Lipopolysaccharide of Klebsiella pneumoniae attenuates immunity of Caenorhabditis elegans and evades by altering its supramolecular structure
Arumugam Kamaladevi and
Krishnaswamy Balamurugan*
Department of Biotechnology, Science Campus, Alagappa University, Karaikudi 630 004, India. E-mail: bsuryar@yahoo.com; Fax: +91 4565 225202; Tel: +91 4565 225215
First published on 10th March 2016
Abstract
Given the prominence of lipopolysaccharide (LPS) in the pathogenesis of Gram-negative bacteria, investigations at the molecular level in in vivo conditions are in dire need to understand its role in provoking infection. Therefore, the current study was intentionally focused on LPS of Klebsiella pneumoniae to shed more light on its role in pathogenesis using an in vivo model system, Caenorhabditis elegans. In the killing assay, LPS showed a dose-dependent mortality in C. elegans. The gene expression analysis in wild-type (N2), sek-1, pmk-1 and tol-1 mutants unveiled that K. pneumoniae-LPS targeted the p38 MAPK pathway. In unison with this, the immunoblotting analysis further confirmed the requirement of a toll-dependent p38 MAPK pathway in nematode defense against K. pneumoniae-LPS. The MALDI-TOF analysis signified that K. pneumoniae escapes from the host defense by shuttering the rapid and prolonged immune activation by modifying its lipid A structure from the hexa-acylated (potent antagonist) to hepta-acylated form (weak antagonist). In addition, the increased number of acyl chain in fatty acids and the presence of palmitate, 4-aminoarabinose along with hepta-acylated lipid A (12 and 24 h) augmented the bacterial resistances against immune defense, which further supported the survival as well as multiplication of pathogen inside the host. Furthermore, the data on FT-IR and XRD unveiled that K. pneumoniae attaches to the host by establishing a strong hydrogen bond and thereby modifying its supramolecular structure of LPS from multilamellar (biologically non-active) to cubic/hexagonal (active), to evade the immune defense of the host and cause fatal infection.
Introduction
Globally, neonatal mortality is most frequently mediated by microbial sepsis, which poses a huge public health problem in developing countries. Around 25–30% of neonatal mortality has been documented through nosocomial infection amongst infants treated in hospitals.1 Klebsiella pneumoniae, a member of the Enterobacteriaceae family has successfully emerged as the important pathogen of nosocomial septicemia in immunocompromised patients. Therefore, most studies have only focused on K. pneumoniae as a single entity, due to its clinical significance in causing infections. In addition, it is renowned to express a variety of virulence factors, including a thick polysaccharide capsule and lipopolysaccharide (LPS). LPS, the chief pathogen associated molecular patterns (PAMPs), trigger the host immune response and cause fatal shock syndrome when it is phlegmatic.LPS found on the outer membrane of Gram-negative bacteria is the key virulent determinant of septicemic infections in mammals.2–5 In general, K. pneumoniae releases a diminutive quantity of LPS into the blood stream once it enters into the host system.5 The injected LPS alters its molecular structure to elicit or to evade the immune response of the host.3–8 Previous reports from our group on P. aeruginosa,8 Shigella spp. and Cronobacter sakazakii9 have also documented the alterations of LPS structure during its infection with host. These uncontrolled elicitations of pro-inflammatory response raises lethal symptoms such as a deficit in the number and functions of neutrophil, an altered response of cytokine that leads to increased pro-inflammatory, immature anti-inflammatory responses, and an increase or decrease in toll-like receptor (TLR) signaling inside the host.10 Although the role of K. pneumoniae-LPS in bacterial pathogenicity is well understood in an in vitro system, their possible mechanism of structural modification and immune evasion is largely unknown in in vivo conditions.
Caenorhabditis elegans, a free-living soil nematode, has widely been used as a model to study host–pathogen interactions. It has also been used to identify the virulence genes that are involved in causing infections by a high-throughput system.11 Mammalian cells recognize the bacterial components by TLRs and activate conserved immune pathways, viz., p38 mitogen activate protein kinase (p38 MAPK), extracellular signal-regulated kinase (ERK), and the daf-2/daf-16 pathway, which secrete anti-inflammatory cytokines against bacterial infections.12 With these facts in mind, the current study was intentionally focused to decipher the alterations in the immune system of C. elegans and the structural modifications of LPS during host–pathogen interaction.
Materials and methods
Maintenance of C. elegans
C. elegans wild type (WT) N2 and mutant strains were obtained from the Caenorhabditis genetics center (CGC), Minnesota, USA and grown on nematode growth agar medium (NGM) plates using Escherichia coli OP50 as the standard food source. The strains used in this study were N2 Bristol (wild type), MAP kinase pathway mutants [KU25 pmk-1 (km25), AU37 sek-1 (km4) and IG10 tol-1 (nr2033)]. L4 young adult nematodes were used in all assays. To obtain an age-synchronized population of L4 worms, the egg-laying nematodes were treated with commercial bleach solution containing 5 M potassium hydroxide in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio, and then the eggs were allowed to hatch on seeded plates at 20 °C. These stage-synchronized worms were used in all assays.
1 ratio, and then the eggs were allowed to hatch on seeded plates at 20 °C. These stage-synchronized worms were used in all assays.
Bacterial culture and growth condition
E. coli OP50 was provided by the CGC and K. pneumoniae was obtained from American type cell culture (ATCC no. 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 031). Both bacterial strains were grown in Luria Bertani (LB) broth at 37 °C in aerobic conditions.
031). Both bacterial strains were grown in Luria Bertani (LB) broth at 37 °C in aerobic conditions.
Isolation, purification and quantification of LPS
Lipopolysaccharide was isolated by a well-established hot water-phenol method.13 In brief, 20 mL of K. pneumoniae cultured overnight in LB was collected and resuspended in 1 mL of hot water. To the aqueous mixture, an equal volume of hot phenol (99%) was added and mixed vigorously by vortexing the solution at high speed. Then, the solution was incubated at −20 °C overnight and followed by centrifugation to remove the bacterial cells. The obtained supernatant was collected and added with 5 μL (10 mg mL−1) of DNase and RNase and 10 μL (10 mg mL−1) of proteinase K. This solution was incubated at 37 °C for 30 min and then at 59 °C overnight to remove contamination by nucleic acid and proteins found in the isolated LPS.14 Furthermore, the contamination-free supernatant was lyophilized at −80 °C using a freeze dryer [alpha 2-4LD plus, Christ, Germany] and the concentration of LPS was determined by measuring the oxidation of unsubstituted terminal vicinal glycol (UTVG) groups in 2-keto-3-deoxy-D-manno-octulosonic acid (KDO) and L-(or D-)glycero-D-manno-heptose of LPS molecules by the Purpald reagent (Sigma Inc., USA) method using KDO (Sigma) as a standard. The absorbance of the mixture was measured at 565 nm. Finally, the concentration of LPS was calculated by dividing the theoretical number by the UTVG of K. pneumoniae.15Killing assay
A batch of ten age synchronized nematodes was transferred in to M9 buffer containing different concentration of LPS (0.1, 0.25 and 0.5 μg mL−1), in a sterile 24-well culture plate and maintained at 20 °C. Every two hours the experimental nematodes were monitored for their survival. The nematodes that did not exhibit any response to touch and pharyngeal contraction were scored as dead. Herein, E. coli OP50 served as the control. Each experiment was performed at least three times independently under the same conditions.Reverse transcription PCR and real time PCR
For total RNA isolation, N2 and mutants C. elegans were exposed to LPS at different time points (6 and 12 h). After experimental exposure, the C. elegans were washed thrice with M9 and subjected to total RNA isolation by following the TRIzol method. For RT-PCR, the oligo-dT primer was used to synthesize a cDNA. According to the instruction of SuperScript III (Invitrogen Inc. USA), the cDNA templates were generated from 100 ng of total RNA isolated from C. elegans exposed to K. pneumoniae-LPS at different time points (6 and 12 h at 0.5 μg mL−1), as described above. Quantitative real time PCR (qRT-PCR) was conducted using the Applied Biosystems SYBR Green fluorescence dye kit. Independent RNA preparations were carried out and the cDNA was converted and the expression of candidate genes was calculated by normalizing the relative expression of β-actin-2. Gene expression was measured by calculating 2−ΔΔct.SDS-PAGE and immunoblotting
The regulation of p38 MAPK protein was analyzed in the nematodes exposed to LPS for different time points (6 and 12 h). The exposed nematodes were washed thoroughly and homogenized in ice-cold PBS buffer containing protease inhibitor. Soluble fractions of lysate were separated from mixture by centrifugation at 12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm for 5 min at 4 °C. The final protein concentrations of the fractions were determined with Bradford solution (Bio-Rad). For each assay, equal concentration of proteins samples were taken and mixed with SDS-loading dye. After heat-denaturation, the protein samples were subjected to polyacrylamide gel electrophoresis, followed by electrophoretic transfer onto the nitrocellulose membrane. Then, the membrane was incubated with rabbit polyclonal anti-p38 MAPK (Santa Cruz Biotechnology. Inc.) or mouse monoclonal anti-β actin (Sigma-Aldrich) at 4 °C for 6 h. Herein, the β actin was served as a house-keeping protein. After incubation, the membrane was washed twice with TBST buffer and transferred to the solution containing alkaline phosphatase conjugated secondary antibody for 4 h at 4 °C. Finally, the membrane was transferred to the developing solution containing nitro-blue tetrazolium and 5-bromo-4-chloro-3-indolylphosphate and observed until intense bands appeared.
000 rpm for 5 min at 4 °C. The final protein concentrations of the fractions were determined with Bradford solution (Bio-Rad). For each assay, equal concentration of proteins samples were taken and mixed with SDS-loading dye. After heat-denaturation, the protein samples were subjected to polyacrylamide gel electrophoresis, followed by electrophoretic transfer onto the nitrocellulose membrane. Then, the membrane was incubated with rabbit polyclonal anti-p38 MAPK (Santa Cruz Biotechnology. Inc.) or mouse monoclonal anti-β actin (Sigma-Aldrich) at 4 °C for 6 h. Herein, the β actin was served as a house-keeping protein. After incubation, the membrane was washed twice with TBST buffer and transferred to the solution containing alkaline phosphatase conjugated secondary antibody for 4 h at 4 °C. Finally, the membrane was transferred to the developing solution containing nitro-blue tetrazolium and 5-bromo-4-chloro-3-indolylphosphate and observed until intense bands appeared.
Fourier transform infrared spectroscopy (FT-IR) analysis
The modification of LPS at different time points during host interaction was analyzed by non-destructive FTIR spectroscopy, a Bruker Tensor 27 equipped with a mallard I-alanine doped deuterated triglycine sulfate (DLaTGS) detector, using potassium bromide (KBr) pellets. The isolated LPS were mixed with 40 mg of KBr and the mixture was converted into pellet using a pellet-maker and then subjected to spectral analysis. To avoid absorption from the ambient, the system was purged using dry nitrogen. For each spectrum, 64 scans were collected at a 4 cm−1 resolution. The spectra covered the wavenumber ranging from 4000 to 400 cm−1. The variations in the frequencies and band area of all sharp bands were determined accurately from the original base-line corrected spectra of the corresponding group using OPUS 6.5 software. The graph for different FT-IR spectra obtained from the LPS isolated at different time points was plotted with Origin 8.0. Because the analysis of FT-IR spectroscopy data was complicated due to superimposition of absorption peaks, the secondary derivative was calculated for the most variable region (12 h) and the modifications were analyzed.16MALDI-TOF analysis
Negative-ion matrix assisted laser desorption ionization time of flight spectra acquired on an Axima Performance (Shimadzu Biotech) equipped with both linear and reflector modes was used to further analyze the structural modifications in LPS isolated from K. pneumoniae at different time points (0, 6, 12, 24 and 36 h). Lyophilized LPS were irradiated at a 10 Hz frequency of with a 230 kV acceleration voltage by a pulsed nitrogen laser. A solution of 2,5-dihydroxybenzoic acid in chloroform/methanol/water in a 3![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1.5
1.5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 0.25 ratio was used as a MALDI matrix. Matrix mixture and samples were taken in a 1
0.25 ratio was used as a MALDI matrix. Matrix mixture and samples were taken in a 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 ratio and coated on the target sample plate. The sample coated on a target plate was allowed to dry at an ambient temperature. Each spectrum was an average of 300 shots.8 The spectra were further calibrated and processed under computer control using the Axima explorer software.
1 ratio and coated on the target sample plate. The sample coated on a target plate was allowed to dry at an ambient temperature. Each spectrum was an average of 300 shots.8 The spectra were further calibrated and processed under computer control using the Axima explorer software.
X-ray diffraction analysis
Modification that occurred in the supramolecular structure of LPS was analyzed by an X-ray diffraction method. K. pneumoniae grown at 37 °C was allowed to infect the C. elegans at 20 °C at different time points. LPS was isolated from host exposed K. pneumoniae at different time points (0, 6, 12, 24 and 36 h) and used for analysis. To exclude the modifications induced by external stimuli, such as temperature, ion concentration and the change in growth conditions, respective control LPS were also included and analyzed. XRD measurements were performed using a XPERT-PRO diffractometer system. Small angle X-ray diffraction measurements of LPS aggregate preparations were performed using an X'pert PRO PANalytical diffractometer. Scattering patterns in the range of the scattering vector 0.1 < s < 1.0 nm−1 (s = 2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ/λ, 2θ-scattering angle and λ the wavelength = 0.15 nm) were obtained between 10 and 80 °C with a 2 s exposure time at 40 kV. The diffraction patterns were evaluated as described previously17 and the spacing ratios of the main scattering maxima to define three-dimensional structures were assigned according to previous reports.7,18–21
θ/λ, 2θ-scattering angle and λ the wavelength = 0.15 nm) were obtained between 10 and 80 °C with a 2 s exposure time at 40 kV. The diffraction patterns were evaluated as described previously17 and the spacing ratios of the main scattering maxima to define three-dimensional structures were assigned according to previous reports.7,18–21
Statistical analysis
All experiments were performed with three independent biological replicates and are expressed as means with standard deviations. For the killing assay, Kaplan–Meier survival analysis (Graphpad Prism 5 statistical software) was performed to compare the mean lifespan of nematodes exposed to LPS versus the E. coli OP50 control group. The significant differences between the survival curves were analyzed by the Log-rank (Mantel-Cox method) test. For all other experiments, statistical comparisons between the groups were analyzed by one-way analysis of variance (ANOVA) and significant differences between the means of the considered parameters were assessed by Duncan's post hoc analysis using the software SPSS Ver.17.0 (SPSS Inc., Chicago, IL, USA). A p-value of <0.05 and <0.005 was considered to be significant.Results
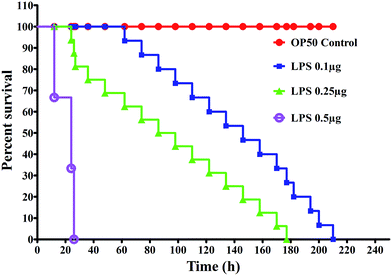
K. pneumoniae-LPS affect survival in nematodes
The severity of K. pneumoniae-LPS pathogenicity in C. elegans was determined by measuring the end-points such as survival, feeding and reproduction. In the liquid killing assay, the nematodes exposed to K. pneumoniae-LPS displayed a complete killing (P < 0.05) at 200 ± 10 h, 177 ± 5 h and 24 ± 3 h at 0.1, 0.25 and 0.5 μg mL−1, respectively (Fig. 1).K. pneumoniae-LPS suppress the immune genes responsible for host defense in C. elegans
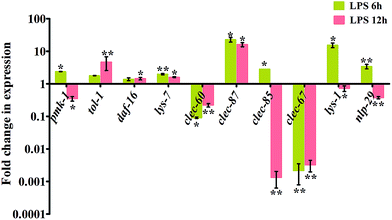
Recognition of PAMPs by the pattern recognition receptors (PRRs) such as TLRs could trigger the immune response of the host against invading foreign molecules. In C. elegans, TLR is encoded by the tol-1 gene. During interaction with K. pneumoniae-LPS, the expression of tol-1 and clec-87 were observed to be significantly (P < 0.05) up-regulated. In addition, clec-60 and clec-67, active members of the C-type lectin family were significantly (P < 0.005) down-regulated (Fig. 2). The cluster of clec-60/67 was studied to increase the nematode survival by increasing the host resistance.12,22 However, the down-regulation of clec-60 and 67 against K. pneumoniae-LPS indicated the succumbing of host immune defense to K. pneumoniae-LPS infection (Fig. 2). According to Pukkila-Worley et al., 2011,23 lys-1 and nlp-29 were particularly regulated by the p38 MAPK pathway during microbial infection. Therefore, the increased expression of pmk-1 along with lys-1 and nlp-29 at 6 h of infection and decrease in subsequent later hour (12 h) revealed that the p38 MAPK pathway appears to have a significant role in C. elegans against K. pneumoniae-LPS infection (Fig. 2).Requirement of toll-like receptor and p38 MAPK in host against K. pneumoniae-LPS
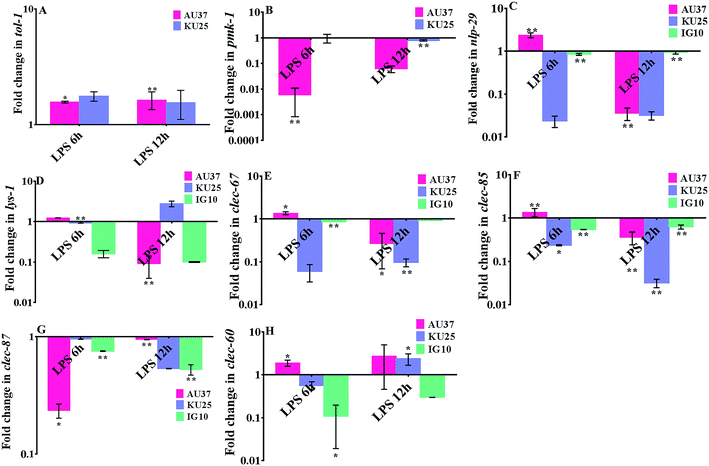
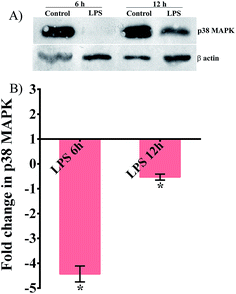
To establish the involvement of the PMK pathway and toll receptor against K. pneumoniae-LPS infection, gene expression analysis was performed in the p38 MAPK pathway mutant (sek-1 and pmk-1) and tol-1 mutant. The upregulation of tol-1 (P < 0.05) in sek-1 and pmk-1 mutants suggested the requirement of a toll-like receptor in C. elegans during K. pneumoniae-LPS infection (Fig. 3A). Infecting sek-1 and tol-1 mutants with K. pneumoniae-LPS, significantly (P < 0.005) down-regulated the pmk-1 expression. Moreover, in tol-1 mutant IG10, there was a lack of pmk-1 activation (Fig. 3B). This data indicated that a lack of toll-like receptor in IG10 botched to activate pmk-1 in nematodes during K. pneumoniae infection. Therefore, it was envisaged that C. elegans required a toll dependent p38 MAPK pathway against K. pneumoniae-LPS, which was further evident by the down-regulation of nlp-29 (Fig. 3C) and lys-1 (Fig. 3D) that are chiefly regulated by the p38 MAPK pathway. Furthermore, the antimicrobial genes clec-67, clec-85 and clec-87 were significantly (P < 0.005) down-regulated during infection, which suggested the succumbing of the host defensive system to K. pneumoniae-LPS pathogenesis (Fig. 3E–H). To further confirm the role of the p38 MAPK pathway and to elucidate whether LPS of K. pneumoniae inhibited the p38 MAPK pathway in C. elegans at protein level, western blot analysis was performed in N2 nematodes (Fig. 4). The results revealed that the level of expression of p38 MAPK protein in nematodes exposed to K. pneumoniae LPS was decreased when compared to control and this suggests that LPS of K. pneumoniae suppressed the level of expression of p38 MAPK pathway (Fig. 4A and B).Structural alteration in K. pneumoniae-LPS during interaction with host
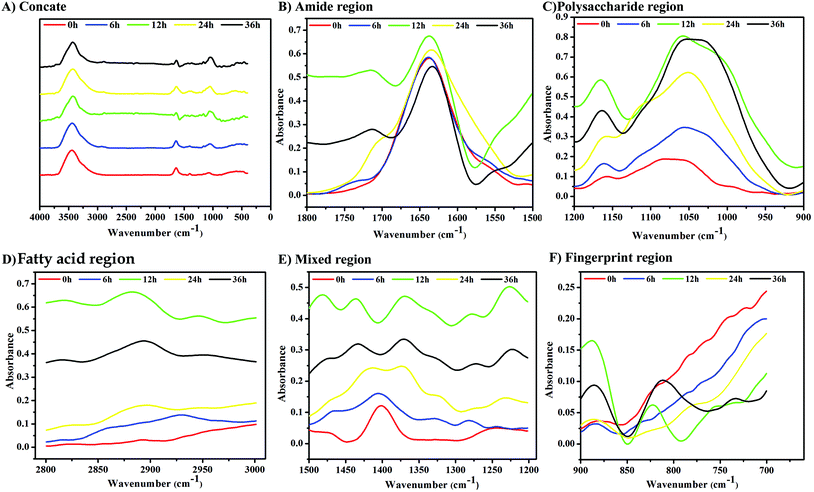
Non-destructive FT-IR was employed to analyze the structural alterations in LPS of K. pneumoniae while interacting with C. elegans. Fig. 5A shows the infrared spectra of LPS isolated from K. pneumoniae exposed to C. elegans at different time points (6, 12, 24 and 36 h). The LPS isolated from K. pneumoniae interacted for 12 h with the host showed significant alterations in their spectra. The observed peak around 1740–1720 cm−1 for 12, 24 and 36 h exposed LPS samples represents the τc![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) o, i.e., out-of-plane twisting of the C
o, i.e., out-of-plane twisting of the C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O group of hydrogen bonded carboxylic acid (Fig. 5B). In addition, the kinetic spectrum of polysaccharide (Fig. 5C), fatty acid (Fig. 5D), mixed region (Fig. 5E) and fingerprint region (Fig. 5F) showed that drastic molecular changes occurred in LPS at 12 h of exposure. Therefore, the FT-IR spectrum of the 12 h LPS sample was further analyzed by calculating the secondary derivative of the spectrum to differentiate the overlapping peaks.
O group of hydrogen bonded carboxylic acid (Fig. 5B). In addition, the kinetic spectrum of polysaccharide (Fig. 5C), fatty acid (Fig. 5D), mixed region (Fig. 5E) and fingerprint region (Fig. 5F) showed that drastic molecular changes occurred in LPS at 12 h of exposure. Therefore, the FT-IR spectrum of the 12 h LPS sample was further analyzed by calculating the secondary derivative of the spectrum to differentiate the overlapping peaks.
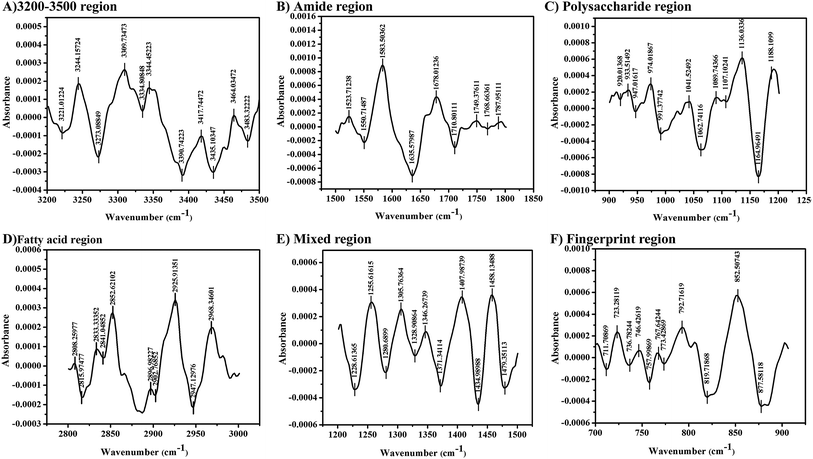
The secondary derivative of the 12 h LPS showed several peaks corresponding to the virulence factor of bacteria. The peaks observed between 3600 and 3100 cm−1 are assigned to O–H and N–H groups and the peak at 3390 cm−1 represents the stretching of O–H group present in the sugars (Fig. 6A). The absorption peak between 1800 and 1000 cm−1 is assigned for the pure O-antigen and more peaks found between these regions indicated the presence of more O-antigen in K. pneumoniae during host interaction. In addition, the intense band at 1062 cm−1 in the polysaccharide region corresponds to the O-antigen. The formation of peaks at 1635 cm−1, 1407 cm−1, 1371 cm−1 and 1255 cm−1 is typically assigned for uronic acids associated with O-acetyl groups (Fig. 6B). The intense bands in the region of 1200–900 cm−1 are assigned to the carbohydrate C–O–C ring absorption. Bands between 1200 cm−1 and 1100 cm−1 are assigned to νC–C and νC–O of the glycosidic linkage (Fig. 6C). The intense peaks at 2925 cm−1, 2843 cm−1 and 2833 cm−1 correspond to the symmetric stretch of νCH2 and νCH3 (Fig. 6D). In addition, the absorption bands at 1305 cm−1 and 1328 cm−1 are assigned to the νC–O of carboxylic acids (Fig. 6E), suggesting that the LPS polymer was acidic.21 The stretching vibrations of νC–H (between 2820 and 2940 cm−1; 1460 and 1470 cm−1) and PO2− (1280 cm−1, 1107 cm−1, 974 cm−1 and 774 cm−1) represent the formation of a lipid A moiety (Fig. 6F).
Modification in chemical structure of LPS during infection
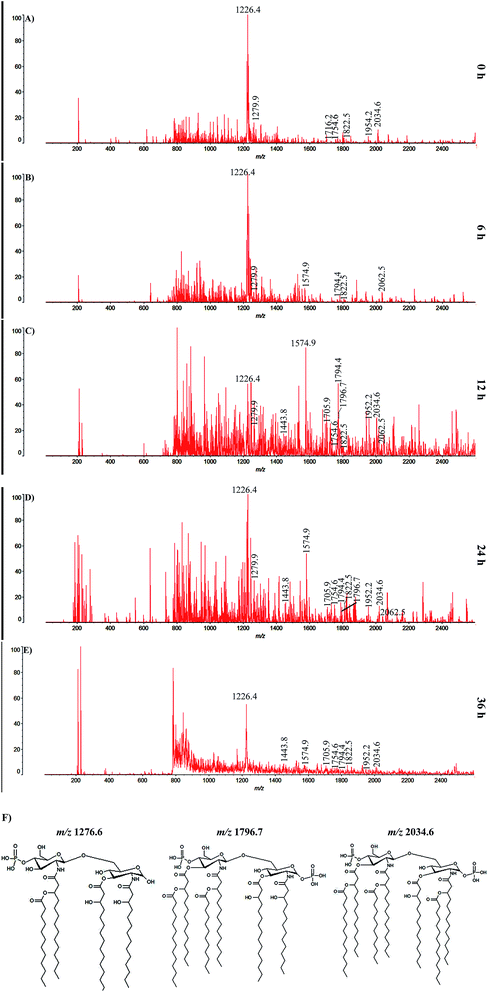
MALDI-TOF analysis was performed to determine the structural modifications in LPS of K. pneumoniae during host interaction. The obtained spectra for LPS isolated at different time points (0, 6, 12, 24 and 36 h) displayed their heterogeneity and identical ion pattern. The intense ions observed at m/z 1822.5 and m/z 1794.4 at all experimental time points are attributed to the signature molecules for K. pneumoniae lipid A such as myristate and laurate, respectively, and thus suggesting a common structure of K. pneumoniae LPS (Fig. 7). According to the chemical analysis and reports on structure of N,O-deacylated enterococcus bacterial LPS,24 the peaks at m/z 1796.4 and m/z 1716.2 at 6 h represent the bis- and mono-phosphorylated hexa-acylated lipid A, respectively (Fig. 7B). In the 12 and 24 h samples, the peaks at m/z 1954.6 and m/z 2034.6 signified the mono- and bis-phosphorylated hepta-acylated forms of lipid A, respectively (Fig. 7C and D). The lower values for those mono-phosphorylated forms for 36 h of LPS samples demonstrated the partial degradation of lipid A (Fig. 7E). Interestingly, the peaks at m/z 2062.5, m/z 1952.2 and m/z 1705.9 signified the peaks for minor lipid A species containing additional palmitate, aminoarabinose and 4-aminoarabinose units (Ara4N), respectively. In addition, the peaks at m/z 1279.6, m/z 1796.7 and m/z 2034.6 in 12 and 24 h samples are assigned to the increased number of acyl chain in the fatty acid molecules found in the LPS. Furthermore, the chemical structures of fatty acids having an added number of acyl chain in their amine residues are illustrated in Fig. 7F.Modification in aggregate structure of LPS during infection
Modification of the aggregate structure of LPS isolated from K. pneumoniae exposed with host for different time points were investigated using XRD. Fig. 8 shows the logarithm of the scattering intensity plotted versus scattering vector S = (2![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) sin
sin![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) θ/λ) for LPS isolated from K. pneumoniae interacted with host for 0, 6, 12, 24 and 36 h. To exclude the modification ensued in LPS was due to external stimuli, such as change in temperature, ion concentration of the used medium and effect of divalent cations, and their respective control LPS were also included in the experimental design. The scattering patterns of LPS (6 h) exhibited a broad maximum between S = 0.1 and 0.35 nm−1, which could be assigned principally to a lamellar structure (Fig. 8C),18 which was absent in the controls (Fig. 8A and B). The peak between 0.4 and 0.55 nm−1 in 12 h exposed LPS indicates a second order maximum, which may result from more than one bilayer (Fig. 8E). Furthermore, the equidistant spacing ratio at 6.80 and the first order periodicity d/2 of 3.4 nm in 6 h mainly indicate the multilamellar aggregate of the LPS (Fig. 8C). A recent study indicated that the multilamellar structure of LPS is biologically inactive.7 The presence of sharper diffraction peaks in 12 and 24 h at 0.3 nm−1 and 0.25 nm−1 could be due to a higher number of lamellae.18 In addition, the d/2 = 5.2 nm, 5.8 nm and 4.6 nm for 12 and 24 h, particularly, indicate the formation of cubic structure with a cylindrical conformation, hydrophilic backbone and the hydrophobic hydrocarbon chains within the molecule aggregate (Fig. 8E and G). The cubic/hexagonal structure of LPS is more virulent and biologically active.7 These results suggest that the LPS of K. pneumoniae were converted from multilamellar aggregate (biologically inactive form) to cubic aggregate (active form) during the host interaction. In contrast, no such distinct bilayer geometries were observed in the control LPS samples at any point of time (Fig. 8D, F and H).
θ/λ) for LPS isolated from K. pneumoniae interacted with host for 0, 6, 12, 24 and 36 h. To exclude the modification ensued in LPS was due to external stimuli, such as change in temperature, ion concentration of the used medium and effect of divalent cations, and their respective control LPS were also included in the experimental design. The scattering patterns of LPS (6 h) exhibited a broad maximum between S = 0.1 and 0.35 nm−1, which could be assigned principally to a lamellar structure (Fig. 8C),18 which was absent in the controls (Fig. 8A and B). The peak between 0.4 and 0.55 nm−1 in 12 h exposed LPS indicates a second order maximum, which may result from more than one bilayer (Fig. 8E). Furthermore, the equidistant spacing ratio at 6.80 and the first order periodicity d/2 of 3.4 nm in 6 h mainly indicate the multilamellar aggregate of the LPS (Fig. 8C). A recent study indicated that the multilamellar structure of LPS is biologically inactive.7 The presence of sharper diffraction peaks in 12 and 24 h at 0.3 nm−1 and 0.25 nm−1 could be due to a higher number of lamellae.18 In addition, the d/2 = 5.2 nm, 5.8 nm and 4.6 nm for 12 and 24 h, particularly, indicate the formation of cubic structure with a cylindrical conformation, hydrophilic backbone and the hydrophobic hydrocarbon chains within the molecule aggregate (Fig. 8E and G). The cubic/hexagonal structure of LPS is more virulent and biologically active.7 These results suggest that the LPS of K. pneumoniae were converted from multilamellar aggregate (biologically inactive form) to cubic aggregate (active form) during the host interaction. In contrast, no such distinct bilayer geometries were observed in the control LPS samples at any point of time (Fig. 8D, F and H).
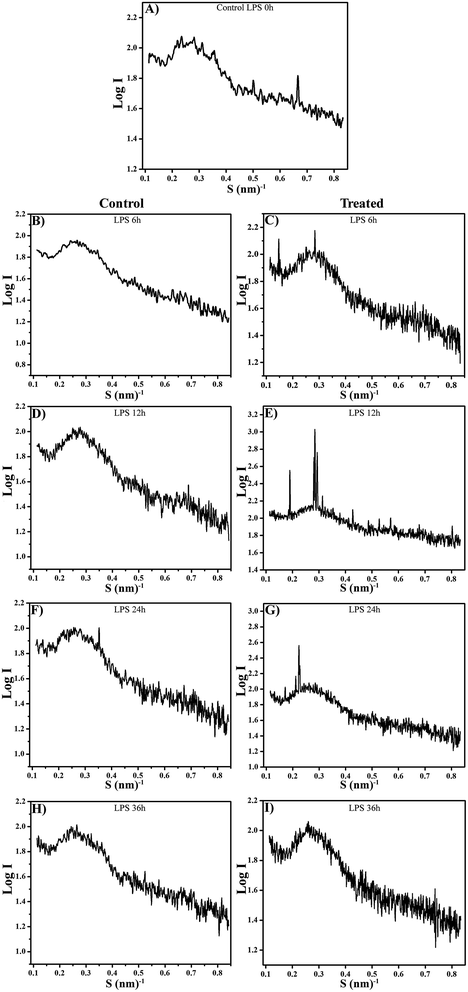 | ||
| Fig. 8 XRD pattern of LPS isolated from control and host interacted K. pneumoniae for different time points showing supramolecular changes. | ||
Discussion
Hitherto, there is an emergent interest in using C. elegans as the model for a variety of biological research.11 K. pneumoniae is an opportunistic pathogen ranked second in causing nosocomial septicemia in humans. LPS is one of the indispensable virulent determinants in K. pneumonia that enhances its pathogenicity to provoke septicemia and resistance against antibiotics.26 Being a potent immune-modulator, LPS has the solitary ability to trigger an innate immune response against various microbial infections at lower concentrations and even leads to multi-organ failure at higher concentrations, e.g. septic shock syndrome. However, the mechanism of immune evasion by LPS to cause fatal shock in an in vivo system is still a great enigma. In this context, the current study is aimed to demonstrate a detailed characterization of K. pneumoniae-LPS-induced modifications in a host immune system as well as the chemical and structural alterations of LPS.LPS of various Gram-negative bacteria has already been well demonstrated to kill C. elegans9,27,28 and Galleria mellonella.29 Congruently, the result of the killing assay indicated that K. pneumoniae-LPS caused mortality in C. elegans. Several studies from the recent past have also suggested the severity of K. pneumoniae-LPS in mice,30–32 G. mellonella29 and HepG2 cells.33 To determine the role of TLR (toll) and p38 MAPK pathway in C. elegans during infection with K. pneumoniae-LPS, gene expression analysis were performed in mutants of PMK (sek-1 and pmk-1) and toll (tol-1). The upregulation of tol-1 in both AU37 and KU25 mutants revealed the plausible role of TLR in C. elegans against K. pneumoniae-LPS. Furthermore, the diminished activation of pmk-1 and its regulated antimicrobial players, nlp-29 and lys-1 in the tol-1 mutant (IG10) suggest the vital role of TLR in activating pmk-1 during K. pneumoniae-LPS infection. Inclusively, the gene expression pattern of the three mutants evidently demonstrated the elicitation of a toll-dependent p38 MAPK pathway in C. elegans. Therefore, the result of the current study agrees well with the previous reports on LPS of Salmonella Typhi,29 Pseudomonas aeruginosa,8 Escherichia coli,30 Shigella Spp.9 Cronobacter sakazakii9 and Salmonella entrica34 infections in C. elegans. Carbohydrate binding proteins or C-type lectins are renowned for their diverse role in host response. C-type lectins were believed to be involved in masking the bacterial attachment (clec-87) and increasing the host resistance (clec-60, clec-67 and clec-85).35–37 The down-regulation of these genes in the present study clearly portrays an increased susceptibility of the mutant host to the K. pneumoniae-LPS infection. These results are in concordance with the findings of a recent study by Yang et al., 2015,38 wherein they reported the role of C-type lectins in C. elegans resistance against B. thuringiensis infection. In addition, the down regulation of p38 MAPK protein in nematodes exposed to LPS substantiated the data of gene expression analysis and further confirmed the vital role of p38 MAPK in nematode defense against K. pneumoniae-LPS mediated infection. Earlier reports have also emphasized the requirement of the p38 MAPK pathway in mice30–32 G. mellonella29 and HepG2 (ref. 33) cells against infection with K. pneumoniae-LPS. Henceforth, the necessity of the conserved p38 MAPK pathway in C. elegans against LPS as that of mice and HepG2 cells clearly signifies that the nematodes and higher model systems share a common mode of defense against K. pneumoniae-LPS.
The modification of LPS seems to be very important criteria for K. pneumoniae to evade the host immune system.3,4,7,8,26 The kinetic analysis of K. pneumoniae-LPS by FT-IR at their macromolecular level showed vast variation at 12 h of host exposed sample. The formation of C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O ester and stretching of –OH and –NH groups in LPS indicates the formation of strong hydrogen bonding and/or electrostatic interactions.39 Likewise, the formation of a hydrogen bond plays a pivotal role in bacteria attachment to the host cell.40,41 The vibrations for uronic acid and glycosidic linkages of carbolic acid moieties suggests that the pathogen created an acidic environment to protect itself from the action of acid hydrolysis by the hosts reactive oxygen species (ROS).21 As a consequence, the chemical property of the LPS might favor the survival of K. pneumoniae at acidic pH similar to the environment they find in phagosomes during host attack, signifying an important mechanism of immune evasion. The mechanism of immune evasion adopted by K. pneumoniae was also confirmed by the supramolecular alteration in the structure of LPS to a biologically inactive multilamellar form, which thereby circumvents the recognition by immune receptor.19 The functional groups CH2 and CH3 relative to C–O and C–O–C, as well as the increased absorption of νC–H and PO2− indicate a progressively larger contribution from lipid A moiety in K. pneumoniae during interaction with C. elegans.
O ester and stretching of –OH and –NH groups in LPS indicates the formation of strong hydrogen bonding and/or electrostatic interactions.39 Likewise, the formation of a hydrogen bond plays a pivotal role in bacteria attachment to the host cell.40,41 The vibrations for uronic acid and glycosidic linkages of carbolic acid moieties suggests that the pathogen created an acidic environment to protect itself from the action of acid hydrolysis by the hosts reactive oxygen species (ROS).21 As a consequence, the chemical property of the LPS might favor the survival of K. pneumoniae at acidic pH similar to the environment they find in phagosomes during host attack, signifying an important mechanism of immune evasion. The mechanism of immune evasion adopted by K. pneumoniae was also confirmed by the supramolecular alteration in the structure of LPS to a biologically inactive multilamellar form, which thereby circumvents the recognition by immune receptor.19 The functional groups CH2 and CH3 relative to C–O and C–O–C, as well as the increased absorption of νC–H and PO2− indicate a progressively larger contribution from lipid A moiety in K. pneumoniae during interaction with C. elegans.
Although LPS has been shown to be an important virulence determinant in various Gram-negative bacterial pathogens, insight knowledge on modifications in its structure in response to host defense is still lacking. To address this issue, a MALDI-TOF analysis was performed for the LPS isolated from K. pneumoniae exposed to C. elegans for different time points (0, 6, 12, 24 and 36 h). The peaks for signature molecules, viz., myristate and laurate, in the spectrum of LPS at all experimental time points were consistent with the previous reports for K. pneumoniae lipid A.42,43 The structural analysis indicated that K. pneumoniae showed a hexa-acylated form of lipid A during the initial hour of infection (6 h). Largely, hexa-acylated form of lipid A has been reported as a potent antagonist for human TLR/MD-2 receptor complex and thereby induces a robust activation of the host immune system.4 Similarly, the hexa-acylated lipid A at 6 h in the present investigation proved the strong and high affinity binding of LPS to the nematodes' TLR receptor and thereby activated robust and prolonged immune activation in the host during the initial phase of pathogenesis. The defined form of lipid A was predominant and identical with those of S. typhimurium,5,44 E. coli,45 Klebsiella oxytoca,46 Serratia marcescens,47 S. flexneri,48 Providencia rettgeri47 and Hafnia alvei49 during activation of TLR receptor and warrants extreme immunostimulatory activities of LPS. Activation of TLR receptor and subsequent synthesis of antimicrobial peptides (AMPs) has been a host defense prominence, which actively eliminates invading pathogens from the cellular system. To avoid LPS being recognized by TLR, Gram-negative bacteria wisely modify its structure to a biologically weak form of lipid A and displayed a weak immune activation.50,51 Herein, the hepta-acylated form of lipid A at 12 and 24 h of LPS has been previously reported to be a weak antagonist and also lost its ability to bind with TLR/MD-2 receptor complex and attributed to a reduced activation of host immunity.49–51 The transformation of lipid A from potent antagonist to weak antagonist (i.e., hexa-acylated to hepta-acylated) for TLR clearly demonstrates the evading strategy of K. pneumoniae during the course of pathogenesis. A similar fashion of escaping strategy has also been practiced by several bacteria, such as E. coli,50 S. Typhimurium51 and H. alvei,49 to protect themselves against TLR mediated immune activation.51 Concurrently, the hepta-acylated (weak antagonist) form of lipid A along with palmitate and Ara4N again substantiates the reduced potential of lipid A in triggering host defense through TLR signaling. Therefore, the prolonged immune activation was shuttered, which eventually supported the survival and multiplication of the pathogen inside the host cell.52 Salmonella utilizes a similar mode of lipid A modification mechanism to survive in the macrophage.52
In general, the innate immune system of host exploits numerous defense mechanisms to eliminate the key factors to get rid of infection. One such factor is the LPS with varied structures in terms of its fatty acyl chain arrangement, length and numbers.3 Addition of more acyl chain in the fatty acids of LPS is the predominant evading and resistant strategy adopted by Gram-negative bacteria. The number of acyl chain units in fatty acids of LPS that dwell on the bacterial outer membrane is directly linked to the resistance of the cell against AMPs, antibiotics, detergents (e.g. bile) and other environmental stressors.25 The increased number of acyl chains in LPS at 12 and 24 h in the present study are in total agreement with the previous finding.25 Therefore, it is envisaged from the current study that K. pneumoniae fortifies its outer membrane and further validated its state of resistance against host immune defense. Furthermore, the transition from non-active multilamellar aggregates to a biologically active virulent cubic structure confirmed the modification adopted by the K. pneumoniae-LPS during interaction with the host, which is in unison with an earlier finding stating the formation of a biologically active cubic structure of LPS during exposure with hemoglobin.7
Conclusion
The current study sheds more light on the in vivo pathogenic role of K. pneumoniae-LPS. Molecular analyses demonstrated the plausible evading strategy adopted by K. pneumoniae-LPS to succumb the host by impeding its TLR dependent p38 MAPK pathway. Extensive characterization studies by employing MALDI-TOF, FT-IR and XRD uncovered the chemical and structural basis for K. pneumoniae-LPS modifications to escape the immune response of C. elegans. Overall, these findings advance our knowledge on in vivo virulence mechanism of K. pneumoniae-LPS and open new avenues on potential therapeutic targets in both K. pneumoniae-LPS and host genes, which could pave the way for novel treatment against K. pneumoniae infection.Abbreviations
| LPS | Lipopolysaccharide |
| XRD | X-ray diffraction |
| FT-IR | Fourier transform infra-red |
| UTVG | Unsubstituted terminal vicinal glycol |
| KDO | 2-Keto-3-deoxy-D-manno-oct-2-ulosonic acid |
| MALDI | Matrix assisted laser desorption ionization |
| TOF | Time of flight |
| PMK | p38 mitogen activated kinase |
| Ara4N-4 | Aminoarabinose |
Acknowledgements
Authors have gratefully thanked the Caenorhabditis Genetics Center, MN, which is funded by the National Institutes of Health, the National Center for Research Resources for providing C. elegans strains. Dr KB thankfully acknowledges the DBT, ICMR, DST-SERB, UGC Major Research Project, CSIR, DST-PURSE [Grant No. SR/S9Z-23/2010/42(G)], FIST (Grant No. SR-FST/LSI-087/2008) and UGC SAP-DRS-I [Grant No. F.3-28/2011 (SAP-II)], New Delhi, India for partial financial assistances obtained through grants. AK thankfully acknowledges the DBT (BT/PR14932/MED/29/233/2010) for financial support through project SRF. Authors also gratefully acknowledge the computational and bioinformatics facility provided by the Alagappa University Bioinformatics Infrastructure Facility (funded by DBT, GOI; Grant No. BT/BI/25/001/2006).References
- S. Roy, R. Gaind, H. Chellani, S. Mohanty, S. Datta, A. K. Singh and S. Basu, Indian J. Med. Res., 2013, 137, 791 CAS.
- M. Matsuura, Front. Immunol., 2013, 4, 109 CrossRef PubMed.
- B. S. Park, D. H. Song, H. M. Kim, B.-S. Choi, H. Lee and J.-O. Lee, Nature, 2009, 458, 1191 CrossRef CAS PubMed.
- J. C. Kagan and R. Medzhitov, Cell, 2006, 125, 943 CrossRef CAS PubMed.
- R. S. Munford and A. W. Varley, PLoS Pathog., 2006, 2, e67 Search PubMed.
- M. W. Hornef, M. J. Wick and M. Rhen, Nat. Immunol., 2002, 3, 1033 CrossRef CAS PubMed.
- S. P. Keese, K. Brandenburg, M. Roessle and A. B. Schromm, Innate Immun., 2014, 20, 787 CrossRef PubMed.
- B. Vigneshkumar, S. Radhakrishnan and K. Balamurugan, Lipids, 2014, 49, 555 CrossRef CAS PubMed.
- A. Kamaladevi, B. S. Sivamaruthi, S. Durai, P. Kesika, B. Vigneshkumar, G. JebaMercy and K. Balamurugan, Tug-of-war between host immunity and microbial pathogenesis using Caenorhabditis elegans as a model system, in The battle against microbial pathogens: basic science, technological advances and educational programs, ed. A. Méndez-Vilas, Formatex Research Center, Spain, 2015, vol. 2, p. 842 Search PubMed.
- E. G. King, G. J. Bauza, J. R. Mella and D. G. Remick, Lab. invest., 2014, 94, 4 CrossRef CAS PubMed.
- D. D. Bolz, J. L. Tenor and A. Aballay, J. Biol. Chem., 2010, 285, 10832 CrossRef CAS PubMed.
- J. E. Irazoqui, E. R. Troemel, R. L. Feinbaum, L. G. Luhachack, B. O. Cezairliyan and F. M. Ausubel, PLoS Pathog., 2010, 6, e1000982 Search PubMed.
- O. Westphal and K. Jann, Methods Carbohydr. Chem., 1965, 5, 83 CAS.
- M. R. Davis and J. B. Goldberg, J. Visualized Exp., 2012, 63, 3916 Search PubMed.
- C. H. Lee and C. E. Frasch, Anal. Biochem., 2001, 296, 73 CrossRef CAS PubMed.
- S. J. Parikh and J. Chorover, Colloids Surf., B, 2007, 55, 241 CrossRef CAS PubMed.
- H. De Cock, K. Brandenburg, A. Wiese, O. Holst and U. Seydel, J. biol. Chem., 1999, 274, 5114 CrossRef CAS PubMed.
- J. Howe, J. Andra, R. Conde, M. Iriarte, P. Garidel, M. H. Koch, T. Gutsmann, I. Moriyon and K. Brandenburg, Biophys. J., 2007, 92, 2796 CrossRef CAS PubMed.
- C. Lopez-Abarrategui, A. Del Monte-Martinez, O. Reves-Acosta, C. L. Franco and A. J. Otero-Gonzalez, Front. Microbiol., 2013, 4, 389 Search PubMed.
- I. Beech, L. Hanjagsit, M. Kalaji, A. L. Neal and V. Zinkevich, Microbiology, 1999, 145, 1491 CrossRef CAS PubMed.
- A. Bosch, D. Serra, C. Prieto, J. Schmitt, D. Naumann and O. Yantorno, Appl. Microbiol., 2006, 5, 736 Search PubMed.
- G. V. Mallo, C. L. Kurz, C. Couillault, N. Pujol, S. Granjeaud, Y. Kohara and J. J. Ewbank, Curr. Biol., 2002, 12, 1209 CrossRef CAS PubMed.
- R. Pukkila-Worley, F. M. Ausubel and E. Mylonakis, PLoS pathog., 2011, 7, e1002074 CAS.
- J. Lukasiewicz, T. Niedziela, W. Jachymek, L. Kenne and C. Lugowski, J. Bacteriol., 2009, 191, 533 CrossRef CAS PubMed.
- M. Boll, A. T. Tucker, D. R. Klein, A. M. Beltran, J. S. Brodbelt, B. W. Davies and M. S. Trent, mBio, 2015, 6, e00478 CrossRef PubMed.
- A. Clements, D. Tull, A. W. Jenney, J. L. Farn, S. H. Kim, R. E. Bishop, J. B. McPhee, R. Hancock, E. L. Hartland, M. J. Pearse, O. L. Wijburg, D. C. Jackson, M. J. McConville and R. A. Strugnell, J. Biol. Chem., 2007, 282, 15569 CrossRef CAS PubMed.
- A. Aballay, E. Drenkard, L. R. Hilbun and F. M. Ausubel, Curr. Biol., 2003, 13, 47 CrossRef CAS PubMed.
- W. Maier, B. Adilov, M. Regenass and J. Alcedo, PLoS Biol., 2010, 8, e1000376 Search PubMed.
- J. K. Bender, T. Wille, K. Blank, A. Lange and R. G. Gerlach, PLoS One, 2013, 8, e73287 Search PubMed.
- S. Shankar-Sinha, G. A. Valencia, B. K. Janes, J. K. Rosenberg, C. Whitfield, R. A. Bender, T. J. Standiford and J. G. Younger, Infect. Immun., 2004, 72, 1423 CrossRef CAS PubMed.
- V. Cano, D. Moranta, E. Llobet-Brossa, J. A. Bengoechea and J. Garmendia, BMC Microbiol., 2009, 9, 156 CrossRef PubMed.
- S. Lawlor, S. A. Handley and V. L. Miller, Infect. Immun., 2006, 74, 5402 CrossRef PubMed.
- J. H. Wu, L. C. Hong, Y. Y. Tsai, H. W. Chen, W. X. Chen and T. S. Wu, Cell. Microbiol., 2006, 8, 1467 CrossRef CAS PubMed.
- J. Kaur and S. K. Jain, Microbiol. Res., 2012, 167, 199 CrossRef CAS PubMed.
- A. Kamaladevi and K. Balamurugan, Pathog. Dis., 2015, 73, ftv021 CrossRef PubMed.
- P. Kesika and K. Balamurugan, Biochim. Biophys. Acta, 2012, 1824, 1449–1456 CrossRef CAS PubMed.
- G. JebaMercy, L. Vigneshwari and K. Balamurugan, Microbes Infect., 2013, 15, 550–568 CrossRef CAS PubMed.
- W. Yang, D. Katja, E. Daniela, T. Andreas, L. Matthias, R. Philip and S. Hinrich, Dev. Comp. Immunol., 2015, 51, 1 CrossRef CAS PubMed.
- D. Naumann, C. Schultz and A. Sabich, J. Mol. Struct., 1989, 214, 213 CrossRef CAS.
- N. Papo and Y. Shai, J. Biol. Chem., 2005, 280, 10378 CrossRef CAS PubMed.
- E. T. Kool, Annu. Rev. Biophys. Biomol. Struct., 2001, 30, 1 CrossRef CAS PubMed.
- S. Sforza, A. Silipo, A. Molinaro, R. Marchelli, M. Parrilli and R. Lanzetta, J. Mass Spectrom., 2004, 39, 378 CrossRef CAS PubMed.
- M. Helander, Y. Kato, I. Kilpelainen, R. Kostiainen, B. Lindner, K. Nummila, T. Sugiyama and T. Yokochi, Eur. J. Biochem., 1996, 237, 272 Search PubMed.
- K. Takayama, N. Qureshi and P. Mascagni, J. Biol. Chem., 1983, 258, 12801 CAS.
- C. Alexander and U. Zähringer, Trends Glycosci. Glycotechnol., 2002, 14, 69 CrossRef.
- A. Silipo, R. Lanzetta, A. Amoresano, M. Parrilli and A. Molinaro, J. Lipid Res., 2002, 43, 2188 CrossRef CAS.
- K. Takayama and N. Qereshi, Chemical structure of lipid A, in Bacterial Endotoxic Lipopolysaccharides, ed. D. C. Morrison and J. L. Ryan, CRC Press, Boca Raton, USA, 1992, p. 43 Search PubMed.
- A. Kussak and A. Weintraub, Anal. Biochem., 2002, 307, 131 CrossRef CAS PubMed.
- J. Lukasiewicz, W. Jachymek, T. Niedziela, L. Kenne and C. Lugowski, J. Lipid Res., 2010, 51, 564 CrossRef CAS PubMed.
- G. Lamarche, S. H. Kim, S. Crepin, M. Mourez, N. Bertrand, R. E. Bishop, J. D. Dubreuil and J. Harel, J. Bacteriol., 2008, 190, 5256 CrossRef PubMed.
- L. Guo, K. B. Lim, C. M. Poduje, M. Daniel, J. S. Gunn, M. Hackett and S. I. Miller, Cell, 1998, 95, 189 CrossRef CAS PubMed.
- R. E. Bishop, Mol. Microbiol., 2005, 57, 900 CrossRef CAS PubMed.
| This journal is © The Royal Society of Chemistry 2016 |