DOI:
10.1039/C5RA20163B
(Paper)
RSC Adv., 2016,
6, 2624-2631
Sampling of dissolved inorganic SbIII by mercapto-functionalized silica-based diffusive gradients in thin-film technique
Received
29th September 2015
, Accepted 21st December 2015
First published on 23rd December 2015
Abstract
The mercapto-functionalized silica (MPS) diffusive gradients in thin-film (DGT) devices, for the first time, were characterized by the determination of dissolved inorganic SbIII. The performance of MPS–DGT was assessed by (1) determining the diffusion coefficient of SbIII in polyethersulfone membrane, (2) assessing the uptake efficiency and digestion efficiency of MPS for SbIII, (3) investigating the effect of pH, ionic strength (as NaNO3) and foreign ions on the performance of MPS–DGT for SbIII species, and (4) assessing the validation of MPS–DGT for the measurement of dissolved inorganic SbIII in spiked water samples. The diffusion coefficient of SbIII measured in the PES membrane by a diffusion cell was (3.05 ± 0.09) × 10−6 cm2 s−1. There was a tendency toward higher adsorption affinity for SbIII compared with SbV. Mass vs. time measurements of MPS–DGT at pH 6 (0.01 mol L−1 NaNO3) demonstrated a linear uptake of SbIII (R2 = 0.9973). The performance of MPS–DGT was independent of ionic strength (0.001–0.7 mol L−1 NaNO3 at pH 5) and pH (3–8, 0.01 mol L−1 NaNO3) for the measurement of SbIII. The presence of foreign ions (such as CaII, MgII, CdII, ZnII, CuII, AsIII and SbV) has no significant influence on the uptake of SbIII by MPS–DGT. MPS–DGT can quantitatively measure the concentration of dissolved inorganic SbIII in spiked etching wastewater. In spiked natural freshwater, the concentration of dissolved inorganic SbIII obtained by MPS–DGT is significantly lower than the concentration of added SbIII due to the presence of natural organic matter (8.7 mg C L−1) which would have complexed a fraction of the added SbIII and thereby changed the speciation of added SbIII. The MPS–DGT device can potentially be used as a tool for speciation measurements of SbIII in aqueous environments.
1. Introduction
Antimony (Sb) is listed as a priority pollutant by the U.S. Environmental Protection Agency due to its toxicity.1 Different species of Sb in the environment may show great differences in chemical behavior, which is a critical factor influencing their toxicity.2 SbIII species are usually more toxic than SbV.3 Determination of the chemical speciation of Sb in the environment is important to obtain additional information about its chemical forms, mobility, availability, geochemical behavior and toxicity.4 It is essential to develop reliable methods for monitoring and sampling the different species of Sb in environmental samples.
The diffusive gradients in thin-films (DGT) technique has been developed by Davison and Zhang5 and become one of the most promising in situ sampling and measurement techniques for trace metals in natural waters, soils and sediments.6 The DGT device contains a diffusion layer (e.g. polyacrylamide hydrogel,7 dialysis membrane,8 nylon membrane9 or chromatography paper10) which allows solute species below a size threshold to pass and a binding layer which is behind the diffusive layer.5 The binding layer usually comprises a binding agent which can bind the metal species across a diffusion layer.5 Garmo and co-workers investigate DGT device with a gel-layer incorporating chelex-100 resin as the binding agent and a hydrated polyacrylamide gel as the diffusion gel (Chelex–DGT) for the measurement of 55 elements, and find that Chelex-DGT is capable of measuring 24 elements accurately, whereas, for the element of SbIII, linear uptake with time is not observed by Chelex–DGT indicating that this metal is not quantitatively collected by Chelex-100 resin and can not be measured by Chelex–DGT.11 Recently, precipitated ferrihydrite has been used as a DGT binding agent for the measurement of SbV, AsV, VV and SeVI.12 Panther and co-workers reported a new DGT device using a titanium dioxide-based adsorbent as the binding agent for the measurement of a variety of anionic species such as SbV, AsV, VV, MoVI and WVI.13 However, the studies on the sampling and measuring of SbIII by DGT technique in water are still insufficient. Such constraint should be rectified by the incorporation of Sb alternative binding agents. Previous researches have reported that the compounds containing thiol groups have strong affinity for SbIII (ref. 14) and can be used for the separation of SbIII from the mixed solution of SbIII and SbV (ref. 15) because the thiol groups as “soft acid” has a high affinity for “soft” SbIII ions and a low affinity for “hard” SbV ions. The objective of this work is to take advantage of mercapto-functionalized silica (MPS) as the DGT binding agents for the selective measurement of SbIII species. MPS has been previously used as a DGT binding agent for AsIII,16 methylmercury.17 However, it has not been investigated for the selective measurement of SbIII by DGT. In this work, the validation of MPS-based DGT device (MPS–DGT) for the measurement of SbIII and the influence of a range of pH (3–8) and electrolyte concentrations (0.0001–0.7 mol L−1 as NaNO3) on its performance were investigated. Finally, the application for the measurement of SbIII in water was assessed.
2. Experimental section
2.1 Reagents, materials, and solutions
All experimental and reagent solutions were prepared with deionized water. SbIII standard was obtained as potassium antimonyl tartrate (Aldrich, 99.95% purity). The stock solutions of SbIII (1000 mg L−1) and SbV (1000 mg L−1) were prepared by potassium antimonyl tartrate and potassium hexahydroxyantimonate (Sigma-Aldrich), respectively. The working solutions were prepared by series dilution of the stock solutions immediately prior to their use. Polyethersulfone membrane (PES, pore diameter 0.2 μm, Φ 25 mm, thickness 165 ± 5 μm) was obtained from Kenker, USA. MPS (200–400 mesh), acrylamide, ammonium peroxydisulfate, bis-acrylamide, tetramethylethylenediamine (TEMED) were purchased from Sigma-Aldrich. The patented agarose-derived cross-linker was purchased from DGT Research Ltd., UK. The other chemicals were obtained from Sinopharm Chemical Reagent Co., Ltd., Shanghai, China. All chemicals used in this work were of analytical grade and used without further purification. Plastic containers and glassware were cleaned by soaking in diluted HNO3 (10%) and were rinsed with deionized water prior to use. All the metal stock solutions (1000 mg L−1) were obtained from the National Research Center for Standard Materials (NRCSM, Beijing, China).
2.2 Preparation of binding layer
MPS binding layer was prepared as described previously.16,17a 1 g of dry mass of MPS was added per 10 mL of pre-gel solution (15% acrylamide and 0.3% DGT cross-linker). 70 μL of 10% freshly prepared ammonium persulfate and 25 μL TEMED were added, and the mixture was stirred well before casting. The mixture solution of gel was cast between two glass plates and placed in an oven at 45 °C for 1 h, afterwards the binding gel was peeled off. It required careful handling to avoid breakage. The 2.5 cm diameter disks of binding gel was cut and stored in deionized water. The thickness of hydrated gels was 0.50 ± 0.05 mm.
2.3 Uptake and digestion
Uptake of disks of MPS binding gel for SbIII was investigated in batch experiments. 10 mL of aqueous solutions containing different initial concentrations of SbIII (0.1, 0.5, and 1 mg L−1) and 0.01 mol L−1 NaNO3 at pH 5 were equilibrated with individually exposing the discs of binding gel (n = 5) for at least 24 h, and then the disks were taken to determine the mass of SbIII remaining in solution. The uptake efficiency of the disks of binding gel for SbIII was calculated as the following equations:| | |
Euptake (%) = 100Ci/Cf
| (1) |
where Euptake represents the uptake efficiency (%); Ci and Cf are the initial and final concentrations of SbIII (mg L−1) in solution, respectively. The SbIII adsorbed onto the disks of MPS binding gel was carried out in a microwave acid digestion unit (Microwave Digestion System Start D, Milestone, Sorisole, Italy). A disk of MPS binding gel was put into PTFE vessels, and 3 mL of suprapure concentrated HNO3, 2 mL of suprapure concentrated HCl, and 2 mL of HF were added. The digestion was allowed to 1200 W of potency and 140 °C over 30 min and then maintained at 140 °C for 45 min. After digestion, the digested solutions were filtered and transferred into a 25 mL volumetric flask, and the volume was completed with a 1% solution of HCl (v/v). The digestion efficiency of SbIII from the disks of MPS binding gel was calculated by the following equations:| |
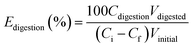 | (2) |
where Edigestion represents the digestion efficiency (%); Vdigested and Vinitial are the volumes of digested solution of disk and initial solution of SbIII, respectively. A similar procedure was followed for the uptake and digestion efficiencies of SbV.
2.4 Assembly of DGT samplers
Pistons and caps were washed in 10% (v/v) HNO3 and then rinsed nine times with deionized water before use. MPS settled on one side of the gel in the forming process, and this side was placed facing up when assembled in DGT devices. The binding gel was covered by PES membranes. The front cap was pressed tightly. Samplers were sealed in plastic bags and stored at 4 °C.
2.5 Measurement of diffusion coefficients
PES membranes were immersed in 1 mol L−1 HNO3 for 24 h before being thoroughly rinsed with deionized water until the pH approached 7, then stored in 0.01 mol L−1 NaNO3 until use. By storing PES membranes in NaNO3 it served to pre-wet the membrane, which aided assembly and facilitated expansion of the membrane.18 The diffusion coefficients of SbIII ions through the PES membrane in 0.01 mol L−1 NaNO3 were determined using a specially designed diffusion cell as described by Zhang et al.19 The diffusion cell comprised two 150 mL of compartments with an interconnecting 20 mm diameter opening. A 25 mm diameter of PES membrane known thickness was placed on the opening, provided the only connection for mass transport between two compartments and allowed the diffusion of SbIII ions from a source solution containing high concentration into a receiving solution which initially contains no SbIII ions. 150 mL of 0.01 mol L−1 NaNO3 containing 100.0 mg L−1 of SbIII ions with pH at 5 as a source solution was put into compartment A, and 150 mL of 0.01 mol L−1 NaNO3 with the same pH as receiving solution was put into compartment B. The high concentration (100.0 mg L−1) of SbIII ions in compartment A was used to ensure that the concentration depletion of metal ions during diffusion process was negligible. Both compartments were stirred continuously at 300 rpm using an overhead stirrer. Samples were taken from both compartments (50 μL for compartment A and 100 μL for compartment B with the same volume of the corresponding original solution replaced in each compartment) at 15 min intervals up to 90 min and measured by AFS. The measurements were repeated for five times. In order to test the influence of a diffusive boundary layer (DBL) on the diffusion coefficients of SbIII through PES membrane, the different stirring rates from 50 to 500 rpm were performed in the source solution. Here, the experimentally determined values of diffusion coefficients, D, were calculated using eqn (3):M is the diffusion mass of SbIII ions from a source solution with analyte concentration (C) into receiving solution, after passing through a diffusive layer of area (A) and thickness (Δg) over a deployment time (t). The diffusion coefficient of SbIII ions at different temperatures can be corrected according to Stokes–Einstein equation.20| |
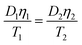 | (4) |
where D1 and D2 are diffusion coefficients at absolute temperature T1 and T2, respectively. η1 and η2 are viscosities of water at T1 and T2, respectively.
2.6 Accumulation over time
To estimate accumulation and measurement of SbIII species over time by MPS–DGT, five sets of triplicate DGT devices were deployed in 40 L of a well-stirred 0.01 mol L−1 of NaNO3 solution spiked with 100 μg L−1 of SbIII at 25 °C. The triplicate probes were removed at 24, 48, 72, 96 and 120 h, while grab samples of deployment the solution were taken at each time point and the concentration of SbIII in the solution were measured by AFS as well as for changes in speciation. The MPS–DGT devices are validated by testing the relationship between the mass of analyte accumulated in the binding layer (M) and the deployment time (t) with a solution of known concentration. CDGT is the concentration measured by the DGT technique in solution and can be predicted by the DGT equation:where, A is diffusive layer of area and Δg is diffusive layer of thickness. The experimental procedures were repeated with SbV to confirm that MPS binding agent would not accumulate SbV from solution.
2.7 Effects of pH, ionic strength and foreign ions
To test the effects of solution pH on the performance of MPS–DGT, fifteen replicates of DGT devices were deployed in 30 L of NaNO3 solutions (0.01 mol L−1) spiked with 100 μg L−1 of SbIII in the pH range 3–8 at 25 °C over periods of time from 24 to 120 h with a 24 hour interval. The pH was adjusted as required using dilute HCl or KOH. The effect of ionic strength of solution on the performance of DGT was investigated by adjusting the ionic strength of a SbIII solution (100 μg L−1) via the addition of NaNO3. The ionic strengths of the deployment solutions were range from 0.001 to 0.7 mol L−1 (0.001, 0.005, 0.01, 0.05, 0.1, 0.2 and 0.7 mol L−1). Most natural waters fall into this range.17a DGT devices were deployed in 30 L of continuously stirred SbIII solutions with different concentrations of NaNO3 at pH 5 for 120 h with a 24 hour interval. To investigate the effect of the foreign ions on the uptake of SbIII, MPS–DGT devices were deployed in 40 L of a well-stirred 100 μg L−1 SbIII of solution containing different foreign ions at 25 °C for 120 h with a 24 hour interval. The foreign ions and their concentrations in this study are listed in Table 1. The deployment solution was equilibrated overnight to obtain a stable pH or ionic strength.
Table 1 Effects of the foreign ions on the uptake of SbIII by MPS–DGT
| Foreign ions |
Concentrations of foreign ions (mg L−1) |
Tolerance ratios (Cforeign ions/CSbIII) |
CDGT (μg L−1) |
CSOLN (μg L−1) |
Ratios of CDGT/CSOLN |
| CaII |
100 |
1000 |
98.7 ± 2.4 |
100.8 ± 1.1 |
0.98 ± 0.03 |
| MgII |
100 |
1000 |
99.8 ± 2.3 |
100.5 ± 1.2 |
0.99 ± 0.03 |
| CdII |
1 |
10 |
96.9 ± 2.4 |
100.1 ± 1.1 |
0.97 ± 0.03 |
| ZnII |
1 |
10 |
96.4 ± 2.0 |
99.8 ± 0.8 |
0.97 ± 0.02 |
| CuII |
1 |
10 |
93.5 ± 3.1 |
100.5 ± 1.0 |
0.93 ± 0.04 |
| PbII |
1 |
10 |
95.1 ± 2.9 |
100.8 ± 0.8 |
0.94 ± 0.03 |
| NiII |
1 |
10 |
99.8 ± 2.5 |
98.8 ± 0.8 |
1.01 ± 0.03 |
| AsIII |
0.1 |
1 |
94.7 ± 2.2 |
99.8 ± 1.8 |
0.95 ± 0.03 |
| AsIII |
1 |
10 |
92.1 ± 3.2 |
100.1 ± 1.8 |
0.92 ± 0.04 |
| SbV |
0.1 |
1 |
98.2 ± 2.3 |
103.4 ± 3.1 |
0.95 ± 0.03 |
| SbV |
1 |
10 |
96.8 ± 2.8 |
105.4 ± 3.4 |
0.92 ± 0.03 |
2.8 Application to waters in the laboratory
The validation of the MPS–DGT were investigated by deploying DGT devices in 30 L of 0.45 μm-filtered natural waters or etching wastewater spiked with 100 μg L−1 SbIII for 24, 48, 72, 96 and 120 h. Grab samples of bulk solution were taken at the beginning and in the end of each deployment. The concentrations of SbIII and total Sb were measured. Prior to deployment of DGT devices, the colloidal material which had coagulated during sample storage was removed from solution by filtering. The natural waters and industrial wastewater were immediately pre-filtered under vacuum through qualitative filter papers before filtering through a 0.45 μm cellulose nitrate membrane in the laboratory. Major cation concentrations, dissolved organic carbon (DOC) and pH of mine wastewater were also shown in Table 2. The concentrations of DOC were measured using a Dohrmanne DC-190 TOC analyzer (USA).
Table 2 Major cation concentrations, dissolved organic carbon and pH value of the Hun river water and etching wastewatera
| Measured parameters |
Water samples |
| Hun river water |
Etching wastewater |
| Major cation concentrations were measured by FAAS after appropriate dilution except Sb. N.D. means not detected. |
| [KI]/mg L−1 |
7.8 ± 1.1 |
2.1 ± 0.6 |
| [NaI]/mg L−1 |
38.4 ± 8.2 |
20.9 ± 7.3 |
| [CaII]/mg L−1 |
62.5 ± 2.5 |
25.5 ± 2.1 |
| [MgII]/mg L−1 |
19.7 ± 1.1 |
8.8 ± 1.0 |
| [CdII]/mg L−1 |
N.D.b |
2.1 ± 0.2 |
| [CuII]/mg L−1 |
N.D. |
58.8 ± 0.2 |
| [PbII]/mg L−1 |
N.D. |
32.7 ± 0.3 |
| [SbIII]/mg L−1 |
N.D. |
N.D. |
| [DOC]/mg C L−1 |
8.7 ± 0.8 |
0.7 ± 0.3 |
| pH |
7.3 ± 0.1 |
4.6 ± 0.1 |
2.9 Detection limit of DGT method
The detection limit of DGT method was determined by calculating 3 times the standard deviation (3σ) of three DGT binding gel blanks and applying eqn (5) for specific time frames (1 day, 3 days, 5 days and 7 days).21
2.10 Antimony analysis
A commercial two channel hydride generation nondispersive atomic fluorescence spectrometer (AFS, Model AFS-2202E, Beijing Haiguang Instrument Co., Beijing, China) equipped with a quartz argon–hydrogen flame atomizer, a quartz gas–liquid separator, and coded high intensity hollow cathode lamps of Sb were used for the measurement of SbIII and total Sb concentrations in solution according to the steps described by Deng et al.22 The concentration of SbIII in solution was determined directly by AFS. The total concentration of SbIII and SbV in solution was measured as follow: in 10 mL aliquot of filtered sample, 2 mL of KI stock solution, 2 mL of 8-hydroxyquinoline stock solutions and 11 mL of HCl (conc.) were added to obtained a 25 mL of final solution.22 This solution was put aside for 20 min at room temperature, and then determined by AFS. The concentration of SbV was obtained by subtracting SbIII from the total inorganic Sb of SbIII and SbV.
3. Results and discussion
3.1 Uptake and digestion
The uptake efficiencies of MPS binding gel disks for SbIII from 0.1, 0.5, and 1 mg L−1 SbIII solution were >99%, while the uptake efficiencies of SbV from 0.1, 0.5, and 1 mg L−1 SbV solution were 8.4 ± 4.6, 9.3 ± 3.8 and 10.5 ± 3.2%. These results indicate that MPS binding gel disks can selectively quantitative accumulate SbIII from aqueous solution. Previous studies have observed the selective adsorption of SbIII by thiol-functionalized sorbents due to the strong interactions of SbIII with the thiolate sulfur.14a,23 Accurate calculation of SbIII by MPS–DGT requires the quantitative and reproducible recovery of metal ions captured in MPS binding gels. Concentrated acids digest and destroy the structure of the gels, with complete release of SbIII from the binding gels disks. Prior to analysis of Sb by AFS, parts of the polyacrylamide re-precipitated, making a filtration or centrifugation step necessary.13 The high acid matrix does not affect the measurement of AFS for SbIII or SbV. The values of Edigestion for SbIII or SbV by MPS binding gels disks were 97.7 ± 2.6 and 98.1 ± 3.3% (mean value ± standard deviation), respectively, which was very close to the digestion efficiency of the precipitated ferrihydrite containing gel described by Luo and co-workers.12 The low standard deviations indicated that the digestion procedure was reproducible.
3.2 Diffusion coefficient in the PES membrane
The DGT-labile concentration is accurately determined relying on the use of diffusion coefficient. For SbIII, applying the Stokes–Einstein equation for temperature correction (eqn (4)), the diffusion coefficient obtained from eqn (3) in PES membrane was (3.05 ± 0.09) × 10−6 cm2 s−1 at pH 5 and temperature 25 °C. There are no published diffusion coefficient data for SbIII measured using a diffusion cell. For SbV, the diffusion coefficient measured using a diffusion cell ((1.71 ± 0.15) × 10−6 cm2 s−1) is ∼19% of the diffusion coefficients in water (9.05 × 10−6 cm2 s−1)24 at 25 °C and lower than the value reported by Panther et al. ((6.04 ± 0.12) × 10−6 cm2 s−1)12 and published by Öterlund et al. ((5.55 ± 0.20) × 10−6 cm2 s−1).25 The differences might arise from the composition variations of diffusion layers.
The diffusion coefficient of SbIII is independent on pH in a 0.01 mol L−1 of NaNO3 matrix (as shown in Fig. 1a). There are only small discrepancies of diffusion coefficient at the different pH values. For a constant pH 5, the diffusion coefficient of SbIII in PES membrane was independent of ionic strength in the range of 1–200 mmol L−1 and decreased at the ionic strength of 700 mmol L−1 (as shown in Fig. 1b), indicating that the high ionic strengths had effects on the diffusion of SbIII through PES membrane as a result of the change of solution viscosity and/or the competition diffusion between SbIII and nitrate.
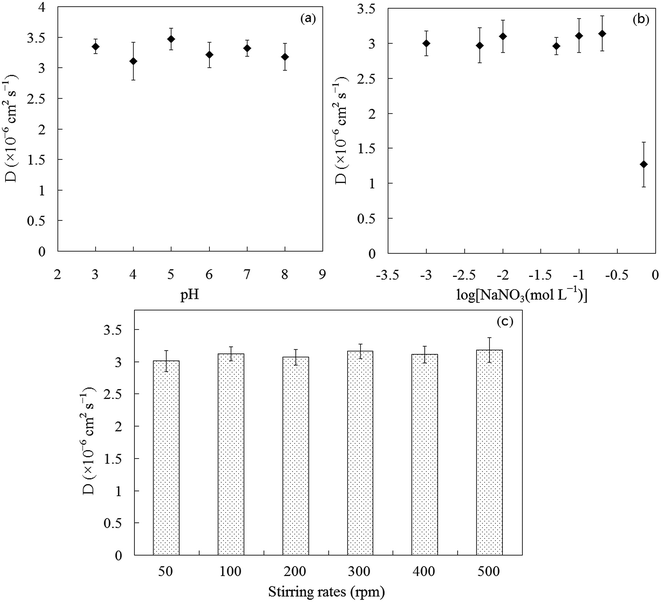 |
| | Fig. 1 Diffusion coefficient as a function of pH (a) and ionic strength (b). Data has been adjusted using eqn (4) for temperature of 25 °C. Error bars represent the standard deviation of repeated experiments. | |
In poorly moving waters, the DBL thickness becomes significant compared with the diffusive layer thickness. This need arises as a result of the diffusion coefficient of metal ions in bulk solution being similar to the diffusion coefficients in the polyacrylamide gels.8 The diffusive gradient within the DBL can also limit the overall mass transport. With the PES membrane diffusive layer described here, changing rates of stirring from 50 to 500 rpm had virtually no influence on the diffusion coefficients of SbIII through PES membrane in the diffusion experiments (Fig. 1c). These experimental results indicate that a DBL does not become significant, even in slower moving waters and the transport across the membrane is the rate-limiting step even under the most critical conditions because the diffusion coefficient in the membrane is lower than the diffusion coefficient in the bulk solution. Although the diffusion coefficient of SbIII in aqueous solution has not been reported in the literature, diffusion coefficient of 7.0 × 10−6 cm2 s−1 for SbIII in 1 mol L−1 of HNO3 solution has been previously reported.26 The diffusion coefficients of metals have been significantly affected by ionic strengths of solution and decrease with the increase of ionic strengths.8,27 In this work, the same trend has been obtained. We may speculate that the diffusion coefficients of SbIII in natural freshwater may be greater than the diffusion coefficients in 1 mol L−1 of HNO3 solution. Diffusion coefficient of SbIII in PES membrane will always be lower than the diffusion coefficient of the metal ions in solution, the formation of a DBL layer at the membrane interface will usually have a very minor contribution to the overall mass transport. This means the thickness of the membrane can be used as Δg in the DGT equation under most deployment conditions without needing to correct for the DBL thickness.
3.3 Accumulation over time
The MPS–DGT was validated by examining the relationship between the mass of SbIII accumulated in MPS and the deployment time with a SbIII or SbV solution with fixed concentration. The measured mass of SbIII accumulated by MPS–DGT increased linearly with deployment time over 120 h (R2 = 0.9973) and fitted the theoretical line which calculated from the known solution concentrations (Fig. 2). Based on these data, the concentrations of SbIII were calculated using eqn (5). The fraction of labile SbIII in synthetic solution was 94.5 ± 3.2% of the soluble SbIII concentration, where free species dominated, however, the linear uptake of SbV with time is not observed by MPS–DGT (Fig. 2). This result is consistent with the low uptake efficiency of MPS for SbV, suggesting that accumulation of SbV is limited by thermodynamic factors.28 Previous studies have observed the weak coordination interactions of SbV by thiol groups.29 These results partially confirm that the new MPS–DGT devices meets the assumptions of the DGT equation, ensuring that the concentration of analyte at the interface between the binding gel and diffusive layer was effectively zero. These results confirm that MPS can be used as an appropriate DGT binding agent for the selective accumulation of SbIII.
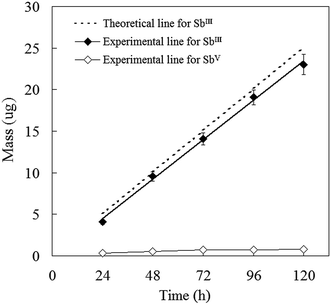 |
| | Fig. 2 Dependence of mass of SbIII and SbV accumulated by MPS–DGT on deployment time. The dashed line is the theoretical slopes calculated from known concentrations of SbIII in solution. | |
3.4 Effect of pH
In order to test the effect of the solution pH on the uptake of SbIII by MPS binding gel, MPS–DGT devices were deployed in synthetic solution at different pH from 3 to 8. Fig. 3a shows the ratio of DGT-measured concentrations (CDGT) to the independently measured concentrations of the bulk solutions (CSOLN) over a range of pH values. The results showed that the uptake of SbIII by MPS–DGT devices was satisfactory in pH range of 3–8, indicating that the charge of bulk solution pH did not significantly affect the uptake performance of SbIII by MPS–DGT. The pH of natural fresh waters normally falls into this range.
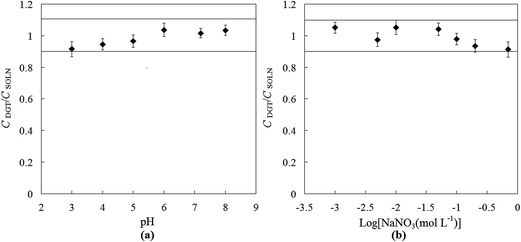 |
| | Fig. 3 (a) Effect of pH on the performance of MPS–DGT, assessed by the ratio of CDGT to CSOLN, accounting for the change with pH at 25 °C. Ionic strength was kept at 0.01 mol L−1 for all experiments. (b) Effect of ionic strengths on the performance of MPS–DGT at pH 5 at 25 °C. Mean values and error bars of triplicate measurements are given. The solid lines indicate ±10% of the CDGT/CSOLN ratio. | |
3.5 Effect of ionic strength
To visualize the effect of the ionic strength on uptake of SbIII by MPS–DGT, the ratios of CDGT/CSOLN is plotted versus the log value of the ionic strength. At ionic strengths range from 0.001 to 0.7 mol L−1, the values of CDGT for SbIII were close to CSOLN (Fig. 3b). This suggests that varying the ionic strength of the solution over 3 orders of magnitude did not have a substantial effect on the uptake of SbIII by the MPS–DGT.
3.6 Interference test
MPS gel probably also retain other elements besides SbIII in water samples, such as ZnII, CuII, PbII, CdII and AsIII.30 The results illustrated in Table 1 show that with one exception, the ratios of CDGT/CSOLN were in the range of 0.9–1.1. The presence of CaII, MgII, CdII, ZnII, PbII, CuII, AsIII and SbV ions in the sample solution had no obvious influence on the uptake of MPS–DGT for SbIII under the selected conditions due to the thermodynamically strong interactions between SbIII ions and thiol groups.14a Thus, MPS–DGT device clearly offers a high selectivity for SbIII ions.
3.7 Detection limit
The standard deviation of SbIII blank values for 3 DGT binding gels was 0.05 μg. The detection limit for each DGT mass is, therefore, 0.15 μg (3 × 0.05 μg). Using eqn (5) with a standard DGT configuration (3.14 cm2 exposure window area, 110 μm diffusive gel), and assuming a pH of 5 and temperature of 25 °C, the method detection limits (MDL) for DGT over time frames of 1 day, 3 days, 5 days and 7 days are 1.99, 0.664, 0.498, and 0.285 ng L−1, respectively. As the environmental concentration of SbIII is likely to be in the ng L−1 range, the data show that low ng L−1 detection limits can be achieved for the deployment time at least 3 days.
3.8 Performance in spiked waters
To evaluate the performance of the DGT method for the uptake of dissolved inorganic SbIII in waters, DGT devices were deployed in natural freshwater and an etching wastewater spiked with SbIII. No SbIII could be detected in the river water and etching wastewater by direct measurement of AFS, and hence samples were spiked with aliquots of SbIII to give a SbIII concentration of ∼100 μg L−1. Reproducibility of MPS–DGT in the spiked waters was very good, with relative standard deviations of less than 6%. For etching wastewater, the value of CDGT/CSOLN (0.95 ± 0.05) between 0.9 and 1.1 is obtained, indicating that the fraction of dissolved inorganic SbIII measured by MPS–DGT is in good agreement with the added SbIII concentration, and MPS–DGT can quantitatively measure the concentration of dissolved inorganic SbIII in etching wastewater over the 5 day deployment. Such good agreement indicates that the coexisting ions in etching wastewater have no influence on the uptake of dissolved inorganic SbIII by MPS–DGT. For natural freshwater, CDGT (58.1 ± 3.4 μg L−1) is significantly lower than CSOLN (90.2 ± 4.2 μg L−1). It is possible that the speciation of added SbIII have be changed by the presence of natural organic matter (such as humic acid) in the natural freshwater, which may be affecting the uptake of MPS–DGT for dissolved inorganic SbIII due to the interactions between SbIII ions and natural organic matter.31 Buschmann et al.32 report that over 30% of total SbIII may be bound to natural organic matter under environmentally relevant conditions. Tella and Pokrovski found that SbIII can form complexes with the natural organic matter having O- and N-functional groups and 35% of total dissolved SbIII binds to aqueous natural organic matter via carboxylic and hydroxy-carboxylic groups.33 The MPS–DGT estimates are in a good agreement with the literature values On the other hand, prior to direct AFS measurements, samples were filtered through a 0.22 μm pore-size membrane and acidified. Acidification may release SbIII binding with natural organic matter which remain in the sample, and contributing to the higher value of CSOLN than CDGT. Thus, these results obtained in spiked waters demonstrate the potential and robustness of MPS–DGT to assess SbIII in common water.
4. Conclusions
In this study, a reliable DGT device with MPS as the binding agents and PES membranes as the diffusion layer was developed for the selective measurement of SbIII in aquatic system. This paper presents the first published measurements of the diffusion coefficient of SbIII in PES membranes. The change in diffusion coefficient at high ionic strength of 0.7 mol L−1 NaNO3 is possibly due to the change of solution viscosity or the competition diffusion. Over the pH range of 3–8 and ionic strengths from 0.001 to 0.7 mol L−1, the mass of SbIII accumulated by MPS–DGT agreed well with the mass of SbIII which predicted from the known solution composition. Furthermore, the presence of cations at concentrations up to 1–3 fold higher than those in synthetic solution, has no significant influence on the uptake of SbIII by MPS–DGT. The precision of the results from replicate DGT devices were generally higher than 90%. The competition adsorption between SbIII and HgII onto MPS affects the accumulation of SbIII by MPS–DGT. When MPS–DGT was applied to spiked etching wastewater in the laboratory, a good agreement was obtained between the CDGT and CSOLN. In spiked natural freshwaters, CDGT is significant lower than CSOLN due to interactions between SbIII ions and natural organic matter. This work has demonstrated that the suitability of MPS–DGT for the measurement of inorganic SbIII species in natural waters is likely to be useful for the assessment of SbIII availability in natural waters. Future research may involve testing the suitability of MPS–DGT to assess SbIII in water under field conditions and determine whether DGT assessment of SbIII matches with availability in waters.
Acknowledgements
The project was sponsored by the National Natural Science Foundation of China (grant no. 21107076 and 21477082), by Liaoning Natural Science Foundation Combined with Open Foundation of Shenyang National Laboratory for Materials Sciences (grant no. 2015021019), and by program for Liaoning Excellent Talents in University of China (grant no. LJQ2015050).
References
- USEPA, Water Related Fate of the 129 Priority Pollutants, USEPA, Washington, DC, 1979, vol. 1 Search PubMed.
- S. Inaba and C. Takenaka, Environ. Int., 2005, 31, 603–608 CrossRef CAS PubMed.
- Z. Fan, Microchim. Acta, 2005, 152, 29–33 CrossRef CAS.
-
(a) M. Fillela, N. Belzie and Y.-W. Chen, Earth-Sci. Rev., 2002, 57, 125–176 CrossRef;
(b) M. C. He and J. R. Yang, Sci. Total Environ., 1999, 243, 189–196 Search PubMed.
- W. Davison and H. Zhang, Nature, 1994, 367, 546–548 CrossRef CAS.
-
(a) W. Li, F. Wang, W. Zhang and D. Evans, Anal. Chem., 2009, 81, 5889–5895 CrossRef CAS PubMed;
(b) D. Xu, Y. Chen, S. Ding, Q. Sun, Y. Wang and C. Zhang, Environ. Sci. Technol., 2013, 47, 10477–10484 CAS;
(c) Y. Tian, X. Wang, J. Luo, H. Yu and H. Zhang, Environ. Sci. Technol., 2008, 42, 7649–7654 CrossRef CAS PubMed.
- H. Zhang and W. Davison, Anal. Chem., 1995, 67, 3391–3400 CrossRef CAS.
- W. Li, P. R. Teasdale, S. Zhang, R. John and H. Zhao, Anal. Chem., 2003, 75, 2578–2583 CrossRef CAS PubMed.
- J. Dong, H. Fan, D. Sui, L. Li and T. Sun, Anal. Chim. Acta, 2014, 822, 69–77 CrossRef CAS PubMed.
- B. L. Larner and A. J. Seen, Anal. Chim. Acta, 2005, 539, 349–355 CrossRef CAS.
- Ø. A. Garmo, O. Røyset, E. Steinnes and T. P. Flaten, Anal. Chem., 2003, 75, 3573–3580 CrossRef PubMed.
- J. Luo, H. Zhang, J. Santner and W. Davison, Anal. Chem., 2010, 82, 8903–8909 CrossRef CAS PubMed.
- J. G. Panther, R. R. Stewart, P. R. Teasdale, W. W. Bennett, D. T. Welsh and H. Zhao, Talanta, 2013, 105, 80–86 CrossRef CAS PubMed.
-
(a) H. Sun, S. C. Yan and W. S. Cheng, Eur. J. Biochem., 2000, 267, 5450–5457 CrossRef CAS PubMed;
(b) I. I. Ozturk, N. Kourkoumelis, S. K. Hadjikakou, M. J. Manos, A. J. Tasiopoulos, I. S. Butler, J. Balzarini and N. Hadjiliadis, J. Coord. Chem., 2011, 64, 3859–3871 CrossRef CAS.
-
(a) F. Shakerian, S. Dadfarnia, A. M. H. Shabani and M. N. Ahmadabadi, Food Chem., 2014, 145, 571–577 CrossRef CAS PubMed;
(b) I. López-García, R. E. Rivas and M. Hernández-Córdoba, Talanta, 2011, 86, 52–57 CrossRef PubMed.
- W. W. Bennett, P. R. Teasdale, J. G. Panther, D. T. Welsh and D. F. Jolley, Anal. Chem., 2011, 83, 8293–8299 CrossRef CAS PubMed.
-
(a) Y. Gao, S. D. Craemer and W. Baeyens, Talanta, 2014, 120, 470–474 CrossRef CAS PubMed;
(b) J. Liu, X. Feng, G. Qiu, C. W. N. Anderson and H. Yao, Environ. Sci. Technol., 2012, 46, 11013–11020 CrossRef CAS PubMed.
- B. L. Larner and A. J. Seen, Anal. Chim. Acta, 2005, 539, 349–355 CrossRef CAS.
- S. Scally, W. Davison and H. Zhang, Anal. Chim. Acta, 2006, 558, 222–229 CrossRef CAS.
- N. E. Dorsey. Properties of ordinary water-substance in all its phases: water-vapor/water and all ices, Reinhold Pub. Co., New York, 1940 Search PubMed.
- A. Lucas, A. Rate, H. Zhang, S. U. Salmon and N. Radford, Anal. Chem., 2012, 84, 6994–7000 CrossRef CAS PubMed.
- T.-L. Deng, Y.-W. Chen and N. Belzile, Anal. Chim. Acta, 2001, 432, 293–302 CrossRef CAS.
-
(a) H. Fukuda, J. Tsunoda, K. Matsumoto and K. Terada, Bunseki Kagaku, 1987, 36, 683–687 CrossRef CAS;
(b) M.-Q. Yu, G.-Q. Liu and Q.-H. Jin, Talanta, 1983, 30, 265–270 CrossRef CAS PubMed;
(c) S. Asaoka, Y. Takahashi, Y. Araki and M. Tanimizu, Anal. Sci., 2011, 27, 25–28 CrossRef CAS PubMed.
- H. Öterlund, S. Chlot, M. Faarinen, A. Widerlund, I. Rodushkin, J. Ingri and D. C. Baxter, Anal. Chim. Acta, 2010, 682, 59–65 CrossRef PubMed.
- Y. H. Li and S. Gregory, Geochim. Cosmochim. Acta, 1974, 38, 703–714 CrossRef CAS.
- J. Schoenleber, N. Stein and C. Boulanger, J. Electroanal. Chem., 2014, 724, 111–117 CrossRef CAS.
- H. Chen, T. Sun, D. Sui and J. Dong, Anal. Chim. Acta, 2011, 698, 27–35 CrossRef CAS PubMed.
- V. K. Jain, R. Bohra and R. C. Mehrotra, Structure and Bonding in Organic Derivatives of Antimony(V), Structure and Bonding 52, Springer-Verlag, Berlin, Heidelberg, 1982, pp. 148–196 Search PubMed.
- C. Ma, Q. Zhang, J. Sun and R. Zhang, J. Organomet. Chem., 2006, 691, 2567–2574 CrossRef CAS.
-
(a) R. Celis, M. C. Hermosian and J. Cornejo, Environ. Sci. Technol., 2000, 34, 4593–4599 CrossRef CAS;
(b) M. Jaber, J. Miehé-Brendlé, L. Michelin and L. Delmotte, Chem. Mater., 2005, 17, 5275–5281 CrossRef CAS;
(c) A. M. Spuches, H. G. Kruszyna, A. M. Rich and D. E. Wilcox, Inorg. Chem., 2005, 44, 2964–2972 CrossRef CAS PubMed.
- J. Pilarski, P. Waller and W. Pickering, Water, Air, Soil Pollut., 1995, 84, 51–59 CrossRef CAS.
- J. Buschmann and L. Sigg, Environ. Sci. Technol., 2004, 38, 4535–4541 CrossRef CAS PubMed.
- M. Tella and G. S. Pokrovski, Geochim. Cosmochim. Acta, 2009, 73, 268–290 CrossRef CAS.
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here.