Effect of humic acid on prometryn bioaccumulation and the induction of oxidative stress in zebrafish (Danio rerio)
Qian Zhao and
Lin Zhu*
MOE Key Laboratory of Pollution Processes and Environmental Criteria, Tianjin Key Laboratory of Environmental Remediation and Pollution Control, College of Environmental Science and Engineering, Nankai University, No. 94 Weijin Road, Nankai District, Tianjin 300071, China. E-mail: zhulin@nankai.edu.cn; Fax: +86-22-23508936; Tel: +86-22-23508807
First published on 29th January 2016
Abstract
Humic acid (HA) is the main component of dissolved organic matter in aquatic ecosystems and affects the bioavailability of contaminants. Prometryn is frequently used to control annual broadleaf and grass weeds and has been widely detected in the aquatic environment. An experiment was conducted to investigate the effect of HA on the chronic toxicity of prometryn in zebrafish. In zebrafish treated with 53.2 μg L−1 prometryn (P), prometryn with 5 mg L−1 HA (P + HA5), or prometryn with 15 mg L−1 HA (P + HA15), catalase (CAT) activity and glutathione (GSH) content in the visceral mass initially increased and then decreased with exposure time. After one day of exposure, malondialdehyde in the visceral mass increased by 58% to 64% in fish treated with prometryn and HA compared to those treated with prometryn alone, however, this difference disappeared during days 10 to 40. Treatment with HA enhanced the bioaccumulation of prometryn by 34% and 40% on day 1 in the P + HA5 and P + HA15 groups, respectively, possibly due to changes in gill membrane permeability. Nevertheless, the opposite result was observed during days 10 to 40, owing to the presence of more excreta and suspended particulate matter resulting in a reduction in the quantity of free dissolved prometryn in the P + HA5 and P + HA15 groups. There were significant correlations between CAT activity and prometryn concentration (0.562**, P < 0.01) and between GSH and prometryn concentration (0.808**, P < 0.01) throughout the first 20 days of the experiment, suggesting a role for these antioxidant systems in the detoxification of prometryn in zebrafish.
1. Introduction
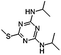
Prometryn (2,4-bis[isopropylamino]-6-methylthio-s-triazine) is an s-triazine herbicide that is widely used to control many annual gramineous, broad-leaved, and perennial terrestrial weeds.1 In addition, prometryn is also used to remove filamentous algae, aquatic weeds, and other harmful algae in the fish, shrimp, crab, shellfish, and sea cucumber aquaculture industries. Prometryn interferes with photosynthesis by inhibiting electron transport.2 Prometryn has been prohibited in Europe since 2004,3 yet it is still widely used in China,4 the United States, Canada, New Zealand, and South Africa.5 Because of its high chemical stability, prometryn persists in the aquatic environment and has the potential to harm non-target organisms. Ren et al.6 detected prometryn in 100% of samples collected from 60 sites in the Bohai sea, with the maximum concentration of prometryn reaching up to 7.12 ± 0.54 μg L−1 in a river in Shanghai.7 Ma et al.8 measured the concentration of prometryn at Shanghai waterworks on the Huangpu river for one year and reported that the average concentration of prometryn was the highest in April (1.25 μg L−1). Furthermore, the detected concentrations of prometryn ranged from 0.91 to 4.40 μg L−1 in surface water and exceeded 1 μg L−1 in ground water in Greece.9 Since its stability and persistence, prometryn is also widely residual in the aquatic environment.Dissolved organic matter (DOM) participates in and is an important regulator of the processes of biogeochemical conversion, which include global nutrient and carbon cycling, metal redox reactions, and cation complexation.10 The concentration of DOM varies greatly in different water bodies and is also dependent upon the season and water depth. Typically, the concentration of DOM ranges from 0 to 15 mg C L−1 (denoted as the dissolved organic carbon) in surface water.11 Humic acid (HA) is the main component of DOM in aquatic ecosystems.12 Previous studies have reported that HA decreases the bioavailability and toxicity of organic contaminants through binding and adsorption.13,14 However, other studies have indicated that HA results in higher bioavailability and toxicity of organic contaminants by increasing the compound solubility15,16 or changing fish gill membrane permeability.17,18 Nevertheless, the mechanism by which HA increases the biological toxicity of organic pollutants remains unclear. Matsuo et al.15 reported that both P450 1A (CYP 1A) expression and 7-ethoxyresorufin-O-deethylase (EROD) activity in the liver of tambaqui (Colossoma macropomum) were induced 2-fold by crude oil in combination with HA versus crude oil alone. Moreover, previous studies have indicated that DOM enhances Na+ flux in Daphnia magna and the gills of fish16,19 due to increased membrane permeability as a result of DOM adsorption,18,20 demonstrating that DOM has direct effects on these organisms.21,22 Galvez et al.17 suggested that the gill epithelium of rainbow trout (Oncorhynchus mykiss) developed a hyperpolarized transepithelial potential (TEP) following exposure to HA (10 mg L−1). This phenomenon is likely the result of a reduction in the concentration of certain ions, such as Ca2+, which could affect the biological membrane permeability.
Previous studies have mainly focused on the effect of prometryn alone on fish,23,24 however, the effect of HA on prometryn toxicity in fish has not been addressed. Our previous research demonstrated that 15 mg L−1 of HA increased the acute toxicity of prometryn in Danio rerio (zebrafish) during a 96 h exposure.25 An HA-accelerated bioaccumulation of prometryn in zebrafish was proposed as an explanation for this increased toxicity. However, this result was inconsistent with other reports,26–28 in which HA was observed to reduce the bioavailability of organic contaminants and the associated toxicity.
In this study, a chronic exposure experiment was conducted to investigate the toxicity of prometryn in the presence of HA. It was hypothesized that the chronic toxic effects of prometryn combined with HA would be consistent with the acute effects that were previously observed. Indicators of oxidative stress (malondialdehyde [MDA] content, catalase [CAT] activity, and glutathione [GSH] content) and the bioaccumulation of prometryn were investigated in zebrafish exposed to a combination of prometryn and HA. Additionally, the gill Na+/K+–ATPase activity and the electrical conductivity of the exposure solution were also evaluated. This work was aimed at providing strong evidence for the enhanced toxicity of an organic contaminant in the presence of DOM in aquatic ecosystem.
2. Materials and methods
2.1. Animals
Zebrafish (body length of 4.6 ± 0.6 cm, weight of 0.49 ± 0.09 g) were bred and maintained in the laboratory in a tank (95 × 43 × 45 cm) containing dechlorinated tap water (pH of 7.5 ± 0.3, temperature of 24 ± 2 °C, dissolved oxygen concentration of 7.83 ± 0.2 mg L−1, DOM ≪ 1 mg L−1) that was continuously aerated. All fish were fed with a commercial fish food (Inch-gold Fish Food Limited Company, Shenzhen, China) at a rate of 1% of body weight per day. The excreta at the bottom of the tank were removed in time. The natural mortality rate was <1% prior to the beginning of the experiment. Feeding was suspended for 24 h before the exposures. All animal experiments were carried out according to the “Measures for the Administration of Experimental Animals Permit” and the Guidelines of Science and Technology Committee, Tianjin, China.2.2. Toxicity tests
Prometryn (purity > 97%) was purchased from Zhongshan Import and Export Corporation (Zhejiang, China). The stock solution of prometryn was prepared with dimethyl sulfoxide (DMSO) as the carrier solvent, sonicated for 30 min at 45 °C, filtered through a 0.45 μm membrane, and stored at 4 °C. All experimental prometryn solutions were prepared by diluting the stock solution in water. The final concentration of DMSO in the water was always less than 0.05%. The prometryn standard curve was prepared using a prometryn standard substance (Aladdin, USA, purity > 99%, CAS: 7287-19-6). The properties of prometryn were shown in Table 1.The stock solutions of HA (Sigma, St. Louis, MO, USA; CAS: 1415-93-6) were prepared by dissolving HA into deionized water, stirring for 24 h, standing for 12 h, and filtering through a 0.45 μm membrane. The concentration of total organic carbon (TOC, mg C L−1), which corresponds to the concentration of HA, was measured using an Analytik Jena multi N/C 3100 (Jena, Germany).
On days 1, 5, 10, 20, and 40, six zebrafish were randomly sampled from each group. Every three fish were dissected and pooled all organs together (visceral mass) for the analysis of CAT, GSH, and MDA. In addition, on days 1, 5, 10, 20, 30, and 40, six fish were collected, rinsed with deionized water, dried with filter paper, and stored at −80 °C for future determination of prometryn bioaccumulation.
The prometryn concentration in the exposure solution was evaluated at 0, 1, 4, 8, 12, 24, 48, and 96 h, and also on days 5, 10, and 15. Furthermore, the zebrafish excreta was collected on days 5, 10, 15, 20, and 25 before renewing the exposure solution. Following collection, the excreta were freeze-dried and weighed.
2.3. Determination of prometryn concentrations
After freeze-drying for 48 h, the zebrafish were ground to a powder and then 0.2 g of the sample (mixed with 2 g of quartz sand) was extracted using accelerated solvent extraction (ASE) with a Speed Extractor E-916 (BUCHI, Switzerland). Acetonitrile (analytical grade; Kangkede, Tianjin, China) was the extraction solvent. The operating conditions of the ASE were as follows: extraction temperature = 100 °C, pressure = 100 bar, heating time = 5 min, static extraction time = 8 min, extraction pool size = 20 mL, purging time = 120 s, and number of extraction cycles = 3. The extract was transferred to pear-shaped bottle and evaporated at 75 °C using a rotary evaporator (Heidolph Laborota 4000 efficient; Heidolph, Schwabach, Germany). The residue was dissolved in 2 mL of a mixture of n-hexane and ethyl acetate (v/v = 3/2) and filtered through florisil columns (1 g, 6 mL; CNW Technologies, Düsseldorf, Germany). The collected filtrate was dried using a pressure blowing concentrator (Huaruiboyuan MTN-2800W; Huaruiboyuan, Beijing, China). The dried residue was dissolved in 2 mL of methanol and filtered through a 0.45 μm organic membrane filter. Collected water samples were filtered through a 0.45 μm organic membrane filter without any pretreatment.Prometryn concentrations were determined using ultra performance liquid chromatography-tandem mass spectrometry (UPLC-MS) (OA_SPE Waters Xevo TQ-S; Waters Corporation, Milford, MA, USA). The UPLC-MS was performed on an ACQUITY UPLC® BEH C18 column (1.7 μm, 2.1 × 50 mm, Waters, USA) at a temperature of 55 °C in a 75%/25% mixture (v/v) of methanol (chromatography grade; Merck, Germany) (A) and 0.1% formic acid aqueous solution (chromatography grade; CNW Technologies; Milli-Q water) (B) at a flow rate of 0.3 mL min−1, using an injection volume of 10 μL, a retention time of 0.79 min, and a pressure ripple of 3939–4922 psi. Mass spectrometry conditions were as follows: ion mode of ESI+, ionization voltage = 3.0 kV, cone voltage = 25 V, source temperature = 150 °C, desolvation temperature = 350 °C, cone gas flow = 144 L h−1, desolvation gas flow = 596 L h−1, collision gas flow = 0.14 mL min−1, and prometryn detection pair of 242 > 158, 242 > 200 (quantitative ion pair). The correlation coefficient of the prometryn standard curve obtained was >0.99.
2.4. Index of toxicology
Six fish from each group were dissected on an ice-cold plate and the visceral mass was excised, washed immediately, and homogenized in a 0.86% physiological saline solution (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 9, w/v). The homogenate was centrifuged at 2500 g min−1 for 15 min (Hettich Mikro 200R; Hettich, Tuttlingen, Germany). The supernatant fluid was diluted to appropriate concentrations for further analysis.
9, w/v). The homogenate was centrifuged at 2500 g min−1 for 15 min (Hettich Mikro 200R; Hettich, Tuttlingen, Germany). The supernatant fluid was diluted to appropriate concentrations for further analysis.
MDA content of the supernatant was analyzed using the thiobarbituric acid reactive substances (TBARS) method to evaluate lipid peroxidation. GSH and protein content, and the activities of CAT and Na+/K+–ATPase, were measured using commercial kits (Jiancheng Bioengineering Institute, Nanjing, China).
2.5. Statistical analysis
The results in the figures and tables were reported as the mean ± standard error (SE). Statistical analysis of all data was performed using one-way ANOVA with SPSS 20 software. Multiple comparisons were conducted using Duncan's multiple range test and differences were considered significant when P < 0.05. Figures were constructed using Origin 8.5.3. Results and discussion
3.1. Toxicological parameters
3.2. Prometryn accumulation in zebrafish
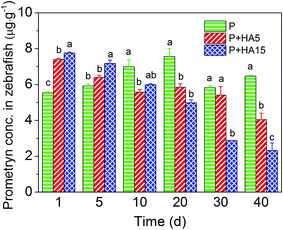
The recovery of prometryn from zebrafish was between 90% and 115%. Prometryn was not detected in zebrafish from the CK or HA15 treatment groups throughout the 40 days of exposure. On days 1 and 5, prometryn accumulation in zebrafish in the P + HA5 group increased by 34% and 8%, respectively, while in the P + HA15 group it increased by 40% and 21%, respectively, compared to those in the P group. However, prometryn accumulation in the P + HA5 and P + HA15 groups decreased by 37% and 64%, respectively, relative to the P group during days 10 to 40 and these differences were significant (P < 0.05, Fig. 5).3.3. Prometryn concentrations in the exposure system
The decrease of prometryn concentration in the exposure solution was less than 10% after five days in all groups. The exposure concentration of prometryn in the P, P + HA5, and P + HA15 groups gradually decreased over time. Prometryn concentrations in the HA groups were lower than that in the groups without HA, and this difference increased gradually over time (Fig. 6). On day 15, prometryn concentrations in the P + HA5 and P + HA15 groups decreased by 8% and 12% relative to the P group, respectively (Fig. 6). It was noteworthy that an opposite trend was found in the dry weight of fish excreta; the dry weight of fish excreta increasing over time in the HA groups compared to the non-HA groups. On day 25, the dry weight of fish excreta in the P + HA15 group was 1.96 times higher than that in the P group (Fig. 7). | ||
| Fig. 6 Prometryn concentrations in the exposure systems containing zebrafish treated with prometryn (P) and humic acid (HA) for 15 days. | ||
 | ||
| Fig. 7 Dry weight of excreta collected from exposure systems containing zebrafish treated with prometryn (P) and humic acid (HA) for 25 days. | ||
3.4. Correlation analysis
Fish metabolic processes result in the production of reactive oxygen species in the presence of stressors such as herbicides, including prometryn, which leads to oxidative stress and damage to the fish (e.g. lipid peroxidation, as indicated by MDA). In response this stress, the antioxidant defense system (CAT and GSH) is activated to clear the reactive oxygen species in order to maintain homeostasis. Accordingly, MDA, CAT, and GSH are typically used as indicators of oxidative stress in order to evaluate the biological toxicity of environmental contaminants. In the P, P + HA5, and P + HA15 groups, the changes in the trends of CAT activity and GSH content in the visceral mass were coincident with those of prometryn accumulation in zebrafish, suggesting a role for these factors in the process of prometryn detoxification. Pearson correlation analysis indicated that there were significant correlations between prometryn accumulation and CAT activity (0.562**, P < 0.01) and GSH content (0.808**, P < 0.01) during the first 20 days of the experiment (Table 2). Antioxidant parameters are meaningful for the investigation of the presence of, and the response to, certain environmental contaminants that are easily degraded and thus difficult to measure. Therefore, these parameters can be chosen as indicators to evaluate the toxicity of contaminants during short-term exposures.| CAT | GSH | P | MDA | EC | WE | Pw | Na+–K+–ATPase | |
|---|---|---|---|---|---|---|---|---|
| a P, prometryn concentration in zebrafish; EC, electrical conductivity; WE, dry weight of excreta; PW, prometryn concentrations in the exposed solution; Na+–K+–ATPase, Na+–K+–ATPase activity in zebrafish gills.b n = 24.c n = 18.d n = 6.e n = 12.f *, correlation is significant at the 0.05 level (2-tailed); **, correlation is significant at the 0.01 level (2-tailed). | ||||||||
| CATb | 1 | |||||||
| GSHc | 0.696** | 1 | ||||||
| 0.001 | ||||||||
| Pb | 0.562** | 0.808** | 1 | |||||
| 0.004 | 0.000 | |||||||
| MDAb | 0.418* | 0.351 | 0.114 | 1 | ||||
| 0.042 | 0.153 | 0.596 | ||||||
| ECd | −0.918** | −0.885* | −0.980** | −0.833* | 1 | |||
| 0.010 | 0.019 | 0.001 | 0.039 | |||||
| WEb | −0.330 | −0.598* | −0.430 | −0.491* | −0.719 | 1 | ||
| 0.115 | 0.009 | 0.036 | 0.015 | 0.108 | ||||
| PWe | −0.108 | −0.804 | −0.491 | −0.223 | 0.962** | −0.815** | 1 | |
| 0.739 | 0.054 | 0.105 | 0.485 | 0.002 | 0.001 | |||
| Na+–K+–ATPased | 0.585 | 0.314 | 0.713 | 0.666 | −0.617 | 0.981** | −0.775 | 1 |
| 0.222 | 0.545 | 0.112 | 0.149 | 0.192 | 0.001 | 0.070 | ||
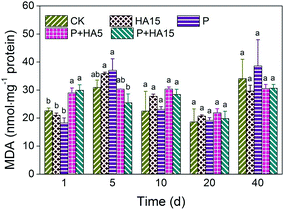
In the early stages of the exposure (day 1), the content of MDA in the visceral mass of zebrafish in the P + HA5 and P + HA15 groups were higher than those in any other group. This suggested that damage as a result of lipid peroxidation was enhanced by HA in fish exposed to prometryn. This result was consistent with that of our previous experiment in which the 96 h acute lethality of prometryn in zebrafish was increased by treatment with 15 mg L−1 HA.25 Meanwhile, prometryn accumulation in fish from the P + HA5 and P + HA15 groups increased by 34% and 40%, respectively, compared with those in the P group. Correspondingly, GSH content and CAT activity also increased on the first day of exposure, indicating that GSH and CAT play roles in the detoxification of prometryn in the early stages of exposure. Nevertheless, during days 10 to 40, prometryn accumulation, GSH content, and CAT activity in the P + HA5 and P + HA15 groups decreased relative to the P group. However, MDA levels in the zebrafish did not change significantly during this time. A possible explanation for these changes is homeostatic processes that zebrafish set up the detoxification mechanisms for defense and discharge of prometryn. Eventually, the physiological metabolism processes of the fish would reach a new level of homeostasis through these mechanisms. In addition, the concentration of free dissolved prometryn gradually decreased in the exposure solution, possibly a result of the adsorption of prometryn to the remaining fecal matter and other suspended particulate matters, which may have also eased the level of lipid peroxidation experienced by the zebrafish.
3.5. Effect of HA on prometryn toxicity in zebrafish
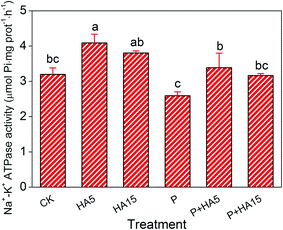
Humic acid is an important type of DOM in aquatic ecosystem and influences the environmental behavior of hydrophobic organic contaminants according to a variety of functional groups such as hydroxyl, carboxyl, phenolic hydroxyl, and enol hydroxyl groups.29,30 Previous studies have demonstrated that pollutant bioaccumulation and toxicity, in addition to organismal antioxidant capacity, were modified by HA.26,31 On days 1 and 5, prometryn accumulation in zebrafish from the groups treated with HA (P + HA5 and P + HA15) increased compared to the group exposed to prometryn alone. This may have been due to changes in gill membrane permeability caused by HA.17,18 Steinberg et al.32 suggested that DOM enhanced the bioaccumulation of terbuthylazine (TBA) in fish by interfering with the permeability of the cell membrane. In this study, gill Na+–K+–ATPase was higher in the P + HA5 and P + HA15 groups than in the P group, indicating that the permeability of the gill cell membrane was altered by HA. Galvez et al.17 reported that there was a direct effect on ion transfer and penetration function in the gills of rainbow trout treated with 10 mg C L−1 HA due to the enhancement of transepithelial hyperpolarization through the complexation of Ca2+. A reduction in Ca2+ concentration, therefore, can lead to changes in the permeability of the gills and affect other ions such as Cl− and Na+. Loice et al.18 indicated that the permeability of a simulated biological membrane was increased by HA. Other scholars have also suggested that the presence of HA resulted in changes in sodium metabolism33 or promoted the resistance to adverse environmental conditions by regulating Na+ flux in fish gills.17 The electrical conductivity of the exposure solution was measured in the current experiment as there is a close relationship between the permeability of a biological membrane and the ion content of the environment. The results showed that the electrical conductivity of the exposure solution decreased in the presence of HA. Compared with CK, a 7% decrement of electrical conductivity was observed in the solution of P + HA15 (Fig. 8). A decreased ion concentration in the exposure solution in the presence of HA likely contributed to changes in the permeability of the biological membranes of the zebrafish.Xia et al.30 reported that HA (1 mg L−1) enhanced the bioaccumulation of perfluoroalkyl substances (PFAS) in D. magna by increasing the rate of uptake above the rate of depuration. In addition, the molecular mass of DOM also affects the bioaccumulation of pollutants in organisms. Hudson et al.34 suggested that high molecular weight (>1 kDa) of DOM increased the absorption of dissolved cadmium, silver, and mercury in zebra mussels (Dreissena polymorpha) compared with low molecular weight of DOM (<1 kDa).
When the exposure time was prolonged, prometryn bioaccumulation in zebrafish in the three treatment groups (P, P + HA5 and P + HA15) was changed in the presence of HA. During days 10 to 40 of the current experiment, prometryn bioaccumulation in zebrafish in the group without HA (P) was reduced compared to the groups treated with HA (P + HA5 and P + HA15). Two potential explanations for this result included an increased metabolic rate and increased depuration dose of prometryn in zebrafish. Previous studies have confirmed that HA can be used as an indirect or direct energy source by fish and enhance the lifespan and fertility of Daphnia magna under extreme circumstances.22 In addition, increased fish activity has been reported following treatment with HA.32 A higher quantity of excreta and suspended particulate matter were formed in the exposure solution containing fish treated with HA (P + HA5 and P + HA15), suggesting a higher metabolic rate among these fish. In the exposure system, the excreta and suspended particulate matter may have interacted with HA and prometryn, resulting in the adsorption of prometryn and thus causing a decrease in the concentration of prometryn in the exposure solution. This reduction of prometryn in the exposed system would eventually reduce the uptake of prometryn by zebrafish. This relationship between prometryn concentrations and quantity of fish excreta was supported by correlation analysis, in which excreta weight was significantly negatively related with the prometryn concentration in the exposure solution (−0.815*, P < 0.05) (Table 2). Furthermore, the gradual adaptation of zebrafish to the exposure environment may also have resulted in the formation of the corresponding detoxification mechanisms. This mechanism may have resulted in an increase in the depuration dose of prometryn in zebrafish that eventually became greater than the uptake dose, especially among fish in the groups treated with HA.
3.6. Biological effects of HA
A wide variety of physiological effects have been reported as a result of DOM exposure, including changes in biological membrane permeability owing to surface adhesion,17,18 induction of heat shock protein expression,35 and activation of glutathione-S-transferase (sGST)36 and cytochrome oxidase (CYP 1A).15 The source, concentration, and molecular weight of DOM also exert an important influence on the response of aquatic organisms. Matsuo et al.15 found that CYP 1A expression in tambaqui was induced by HA (20 to 80 mg L−1) and that commercial preparations of HA were more effective than natural organic matter in eliciting this response. It is likely that HA includes certain components that may function as aryl hydrocarbon receptor agonists and result in the induction of CYP 1A expression. Wiegand et al.26 reported that sGST was increased in Lumbriculus variegatus following treatment with 25 mg L−1 natural DOM, but not with 5 mg L−1 natural DOM, while peroxidase activity obviously increased at both concentrations of DOM. In the current study, there was little effect on the antioxidant system in zebrafish exposed to 15 mg L−1 HA alone, and the reasons for this need to be studied further.4. Conclusion
In the early stages of HA and prometryn exposure (day 1), an increased bioaccumulation of prometryn resulted in an increased level of lipid peroxidation in zebrafish. However, during days 10 to 40 of prometryn and HA exposure, HA decreased the uptake of prometryn by zebrafish. During this time HA appeared to alleviate the toxic effects of prometryn exposure on zebrafish. Furthermore, the effects of HA in combination with prometryn were more pronounced at 15 mg L−1 HA than at 5 mg L−1 HA. Based upon the results of this experiment, CAT activity and GSH content are involved in the detoxification of prometryn in zebrafish. Further research is needed to understand the molecular mechanisms of the effects of HA on the toxicity of prometryn in zebrafish.Acknowledgements
This work was financially supported by Major Science and Technology Program for Water Pollution Control and Treatment (grant No. 2012ZX07501-003).References
- L. Jiang, L. Ma, Y. Sui, S. Han and H. Yang, J. Environ. Monit., 2011, 13, 1935–1943 RSC.
- J. Zhou, X. Li, Y. Jiang, Y. Wu, J. Chen, F. Hu and H. Li, J. Hazard. Mater., 2011, 192, 1243–1249 CrossRef CAS PubMed.
- J. Zhou, F. Hu, J. Jiao, M. Liu and H. Li, J. Soils Sediments, 2012, 12, 576–585 CrossRef CAS.
- J. Zhou, J. Chen, Y. Cheng, D. Li, F. Hu and H. Li, Talanta, 2009, 79, 189–193 CrossRef CAS PubMed.
- S. E. Kegley, B. R. Hill, S. Orme and A. H. Choi. PAN Pesticide Database, Pesticide Action Network, North America, San Francisco, CA, 2010 Search PubMed.
- C. Ren, X. Tian, H. Zhang, Y. Liu, Y. Sun, Y. Xu, X. Gong and M. Wang, J. Chin. Mass Spectrom. Soc., 2013, 34, 353–361 CAS , in Chinese.
- Z. Li, L. Chen, H. Gao, L. Dong and J. Zhao, Chin. J. Chromatogr., 2006, 24, 267–270 CAS , in Chinese.
- X. Ma, N. Gao, Q. Li, B. Xu, L. Le and J. Wu, China Water Wastewater, 2006, 22, 1–4 CAS , in Chinese.
- Z. Vryzas, C. Alexoudis, G. Vassiliou, K. Galanis and M. E. Papadopoulou, Ecotoxicol. Environ. Saf., 2011, 74, 174–181 CrossRef CAS PubMed.
- C. E. Steinberg, N. Saul, K. Pietsch, T. Meinelt, S. Rienau and R. Menzel, Ann. Environ. Sci., 2007, 1, 81–90 CAS.
- E. M. Thurman, Organic geochemistry of natural waters, Springer Science & Business Media, 2012, vol. 2 Search PubMed.
- X. Hu, L. Mu, J. Kang, K. Lu, R. Zhou and Q. Zhou, Environ. Sci. Technol., 2014, 48, 6919–6927 CrossRef CAS PubMed.
- G. Chen, C. Lin, L. Chen and H. Yang, Chemosphere, 2010, 79, 1046–1055 CrossRef CAS PubMed.
- N. H. Song, L. Chen and H. Yang, Geoderma, 2008, 146, 344–352 CrossRef CAS.
- A. Y. Matsuo, B. R. Woodin, C. M. Reddy, A. L. Val and J. J. Stegeman, Environ. Sci. Technol., 2006, 40, 2851–2858 CrossRef CAS PubMed.
- C. N. Glover and C. M. Wood, Physiol. Biochem. Zool., 2005, 78, 1005–1016 CrossRef CAS PubMed.
- F. Galvez, A. Donini, R. C. Playle, D. S. Smith, M. J. O'Donnell and C. M. Wood, Environ. Sci. Technol., 2008, 42, 9385–9390 CrossRef CAS PubMed.
- L. M. Ojwang' and R. L. Cook, Environ. Sci. Technol., 2013, 47, 8280–8287 Search PubMed.
- A. Y. Matsuo, R. C. Playle, A. L. Val and C. M. Wood, Aquat. Toxicol., 2004, 70, 63–81 CrossRef CAS PubMed.
- B. Vigneault, A. Percot, M. Lafleur and P. G. Campbell, Environ. Sci. Technol., 2000, 34, 3907–3913 CrossRef CAS.
- N. J. Fabian, L. B. Albright, G. Gerlach, H. S. Fisher and G. G. Rosenthal, J. Chem. Ecol., 2007, 33, 2090–2096 CrossRef CAS PubMed.
- R. Bouchnak and C. E. Steinberg, Limnologica, 2010, 40, 86–91 CrossRef CAS.
- A. Stara, J. Kristan, E. Zuskova and J. Velisek, Pestic. Biochem. Physiol., 2013, 105, 18–23 CrossRef CAS PubMed.
- A. Stará, A. Kouba and J. Velíšek, BioMed Res. Int., 2014, 2014, 1–6 CrossRef PubMed.
- Q. Zhao, C. Wang, X. Yuan and L. Zhu, J. Agro-Environ. Sci., 2015, 34, 653–659 CAS , in Chinese.
- C. Wiegand, S. Pehkonen, J. Akkanen, O. P. Penttinen and J. V. Kukkonen, Chemosphere, 2007, 66, 558–566 CrossRef CAS PubMed.
- M. Haitzer, S. Höss, W. Traunspurger and C. Steinberg, Aquat. Toxicol., 1999, 45, 147–158 CrossRef CAS.
- P. Qiao and A. Farrell, Comp. Biochem. Physiol., Part C: Toxicol. Pharmacol., 2002, 133, 575–585 CrossRef CAS.
- D. L. Norwood, R. F. Christman and P. G. Hatcher, Environ. Sci. Technol., 1987, 21, 791–798 CrossRef CAS PubMed.
- X. Xia, Z. Dai, A. H. Rabearisoa, P. Zhao and X. Jiang, Chemosphere, 2015, 119, 978–986 CrossRef CAS PubMed.
- J. Akkanen, S. Penttinen, M. Haitzer and J. V. Kukkonen, Chemosphere, 2001, 45, 453–462 CrossRef CAS PubMed.
- C. Steinberg, C. Mayr, R. Lorenz, O. Spieser and A. Kettrup, Naturwissenschaften, 1994, 81, 225–227 CrossRef CAS PubMed.
- A. Bianchini and C. M. Wood, Environ. Toxicol. Chem., 2003, 22, 1361–1367 CrossRef CAS PubMed.
- H. A. Roditi, N. S. Fisher and S. A. Sañudo-Wilhelmy, Nature, 2000, 407, 78–80 CrossRef CAS PubMed.
- M. A. Timofeyev, C. Wiegand, B. K. Burnison, Z. M. Shatilina, S. Pflugmacher and C. E. Steinberg, Sci. Total Environ., 2004, 319, 115–121 CrossRef CAS PubMed.
- N. Meems, C. Steinberg and C. Wiegand, Sci. Total Environ., 2004, 319, 123–136 CrossRef CAS PubMed.
- A. Kaune, R. Brüggemann and A. Kettrup, J. Chromatogr. A, 1998, 805, 119–126 CrossRef CAS.
- K. V. Plakas and A. J. Karabelas, Sep. Purif. Technol., 2011, 80, 246–261 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |