. Biophys. Acta, 2007, 1768, 2246–2255 CrossRef PubMed.
- A. Gidwani, D. Holowka and B. Baird, Biochemistry, 2001, 40, 12422–12429 CrossRef CAS PubMed.
- S. K. Dash, S. S. Dash, S. Chattopadhyay, T. Ghosh, S. Tripathy, S. K. Mahapatra, B. G. Bag, D. Das and S. Roy, RSC Adv., 2015, 5, 24144–24157 RSC.
- M. M. Rahaman Mollick, B. Bhowmick, D. Mondal, D. Maity, D. Rana, S. K. Dash, S. Chattopadhyay, S. Roy, J. Sarkar, K. Acharya, M. Chakraborty and D. Chattopadhyay, RSC Adv., 2014, 4, 37838–37848 RSC.
- F. Denizot and R. Lang, J. Immunol. Methods, 1986, 89, 271–277 CrossRef CAS PubMed.
- G. Angelini, M. Chiarini, P. De Maria, A. Fontana, C. Gasbarri, G. Siani and D. Velluto, Chem. Phys. Lipids, 2011, 164, 680–687 CrossRef CAS PubMed.
- J. Marcelino, J. L. Lima, S. Reis and C. Matos, Chem. Phys. Lipids, 2007, 146, 94–103 CrossRef CAS PubMed.
- L. Lopes, M. Scarpa, G. Silva, D. Rodrigues, C. Santilli and A. Oliveira, Colloids Surf., B, 2004, 39, 151–158 CrossRef CAS PubMed.
- M. Claessens, B. Van Oort, F. Leermakers, F. Hoekstra and M. Cohen Stuart, Biophys. J., 2004, 87, 3882–3893 CrossRef CAS PubMed.
- F. Quemeneur, M. Rinaudo, G. Maret and B. Pépin-Donat, Soft Matter, 2010, 6, 4471–4481 RSC.
- H. Ferreira, T. Matamá, R. Silva, C. Silva, A. C. Gomes and A. Cavaco-Paulo, React. Funct. Polym., 2013, 73, 1328–1334 CrossRef CAS.
- S. Salgın, U. Salgın and S. Bahadır, Int. J. Electrochem. Sci., 2012, 7, 12404–12414 Search PubMed.
- S. A. Surwase, J. P. Boetker, D. Saville, B. J. Boyd, K. C. Gordon, L. Peltonen and C. J. Strachan, Mol. Pharm., 2013, 10, 4472–4480 CrossRef CAS PubMed.
- N. V. Gupta, D. Gowda, V. Balamuralidhara and M. Khan, J. Pharm., 2012, 2013, 109837 Search PubMed.
- S. Qi and D. Q. Craig, Mol. Pharm., 2012, 9, 1087–1099 CrossRef CAS PubMed.
- E. Sparr and H. Wennerström, Curr. Opin. Colloid Interface Sci., 2011, 16, 561–567 CrossRef CAS.
- M. Lúcio, F. Bringezu, S. Reis, J. L. Lima and G. Brezesinski, Langmuir, 2008, 24, 4132–4139 CrossRef PubMed.
- K. J. Crowley and G. Zografi, J. Pharm. Sci., 2002, 91, 492–507 CrossRef CAS PubMed.
- C. Yang, Y. Liu, Q. Li and L. Li, Acta Phys.-Chim. Sin., 2007, 23, 635–639 CrossRef CAS.
- B. D. Wagner, Molecules, 2009, 14, 210–237 CrossRef CAS PubMed.
- P.-L. Bardonnet, V. Faivre, F. Pirot, P. Boullanger and F. Falson, Biochem. Biophys. Res. Commun., 2005, 329, 1186–1192 CrossRef CAS PubMed.
- P. Nahak, G. Karmakar, B. Roy, P. Guha, M. Sapkota, S. Koirala, C.-H. Chang and A. K. Panda, RSC Adv., 2015, 5, 26061–26070 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra17774j |
| This journal is © The Royal Society of Chemistry 2016 |