Open Access Article
Open Access ArticleCreative Commons Attribution 3.0 Unported Licence
Effect of the reactive amino and glycidyl ether terminated polyethylene oxide additives on the gas transport properties of Pebax® bulk and thin film composite membranes
Jelena
Lillepärg
,
Prokopios
Georgopanos
,
Thomas
Emmler
and
Sergey
Shishatskiy
*
Helmholtz-Zentrum Geesthacht, Institute of Polymer Research, Max-Planck-Str. 1, 21502 Geesthacht, Germany. E-mail: sergey.shishatskiy@hzg.de; Fax: +49 (0)4152 87 2499; Tel: +49 (0)4152 87 2467
First published on 20th January 2016
Abstract
This paper considers Pebax® MH 1657 as a material for the CO2/N2 separating layer in thin film composite (TFC) membranes. The CO2 permeability of Pebax® can be improved via blending with various poly(ethylene oxide) (PEO) based materials without loss of CO2/N2 selectivity. Analogous blends containing PEOs with reactive end groups have been investigated for the possibility of a network formation within the Pebax® matrix. The formation of network is possible through the reaction between two types of additives containing two reactive end groups. The thick film samples and TFC membranes were prepared from mixtures of Pebax® MH 1657, PEG DG526 and JEFFAMINE® with different molecular weights. The samples were characterized by single gas permeation measurements, DSC, and NMR. The samples with incorporated networks show improved and stable gas transport properties compared to the original polymer for both thick films and TFC membranes.
Introduction
Carbon dioxide (CO2) is considered as a major anthropogenic greenhouse gas affecting global climate change. In 2014 the global carbon dioxide emission stood at 32.3 billion tons, the same amount as in 2013.1 Coal-fired power plants are responsible for 28% of this enormous value.2 Among the activities to minimize the harmful effect of greenhouse gas emission on the climate the approach of CO2 capture and storage (CCS) during energy production is one of the most crucial since such types of emission are very localized and constant.For the implementation in existing fossil fueled power plants the post-combustion approach of CCS based on either absorption or separation with polymeric membranes is among the most analyzed. The use of separation systems based on polymeric membranes with high CO2/N2 selectivity and high CO2 permeance as well as implementation of developed systems into the post-combustion separation process can lead to significant CO2 emission reduction but, unfortunately will reduce the efficiency of the energy production process.3,4
The current trend in polymeric membrane development is the use of materials able to transport CO2 either by active transport or by simple solution-diffusion mechanisms. Active transport membranes are currently prepared in small pilot scale and cannot be considered as materials of immediate or near future availability.5 Polymeric membranes based on poly(ethylene oxide) (PEO) have come to larger pilot scale production and are tested at several industrial sites for performance and stability in the environment of natural gas or coal fired power plants flue gas streams.6,7 The permeance and selectivity of developed PEO membranes lead to effective extraction of CO2 from the flue gas while the modelling of separation process economy shows that membrane separation systems are competitive to other considered methods of CCS.8
The selectivity of the developed membranes has reached the limit defined by the ideal selectivity of PEO based materials used for formation of the selective layer. At the same time the permeance can be varied by either reduction of the selective layer thickness or by development of new materials with higher CO2 permeability coefficient. The selective layer thickness of currently available thin film composite membranes has reached approximately 70 nm and hardly can be significantly reduced, meaning that new materials with high CO2 permeability coefficients are needed.
It was shown that blending of PEO containing block copolymers with low molecular weight PEO can significantly increase CO2 permeability coefficient without loss of the CO2/N2 selectivity.9 For example experiments with physical blending of low molecular weight poly(ethylene glycol)s (PEG) with PEO based Pebax® MH 1657 showed that permeability of the blend material at room temperature can be increased by 4–5 times.10,11 The increase in permeability at the same loading level depends on the molecular weight and end group nature of the additive to the polymer. The largest increase in permeability was observed for the materials containing PEG additive with the lowest molecular weight or shortest PEO chain length. The influence of PEO chain length on the CO2 solubility was studied in detail.12 However, stability tests of these blend materials at 50 °C, which is considered to be the operating conditions of CCS post-combustion process, showed significant leaching out of the PEO additive from the polymer matrix resulting in decay of gas transport properties. It was shown13 that the best results in terms of material's gas transport properties and stability can be achieved when additives having molecular weight more than 500 g mol−1 are used.
Alternatively compositions containing PEO blocks have been used in the studies of organic/inorganic hybrid membranes prepared from γ-glycidyloxypropyltrimethoxysilane (GPTMS) and poly(ether) diamines (PAPE). In this case the materials with the longest (2000 g mol−1) PEO chain have shown the highest CO2 permeability. The CO2/N2 selectivity was increased for the membranes containing amine functionalized compounds.14,15
Current work was targeted at development of materials for CO2 separation membranes with increased permeability and optimized selectivity. JEFFAMINE® poly(ether) diamines, as well as glycidyl ether terminated polyethylene oxides were considered as suitable materials for Pebax® MH1657 modification. JEFFAMINE® poly(ether) diamines in combination with PEO containing reactive end groups such as diglycidyl ether could lead to the formation of a polymer within the Pebax® matrix which can be considered as a network similar to certain extent to the concept of the interpenetrating networks.16 In this work the incorporation of the network into the matrix of Pebax® MH 1657 is presented. The membrane materials and thin film composite membranes were characterized using single gas permeation measurements, thermal and spectroscopy methods.
Experimental
Materials
The Pebax® MH 1657 (further Pebax®) was received in a pellet form from Arkema. PEG diglycidyl ether DG526 (Mw: 526 g mol−1), JEFFAMINE® ED series (O,O′-bis(2-aminopropyl)polypropylene glycol-block-polyethylene glycol-block-polypropylene glycol) ED600 and ED2003 having molecular weights of 600 and 2000 g mol−1 respectively were obtained from Sigma-Aldrich. Ethanol (Th. Geyer, 99.9%) and high purity deionized water (0.06 μS cm−1) were used as solvents. A porous PAN membrane developed at HZG17 with average pore size 20 nm and 15% surface porosity coated with a 150 nm layer of PDMS was used for formation of thin film composite membranes.Membrane preparation
Films having thickness of approximately 100 μm were prepared according to the procedure reported elsewhere13 by casting from a 3 wt% Pebax® solution in a solvent mixture of ethanol and water (EtOH/H2O) with a ratio of 70/30 (wt%). For the preparation of binary polymer compositions, different amounts of JEFFAMINEs® and PEG DG526 were added to a 3 wt% Pebax® solution and stirred at room temperature for 20 min. The prepared solution was poured on a Teflon® coated glass plate and the ethanol/water solvent was evaporated at 30 °C within 36–48 h.Samples with incorporated network structures formed by reaction of amino- and glycidyl-functionalized PEGs were prepared as follows: equimolar mixtures of JEFFAMINE® and PEG DG526 were stirred for 20 min at 40 °C in EtOH/H2O mixtures used as solvent. A predetermined amount of the prepared JEFFAMINE®/PEG DG526 solution was added to the Pebax® solution and stirred at room temperature for 20 min. Thick films were cast from the resulting Pebax®/JEFFAMINE/PEG solution in the same way as described above. The mechanism of amine–glycidyl ether reaction at an initiation temperature 50 °C is known since decades and thus no reaction has happened during the film preparation.18 In the current study the reaction in the prepared polymer films was initialized by membrane sample conditioning at 80 °C for 48 h in a vacuum oven equipped with a turbo-molecular pumping unit. It ensured complete degassing of the sample from traces of solvents or other volatile compounds.
The thin film composite membrane (TFCM) has been prepared from a 0.5 wt% polymer solution in EtOH/H2O. Binary blends of Pebax® with JEFFAMINE® ED600, ED2003 and PEG DG526 were used for selective layer formation along with three-component blends of Pebax® and an equimolar mixture of ED600/DG526. TFCMs were further treated for 48 h in the vacuum oven at 80 °C in order to achieve the amine–glycidyl reaction. As supporting layer for the preparation of TFCMs polyacrylonitrile (PAN) microporous membrane prepared on non-woven polyester, coated with a poly(dimethylsiloxane) (PDMS) layer,19 was used. PDMS served as a “gutter layer” distributing gases penetrating through the selective layer to the pores of the support and protecting the porous support from impregnation with components of the selective layer during the TFCM preparation.20,21 Additionally, a thin layer of ethyl cellulose was deposited on the surface of the PDMS layer to provide better compatibility between PDMS and Pebax®. The permeance of the ethyl cellulose/PDMS/PAN support was significantly higher than the permeance of the final membrane and did not influence significantly the selectivity of the resulting CO2 selective TFCM.
Membrane characterization
For the characterization of the produced thick films, spectroscopy and thermal analysis experiments were accomplished. 1H-NMR and 1H-HR-MAS NMR experiments were performed on the thick films. Thermal tests on the produced film samples as well as on the initial materials were done by differential scanning calorimetry (DSC). The properties obtained in these experiments were correlated to the gas transport measurements at different temperatures, and results were compared to the weight change of film samples studied for gas transport properties obtained from the weighting of films on an analytical balance before and after gas transport measurements at various temperatures. The films thickness was measured with a digital micrometer DELTASCOPE® FMP10.For the NMR experiments Bruker AVIIIHD 500 MHz spectrometer (Bruker, Karlsruhe, Germany) equipped with BBFO and HR-MAS probes was used. The solvent for the membrane swelling measurements was deuterated chloroform with TMS as internal standard (CDCl3, Sigma-Aldrich, Schnelldorf, Germany). The experiments were performed at room temperature. HR-MAS experiments were made using a 4 mm Bruker HR-MAS probe and -rotors at rotation frequencies of 10 kHz. The relaxation delays were chosen in that way that the sample was fully relaxed.
DSC experiments were done with the use of the calorimeter DSC 1, from the company Mettler Toledo (Mettler-Toledo, Greifensee, Switzerland), with temperature measurement range from −60 °C to +280 °C. The heating rate was 10 K min−1. The calculations presented in this work are based on the first cooling and the second heating intervals. The measurement was performed in argon atmosphere. Approximately 10 mg of the polymer were placed in an aluminum pan of 10 μl.
The instrumentation used for the thermogravimetric analysis experiments was the TG 209 F1 Iris (Netzsch, Selb, Germany). The experiment was done in a temperature range from 25 °C to 500 °C, and heating rate of 10 K min−1. The measurement was performed in a nitrogen gas flow.
Gas transport properties
The permeability coefficients of pure carbon dioxide and nitrogen were measured by a constant volume variable pressure technique22 (time-lag) in the temperature range from 30 °C to 90 °C and back from 90 °C to 30 °C. The feed pressure was 600, 450 and 350 Torr, in consecutive measurements for all gases. The permeability coefficient P [cm3 (STP) cm cm−2 s−1 cmHg−1] of single gas was determined as: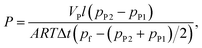 | (1) |
The diffusion coefficient D (cm2 s−1) was calculated from membrane thickness l (cm) and time-lag θ (s) determined graphically as intersection of the line drawn through the linear region of the pressure increase curve to intersection with the time axis:
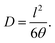 | (2) |
The solubility coefficient S (cm3 (STP) cm−3 cmHg−1) was calculated according to the following equation:
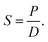 | (3) |
The ideal permeability selectivity of the material can be expressed as the ratio of permeability coefficients of two penetrants and according to eqn (3) it is a function of diffusion and solubility selectivity, leading to the equation:
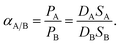 | (4) |
The single gas permeances of prepared TFCMs were determined using the “pressure increase” facility designed and built at HZG. The pressure increase facility19 utilizes the same principle as the aforementioned “time-lag” facility with difference in the data acquisition routine adjusted for investigation of membranes with thin selective layers. In case of the “pressure increase” the aim is the determination of the pressure increase rate in the calibrated permeate volume without considering any effects related to the thickness of the membrane. The basic principle of “pressure increase” measurements was described elsewhere.24 Single gas permeation data were determined at 500 mbar feed pressure and in the temperature range from 30 °C to 80 °C. In order to control the property stability for each defect-free TFCM sample, the measurements were repeated in the temperature range from 80 °C to 30 °C.
The permeance L (m3 (STP) m−2 h−1 bar−1) of the membrane can be calculated using the equation:
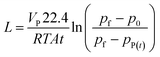 | (5) |
Results and discussion
Preliminary results obtained for the system Pebax® MH1657/LMW PEG have already indicated that the gas transport properties of films containing PEGs with different molecular weights and concentration up to 40 wt% in the polymer matrix can be improved by a factor of 3 to 5. The best results were obtained for Pebax® blended with PEG having molecular weight less than 300 g mol−1, but at temperatures interesting for application (50–80 °C) such low molecular weight compound tends to leave the polymer matrix (leaching-out). This can be explained by the fact that heat excites the mobility of PEO chains and provokes the separation of filler and polymer. On the other hand, membranes containing PEOs with higher molecular weight up to 1000 g mol−1 exhibit increased crystallinity which leads to material brittleness and damage of the thick film sample during the cooling part of gas permeation experiment.The Pebax® MH 1657 used in this study contains 60% of flexible PEO blocks promoting the high CO2 selectivities over other light gases. Commercial availability of this material makes it attractive for use as a selective material of TFCMs for CO2/N2 separation. In the present study JEFFAMINEs® of ED series and their reaction product with epoxy groups containing PEGs were used to increase the permeability of the CO2 selective material.
Binary systems of Pebax® and PEGs
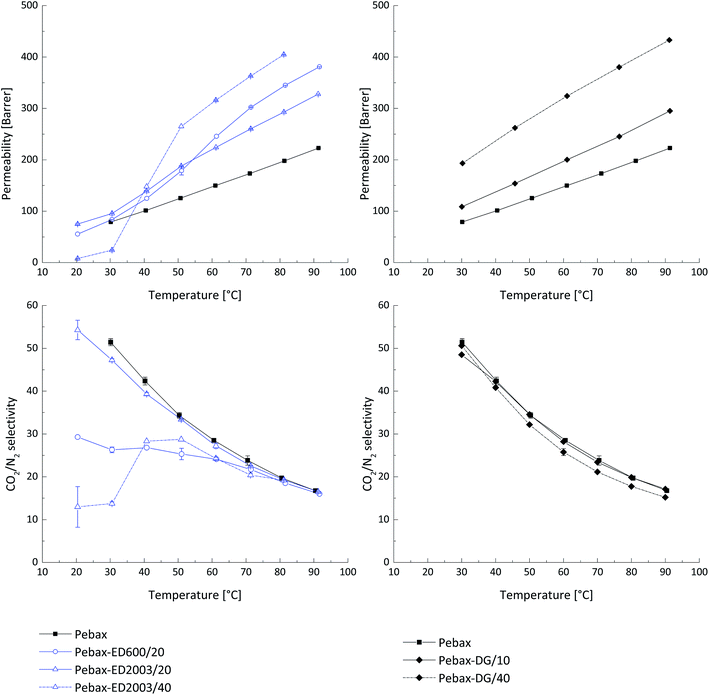
Samples of blended Pebax®MH1657 having thicknesses of about 100 μm were prepared as described above and tested for the gas transport properties. The temperature conditions of measurements in the range 30 °C to 90 °C were chosen according to previous work.13 Addition of 10 wt% PEG DG526 increases the permeability of the material by 15% whilst almost no change in the CO2/N2 selectivity was observed. Increasing of the concentration of PEG DG526 up to 40 wt% resulted in 1.5 times permeability increase whilst the CO2/N2 selectivity was slightly decreased (Fig. 1). The increase of permeability in polymers blended with PEG depends on their molecular weight, end groups and concentration in Pebax® MH 1657. For this work an optimal concentration of 20 wt% filler in the total membrane weight in order to maintain the integrity of the thick films was selected. Thus, for each reagent concentration is only 10 wt%, which explains the increase of the CO2 permeability only 1.5 times. The deviations in CO2 permeability coefficients for pure Pebax® MH 1657 and blends containing 10 wt% and wt 40% of LMW PEG DG526 were 0.7% and 6%, respectively, for the results obtained at the same temperatures during the heating and cooling process.The CO2 permeability for samples containing 20 wt% polyether amines ED600 and ED2003 is depicted in Fig. 1. The permeability increment for samples with lower molecular weight JEFFAMINE® (ED600) is greater than that of the higher molecular weight samples (ED2003) at temperatures above 60 °C. The selectivity of the sample with ED600 decreases significantly compared to the pure Pebax®, which may indicate a surprising incompatibility of the low molecular weight PEG terminated with amino groups. An interesting effect is observed at temperatures above 60 °C, where the ED600 blend shows the same selectivity as other studied materials. This can be considered as complete dissolution of the ED600 in the PEO blocks of Pebax®. Further attempts to increase the concentration of JEFFAMINE® ED600 up to 30 wt% lead to sample's mechanical instability due to strong phase segregation. ED2003 in the PEBAX® matrix shows completely different behaviour. The relatively long PEG chain masks the effect of the presence of amine groups and at concentration 20 wt% the blend shows good CO2 permeability and CO2/N2 selectivity. The increase of ED2003 concentration to 40 wt% still gives one the possibility to handle the prepared thick film indicating good mechanical properties of this blend material. The gas transport properties show that ED2003 starts to behave differently from the surrounding Pebax® matrix: the CO2 permeability temperature dependence has a significant step which starts at 30 °C and ends at 50 °C. At a temperature below 30 °C, the blend shows very low CO2/N2 selectivity which can be explained by the presence of a crystalline ED2003 phase surrounded by highly disordered and loosely packed PEG chains. In the temperature range 30 °C to 50 °C, the ED2003 crystals start to melt and the molten phase becomes available for CO2 transport. At temperatures above 50 °C the blend of ED2003 and Pebax® shows gas CO2/N2 selectivity usual for materials with high PEG content.
Three-component system
A three-component system was prepared by incorporating in Pebax® matrix two additional components that are able to react with each other even in solid (solvent-free) state, resulting in a stable membrane. This approach is different from formation of interpenetrating network within the polymer matrix. Bi-functional PEGs in equimolar mixture can give only linear product since the reactivity decreases significantly from primary to secondary amines. At the same time it was found earlier that incorporation of linear PEGs having molecular weight higher than 1000 does not give the desired effect of gas permeability increase. Additionally, PEGs with the molecular weight above 1500 g mol−1 have strong tendency for crystallization at room temperature. Formation of a linear polymer as a product of a reaction between amino- and glycidyl-end groups of low molecular weight PEGs can lead to interesting results in terms of gas transport properties because when such a compound is embedded into the Pebax® matrix it will depict a lower probability for crystallization, since PEG blocks will be interrupted by blocks formed from the amine–glycidyl reaction. This effect can increase membrane's permeability and reduce the probability for leaching-out of the low molecular weight additive for the Pebax® matrix.Films of Pebax® blended with PEG DG526 and JEFFAMINE® ED600 having equal amount of PEG monomeric units in the chain (n = 9) were prepared following the same procedure as described above. In Fig. 2 the CO2 permeability coefficients for three-component system Pebax®/ED600/DG 526, where the total content of the latter two components was 20 wt% and 30 wt%, is presented. The effect in permeability increase was smaller than anticipated, the CO2/N2 selectivity decreased sharply. In Fig. 2, curves for the heating interval of “time-lag” measurements are presented since material instability leading to sample damage was observed during the cooling down from 90 °C to 30 °C. The increase in permeability was observed for the samples Pebax®/ED600-DG 526 20 wt%, treated at 80 °C for 48 hours; the values of the CO2/N2 selectivity were decreased compared to the neat Pebax®. The deviation of the observed gas transport properties during the heating up and cooling down part of the “time-lag” experiment was less than 1.5% for this sample, indicating the reaction of the amine with the glycidyl ether and formation of network within PEO blends. When the concentration of additives in the Pebax® matrix of the thermally treated samples was raised to 30%, the CO2 permeability has decreased compared to thermally untreated material; but the CO2/N2 selectivity maintained at values of the neat Pebax® MH 1657. The degree of amine glycidyl ether consumption during the reaction in solid polymer matrix was controlled by a NMR study.
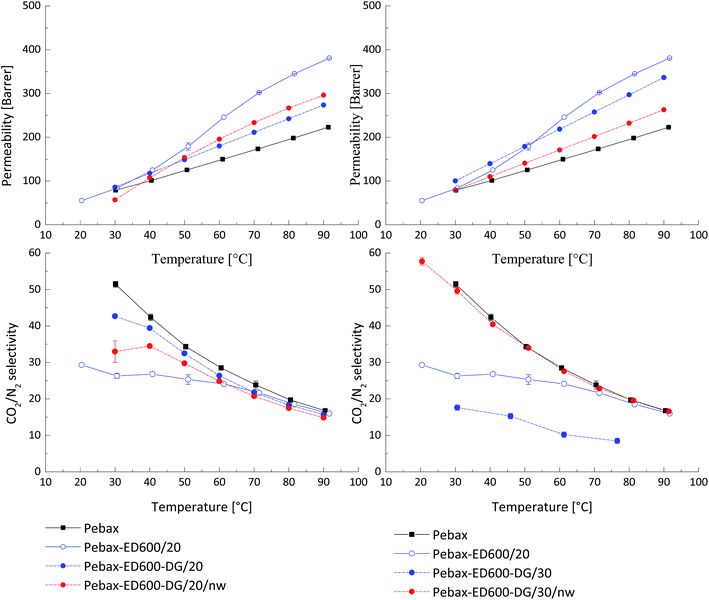 | ||
| Fig. 2 Influence of thermal treatment of Pebax®/ED600/PEG DG526 on the gas transport properties. nw – formation of network through end group reaction in solid PEO containing blends. | ||
To verify the completion of the amine–glycidyl reaction during the thermal treatment of solid films the samples of the three component system were investigated with High Resolution Magic Angle Spinning (1H HR-MAS NMR). The 1H HR-MAS NMR spectra are dominated by the presence of Pebax® 1567, so only slight variations can be seen. At 3.7 ppm all the (–CH2–CH2–O–) signals from JEFFAMINE® ED600, Pebax 1567 and PEG DG526 overlap (Fig. 3), but the signals of the –CH3 group of the JEFFAMINE® are visible below 1.25 ppm. The signals of these –CH3 groups experience – compared to the 1H NMR measurements in liquids of a binary system PEG DG526/JEFFAMINE® ED600 – a small shift due to the Pebax® environment, but nevertheless they still can be identified.
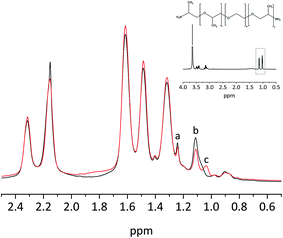 | ||
| Fig. 3 1H HR-MAS NMR of the thick film Pebax® MH 1657/ED600-DG 526 20 wt%: black before and red after heat treatment (80°). The insert shows the solution NMR spectrum of JEFFAMINE® ED600. | ||
Fig. 3 clearly shows, that one of the signals of the –CH3 groups (b, 1.1 ppm) loses intensity and a new signal arises (c, 1.05 ppm). Because of that loss it can be concluded that the JEFFAMINE® and the PEG glycidyl ether have reacted leading to formation of a stable blend system even when not all of the reactive compounds were consumed.
Processes observed by changes in CO2 permeability can be confirmed by DSC analysis. The DSC experiments were carried out for all initial components as well as binary and ternary component systems. The results are summarized in Table 1. For the blend systems, the glass transition of the PEG block is observed at approximately −50 °C, which is comparable with the values of the neat Pebax®. Slightly stronger effect is observed for the crystallization and melting temperatures of the PEG block. The temperatures TPEGc and TPEGm vary from −10 °C to 10 °C and from 15 °C to 40 °C, respectively. The crystallization temperature attributed to the polyamide block seems to be stable with the only exception in the case of the Pebax®/ED600 (20%) blend, where a shift of approximately 20 °C is observed. This could be attributed to the fact that the low molecular weight PEG contains a relatively large amount of –NH2 groups that could form hydrogen bonds with the respective polyamide block. The melting temperature of the polyamide block remains at similar values to that of neat Pebax®, at approximately 205 °C for binary systems.
| Material | T PEGg | T PEGc | T PEGm | T PAc | T PAm |
|---|---|---|---|---|---|
| a T PEGg: glass transition temperature of the polyethylene glycol block, °C, TPEGc: crystallization temperature of the polyethylene glycol block, °C, TPEGm: melting temperature of the polyethylene glycol block, °C, TPAc: crystallization temperature of the polyamide block, °C, TPAm: melting temperature of the polyamide block, °C, n.a.: not applicable, – : no conclusive data, nw: thermally treated at 80 °C. | |||||
| Pebax® MH 1657 | −53 | −20 | 16 | 156 | 205 |
| Jeffamine® – ED600 | −49 | −26 | −12 | n.a. | n.a. |
| Jeffamine® – ED2003 | −56 | −6 | 36 | n.a. | n.a. |
| LMWPEG – DG526 | −72 | −45 | −14 | n.a. | n.a. |
| Jeffamine®ED600 – DG526/nw | −44 | n.a. | n.a. | n.a. | n.a. |
| Jeffamine®ED2003 – DG526/nw | −46 | −2 | 33 | n.a. | n.a. |
| Pebax® – ED600 (20%) | −56 | 3 | 18 | 167 | 204 |
| Pebax® – ED2003 (20%) | −51 | 6 | 37 | 159 | 204 |
| Pebax® – ED2003 (40%) before TL | −51 | 13 | 36 | 161 | 204 |
| Pebax® – ED2003 (40%) after TL | −52 | 14 | 36 | 161 | 203 |
| Pebax® – DG526 (40%) | −55 | −10 | 15 | 151 | 196 |
| Pebax® – ED600-DG526 (20%) | −55 | −9 | 16 | 156 | 203 |
| Pebax® – ED600-DG526/nw (20%) | −50 | −10 | 17 | 156 | 203 |
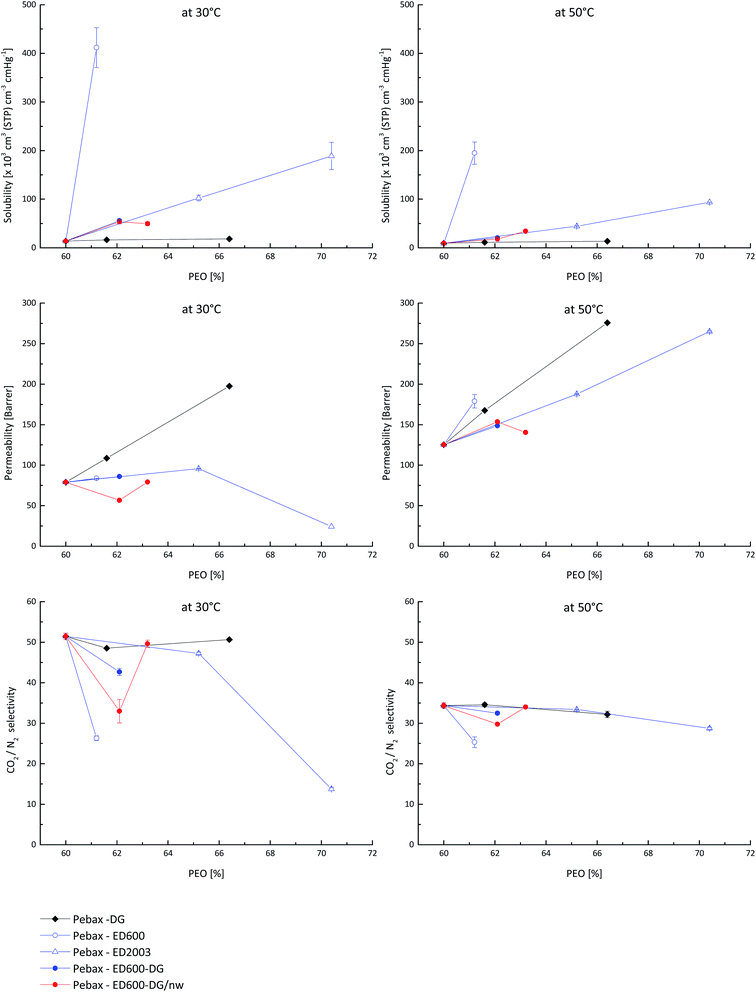
The effect of changes in gas transport properties caused by introduction of additional PEG with different molecular weight and end groups into the Pebax® matrix can be clearly seen in Fig. 4, where the solubility and permeability coefficients of CO2 as well as the CO2/N2 selectivity are plotted against the PEO content in the system. Amino terminated PEO ED600 exhibits the highest CO2 solubility coefficient among the other studied materials but has not the leading permeability coefficient due to incompatibility of this Jeffamine® to the polymer matrix and thus presence of additional resistance for CO2 diffusion between phases. From the low CO2/N2 selectivity of the Pebax®/ED600 system one can conclude that CO2 can be even partially immobilized on amino groups while all N2 molecules have the freedom to pass through the polymer system. As soon as amino groups are consumed in e.g. reaction with glycidyl groups the selectivity of the system becomes similar to the one of neat Pebax®.
The behaviour of the system containing diglycidyl ether DG526 is even more interesting. This system shows the highest permeability at 30 °C amongst the other studied materials, the CO2/N2 selectivity does not differ from the neat Pebax® at all DG526 loadings, but the solubility coefficient is the lowest of all other materials meaning that DG526 significantly and non-selectively increases diffusion coefficients of both carbon dioxide and nitrogen.
Jeffamine® ED2003 when distributed in Pebax® gradually increases the CO2 solubility coefficient but at temperature below melting temperature causes gas diffusion path distortion as well as changes in material packing which results in lower CO2 permeability and CO2/N2 selectivity at high loadings. As soon as the experimental temperature becomes higher than the melting point, the permeability coefficient dependence becomes more gradual and the selectivity reaches the level of neat Pebax®.
Gas transport characteristics of a ternary system Pebax®/ED600/DG526 before and after thermal treatment follow those of the Pebax®/ED2003 at low loadings. The solubility coefficient increases gradually with a rise in PEO content, the permeability of the thermally not treated system does not change, as well as the selectivity. The decrease of the CO2 permeability and CO2/N2 selectivity at low loading in case of the thermally treated system we attribute to too low concentrations of reactive groups dispersed in the polymer matrix. In this case the reaction in solid Pebax® is hindered, and amino containing compounds can form a separate phase as discussed above. When the concentration of both additives to the Pebax® matrix increases, the probability of reaction between amino- and glycidyl-groups increases and the resulting system exhibits properties usual for PEO containing materials. It is interesting to observe, that at 50 °C all studied systems except amino-groups containing have very similar selectivities.
As was discussed above, the presence of the crystalline phase gives strong effects on both permeability and selectivity. Table 2 illustrates that PEO crystallinity of the studied systems obtained from the analysis of DSC results varies significantly. For crystallinity calculations literature value of the PEO melting enthalpy of 8.67 kJ mol−1 have been used.12,25–30 The lowest level of crystallinity was observed for neat Pebax® and for the ternary system. All binary systems demonstrate 5–9% higher crystallinity compared to the neat Pebax®. Since crystallinity values are obtained from the DSC curves and one can consider (to certain extent) this crystallinity as crystallinity at melting temperature for systems with low molecular weight PEO, higher values do not influence the gas transport at temperatures well above the melting point. In case of the system containing ED2003 the crystallinity at 30 °C presents a significant problem for both CO2 permeability coefficient and CO2/N2 selectivity but as soon as the membrane is heated above the melting temperature, the CO2 permeability surpasses those of other studied systems while the selectivity reaches values characteristic for other studied materials.
| Material | PEO [wt%] | X C (PEO) [%] |
|---|---|---|
| Pebax® | 60.0 | 18 |
| Pebax® – DG526 (40%) | 66.4 | 23 |
| Pebax® – ED600 (20%) | 61.2 | 26 |
| Pebax® – ED2003 (20%) | 65.2 | 27 |
| Pebax® – ED600-DG526 (20%) | 62.1 | 17 |
| Pebax® – ED600-DG526 (20%) – nw | 62.1 | 16 |
Thin film composite membranes
A practical application of rubbery polymeric materials for membrane gas separation is possible in form of thin film composite (TFC) membranes. This type of membranes has a long history of successful use in numerous applications.31,32 Currently at least two membranes with selective layers made of materials having high PEG content are undergoing rigorous pilot tests for the separation of CO2 from the off gases of natural gas (Polaris, MTR) and coal fired (PolyActive™, HZG) power plants.7,33 Development of new highly permeable and, if possible, more selective materials as well as their implementation in TFC membranes can facilitate a big advance in membrane separation systems for CO2 capture.The most promising materials investigated in the current study in a form of thick isotropic films have been used for the formation of the selective layer of the TFC membrane. The membrane used as a support consisted of non-woven providing mechanical strength, porous PAN support and PDMS layer serving as a smooth gutter layer. The PDMS layer provides smooth adhesive surface for application of thin selective layer and additionally serves for distributing the gas stream penetrating through the selective layer to the nears pore of the porous support (gutter layer approach).34,35
In order to improve compatibility of the gutter layer and solution used for deposition of the selective layer one more layer of ethyl cellulose was deposited onto the PDMS membrane. The CO2 permeance of the prepared ethyl cellulose TFC membrane was 4.1 Nm3 m−2 h−1 bar−1 and CO2/N2 selectivity 16.8 at 30 °C. This permeance is three times higher than the CO2 permeance of the pure Pebax® TFC membrane (1.37 Nm3 m−2 h−1 bar−1 at 30 °C) and thus layers supporting Pebax® based selective layer did not influence the total membrane selectivity which could occur when inappropriate support is used for TFC membrane formation.36,37 The permeance of the TFC membrane having pure Pebax® selective layer is not competitive compared to other available CO2 selective membranes and should be improved for the use in industrial processes.38 As seen on the Fig. 5, the permeance divergence was observed for the three-component system (not thermally treated before the gas permeation experiment) Pebax®/ED600/DG 526 20% during the heating and cooling parts of measurements in the temperature range from 20 °C to 90 °C. Exposure to the elevated temperatures facilitates leaching out of the low molecular weight components from the selective layer and leads to reduced membrane permeance observed during the cooling from 90 to 20 °C part of the gas transport experiment. The same membrane but thermally treated in vacuum for 10 hours at 80 °C showed stable gas transport properties in the investigated temperature range in case of both heating and cooling parts of the experiment. It is important to mark that the membrane heated to 80 °C relatively fast, with the heating rate provided by the vacuum oven shows higher permeance compared to the membrane which was heated up stepwise during the gas transport experiment. This observation is especially valuable since it is possible to provide intensive thermal treatment of the membrane after the solvent drying procedure in the membrane production facility and by this intensive IR treatment to foster fast reaction between amino- and glycidyl-end groups of low molecular weight PEOs dispersed in the Pebax® matrix. The CO2/N2 selectivity of both ternary system based TFC membranes was identical to the selectivity of the neat Pebax® and reflects the quality of the membrane manufacturing process. The CO2 permeance of the thermally treated membrane sample was above 2.2 Nm3 m−2 h−1 bar−1 at 30 °C and indicates a good potential of the developed material for production of competitive TFC membranes.
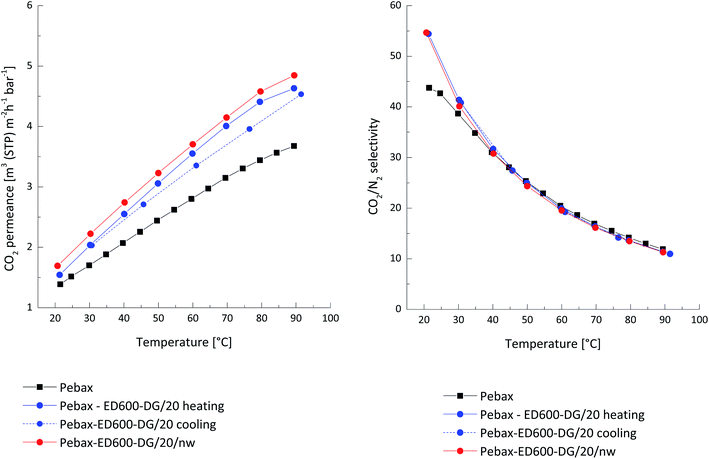 | ||
| Fig. 5 Thin film composite membrane Pebax® MH 1657 as selective layer blended with incorporated network ED600/PEG DG 526; nw – thermal treated at 80 °C. | ||
Conclusions
Two and three-component systems based on the commercially available polymer Pebax® MH 1657, JEFFAMINE® diamine and PEG diglycidyl ether were investigated for their gas transport properties. The JEFFAMINE® and the PEG diglycidyl ether reacted in the polymer matrix and formed a network stably distributed in the Pebax® matrix. Thermally treated samples present stable gas transport properties in comparison with binary blends of PEG or JEFFAMINE® with Pebax®. TFCM with incorporated networks have demonstrated improved CO2 permeance and unchanged CO2/N2 selectivity compared to the TFC membrane with pure Pebax® selective layer. The thick film of a thermally treated ternary system has shown stable gas transport properties after multiple heating/cooling cycles. The same properties were achieved for the thin film composite membranes indicating that proposed ternary system can be used for large scale CO2 selective membrane production. The developed membranes are to be tested at operating conditions temperatures for the removal of CO2 from the flue gas of coal fired power plants.Acknowledgements
The authors would like to thank Volker Abetz, Torsten Brinkmann, Volkan Filiz, Jan Wind, Alberto Tena, Mushfequr Rahman, Silvio Neumann, Ivonne Ternes, Maren Brinkmann and Carsten Scholles for their technical support and valuable discussions. This work was financially supported by the project from the Helmholtz Association of German Research Centres through the Helmholtz Portfolio MEM-BRAIN.References
- International Energy Agency, http://www.iea.org/newsroomandevents/news/2015/march/global-energy-related-emissions-of-carbon-dioxide-stalled-in-2014.html, accessed 13 March 2015.
- EDGAR - Emission Database for Global Atmospheric Research, http://edgar.jrc.ec.europa.eu/news_docs/jrc-2014-trends-in-global-co2-emissions-2014-report-93171.pdf, accessed December 2014.
- D. Y. C. Leung, G. Caramanna and M. M. Maroto-Valer, Renewable Sustainable Energy Rev., 2014, 39, 426–443 CrossRef CAS
.
- X. He and M. B. Hägg, Membranes, 2012, 2, 706–726 CrossRef CAS PubMed
.
- X. He and M.-B. Hägg, Energy Procedia, 2014, 63, 174–185 CrossRef CAS
.
- L. S. White, X. Wei, S. Pande, T. Wu and T. C. Merkel, J. Membr. Sci., 2015, 496, 48–57 CrossRef CAS
.
-
J. Pohlmann and T. Brinkmann, presented in part at the 15th Aachener Membran Koloquium (AMK), Aachen, November, 2014 Search PubMed
.
- H. S. Choi, H. K. Kwon, K. S. Kim, J. S. Park and C. H. Moon, IEEE Trans. Compon., Packag., Manuf. Technol., 2014, 4, 1131–1135 CrossRef CAS
.
- A. Car, C. Stropnik, W. Yave and K.-V. Peinemann, Sep. Purif. Technol., 2008, 62, 110–117 CrossRef CAS
.
- W. Yave, A. Car and K.-V. Peinemann, J. Membr. Sci., 2010, 350, 124–129 CrossRef CAS
.
- W. Yave, A. Car, K.-V. Peinemann, M. Q. Shaikh, K. Rätzke and F. Faupel, J. Membr. Sci., 2009, 339, 177–183 CrossRef CAS
.
- A. Tena, A. Marcos-Fernandez, A. E. Lozano, J. de Abajo, L. Palacio, P. Pradanos and A. Hernandez, Chem. Eng. Sci., 2013, 104, 574–585 CrossRef CAS
.
- J. Lillepärg, P. Georgopanos and S. Shishatskiy, J. Membr. Sci., 2014, 467, 269–278 CrossRef
.
- C. Nistor, S. Shishatskiy, M. Popa and S. P. Nunes, Sep. Sci. Technol., 2009, 44, 3392–3411 CrossRef CAS
.
- T. Kai, T. Kouketsu, S. H. Duan, S. Kazama and K. Yamada, Sep. Purif. Technol., 2008, 63, 524–530 CrossRef CAS
.
- A. Bos, I. G. M. Pünt, M. Wessling and H. Strathmann, J. Polym. Sci., Part B: Polym. Phys., 1998, 36, 1547–1556 CrossRef CAS
.
- N. Scharnagl and H. Buschatz, Desalination, 2001, 139, 191–198 CrossRef CAS
.
- L. Shechter, J. Wynstra and R. P. Kurkjy, Ind. Eng. Chem., 1956, 48, 94–97 CrossRef CAS
.
- W. Yave, A. Car, S. S. Funari, S. P. Nunes and K.-V. Peinemann, Macromolecules, 2009, 43, 326–333 CrossRef
.
- I. F. J. Vankelecom, B. Moermans, G. Verschueren and P. A. Jacobs, J. Membr. Sci., 1999, 158, 289–297 CrossRef CAS
.
-
J. M. S. Henis and M. K. Tripodi, Multicomponent membranes for gas separations, U.S. Pat., 4 230 463, 1980
.
- L. Y. Deng, T. J. Kim and M. B. Hagg, J. Membr. Sci., 2009, 340, 154–163 CrossRef CAS
.
- T. J. Kim, H. Vralstad, M. Sandru and M. B. Hagg, J. Membr. Sci., 2013, 428, 218–224 CrossRef CAS
.
- C. Nistor, S. Shishatskiy, M. Popa and S. P. Nunes, Rev. Roum. Chim., 2009, 54, 603–610 CAS
.
- A. Tena, A. Marcos-Fernandez, L. Palacio, P. Pradanos, A. E. Lozano, J. de Abajo and A. Hernandez, J. Membr. Sci., 2013, 434, 26–34 CrossRef CAS
.
- A. Marcos-Fernández, A. Tena, A. E. Lozano, J. G. de la Campa, J. de Abajo, L. Palacio, P. Prádanos and A. Hernández, Eur. Polym. J., 2010, 46, 2352–2364 CrossRef
.
- J. H. Kim, S. Y. Ha and Y. M. Lee, J. Membr. Sci., 2001, 190, 179–193 CrossRef CAS
.
- Y. Hirayama, Y. Kase, R. Tanihara, Y. Sumiyama, Y. Kusuki and K. Haraya, J. Membr. Sci., 1999, 160, 87–99 CrossRef CAS
.
- A. Marcos-Fernandez, A. E. Lozano, J. G. de la Campa, J. de Abajo, A. Tena, L. Palacio, P. Pradanos and A. Hernandez, Abstr. Pap. Am. Chem. Soc., 2009, 237 Search PubMed
.
- A. Tena, A. E. Lozano, L. Palacio, A. Marcos-Fernandez, P. Pradanos, J. de Abajo and A. Hernandez, Int. J. Greenhouse Gas Control, 2013, 12, 146–154 CrossRef CAS
.
- A. Makaruk, M. Miltner and M. Harasek, Sep. Purif. Technol., 2010, 74, 83–92 CrossRef CAS
.
- M. Czyperek, P. Zapp, H. J. M. Bouwmeester, M. Modigell, K. Ebert, I. Voigt, W. A. Meulenberg, L. Singheiser and D. Stover, J. Membr. Sci., 2010, 359, 149–159 CrossRef CAS
.
- T. C. Merkel, H. Lin, X. Wei and R. Baker, J. Membr. Sci., 2010, 359, 126–139 CrossRef CAS
.
- J. Peter and K. V. Peinemann, J. Membr. Sci., 2009, 340, 62–72 CrossRef CAS
.
-
I. Cabasso and K. A. Lundy, Method of making membranes for gas separation and the composite membranes, US Pat., 4 602 922, 1986
.
- J. Peter and K. V. Peinemann, J. Membr. Sci., 2009, 340, 62–72 CrossRef CAS
.
- H. Mushardt, V. Kramer, D. Hulagu, T. Brinkmann and M. Kraume, Chem. Ing. Tech., 2014, 86, 83–91 CrossRef CAS
.
- B. T. Low, L. Zhao, T. C. Merkel, M. Weber and D. Stolten, J. Membr. Sci., 2013, 431, 139–155 CrossRef CAS
.
| This journal is © The Royal Society of Chemistry 2016 |