A novel bifunctional additive for 5 V-class, high-voltage lithium ion batteries
Jinkui Feng*a,
Xueping Gaoa,
Lijie Cia and
Shenglin Xiongb
aKey Laboratory for Liquid-Solid Structural Evolution & Processing of Materials, School of Materials Science and Engineering, Shandong University, Jinan 250061, China. E-mail: jinkui@sdu.edu.cn
bSchool of Chemistry and Chemical Engineering, Shandong University, Jinan 250100, PR China
First published on 6th January 2016
Abstract
A novel additive is investigated as a bifunctional electrolyte additive for 5 V-class lithium ion batteries. It was found that the additive can be electro-polymerized at 5.05 V (vs. Li/Li+), which would protect the batteries from voltage runaway. Moreover, this additive could lower the electrolyte's self-extinguishing time (SET). The influence of this additive on cycling performance and capacity is positive, which makes it promising.
1. Introduction
The rapid development of portable electronics and electric vehicles demands higher-energy power sources. Recently, owing to their high energy density, 5 V-class cathodes have been considered as promising substitutes for commercial 4 V-class cathodes, such as LiNi0.5M1.5O4, LiCoPO4 and lithium-rich cathodes (Li2MnO3·LiMO2),1–13 the working plateau of these cathodes could be as high as 4.8 V. However, a key hindrance for applications of 5 V lithium batteries is safety concerns. Because electrolytes for lithium ion batteries make use of highly flammable and voltage-sensitive carbonate-based electrolytes, they may cause serious hazards such as firing and explosion under overcharged conditions.14–16 As the cells become thermally unstable at the overcharged state due to heat generation, irreversible electrolyte decomposition, a highly reactive delithiated cathode and excess lithium metal deposited on the anode may ignite or explode.17To solve these problems, much effort has been focused on developing overcharge additives for 4 V-class lithium batteries, such as electropolymerizable additives,18–24 redox shuttles25–30 and electroactive polymer-based separators.31–36 Our groups also have proposed a kind of additive with both flame retardant and overcharge protection ability by combining different functional groups.35,36 Among these many benzene derivatives were verified as efficient safety additives, which act by forming polymers inside the batteries to bypass or interrupt the charge current combined with H2 production to trigger the safety pressure vent. In the commercial electrolyte, overcharge protection additives such as biphenyl (BP)18 or cyclohexylbenzene (CHB)20 have been essential components. However, the working potential of these additives is for 4 V-class lithium batteries, which cannot be used in 5 V-class lithium batteries due to their low oxidation potential (4.6 V for BP and 4.7 V for CHB).18–20 New high-voltage overcharge protection additives for 5 V-class lithium batteries are considered crucial for commercial applications. However, little progress on this front has been reported.
In this study, pentafluoro(phenoxy)cyclotriphosphazene (FPPN) was tested as a safety additive for 5 V Li-ion batteries. We found that by combining the flame-retardant fluoro-substituted phosphazene group37–39 and aryl group, this additive not only provides overcharge protection for 5 V-class batteries but also reduces electrolyte flammability, which makes it a promising bifunctional additive for 5 V-class lithium ion batteries.
2. Experimental
All the chemicals were of analytical grade and used without further purification except as otherwise noted. FPPN was purchased from TCI Corp. To examine flammability of the FPPN electrolyte, a self-extinguishing time (SET) test was applied via a method similar to one described in our previous report.35 The change in morphology of the electrodes after overcharge was examined using field emission scanning electron microscopy (FE-SEM, SU-70, Japan). Conductivity of the electrolyte with different FPPN was measured using a conductivity meter (DDS-11A, Leici).The electrochemical character of FPPN was examined via cyclic voltammetry (CV) on a CHI660E. CV was test using 2016 coin cells with lithium foil as both the anode and reference electrodes and stainless coin shell as the working electrode. The electrochemical compatibility of the FPPN-containing electrolytes was examined by laboratory-made coin type cells. The positive electrode consisted of 85% lab-made LiNi0.5Mn1.5O4, 7% super p and 8% PVDF (wt%). The negative electrode consisted of 90% graphite, 2% super p and 8% PVDF. The base electrolyte was 1 M LiPF6/EC–DMC–DEC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) purchased from Tianci High-Tech Co. Ltd., China. To test the AC impedance of the cell at overcharge states, the cell was fully charged at 0.1 C, and then overcharged for 1 h to reach a stable open-circuit voltage. The impedance was measured in the frequency range of 0.01 Hz to 1 MHz.
1 v/v) purchased from Tianci High-Tech Co. Ltd., China. To test the AC impedance of the cell at overcharge states, the cell was fully charged at 0.1 C, and then overcharged for 1 h to reach a stable open-circuit voltage. The impedance was measured in the frequency range of 0.01 Hz to 1 MHz.
3. Results and discussion
3.1. Physical properties and characterization of FPPN
Fig. 1 shows the SET test result for the1 M LiPF6/EC–DMC–DEC (1/1/1 v/v) electrolyte with different ratios of FPPN. It can be seen that with increasing FPPN content from 0 to 12 wt%, burning time decreased from 57 s to 0 s, proving that FPPN can effectively lower the flammability of the electrolyte.35,37 Phosphorous and fluorine elements have been verified to be flame retarding via the radical adsorption and oxygen isolation mechanism.16 Electrolyte ionic conductivity declined slightly with the addition of FPPN, which may be ascribed to the lower polarity of FPPN. With 12% FPPN added, the electrolyte is nonflammable (Fig. 2(b)), so we selected 12 wt% FPPN content to form a nonflammable electrolyte and measure its electrochemical performance. Future research may focus on optimizing the electrolyte component.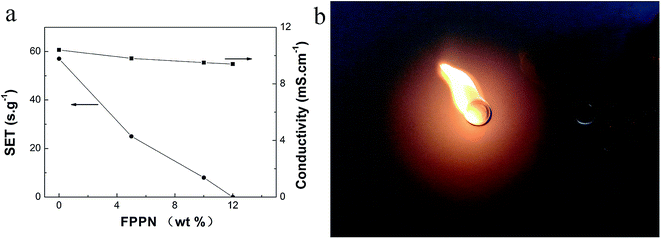 | ||
Fig. 1 Flammability and ionic conductivity of 1 M LiPF6 EC–DMC–DEC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) at different content of FPPN (a) and flame test of electrolyte (b). 1 v/v) at different content of FPPN (a) and flame test of electrolyte (b). | ||
3.2. Electrochemical behaviors of FPPN
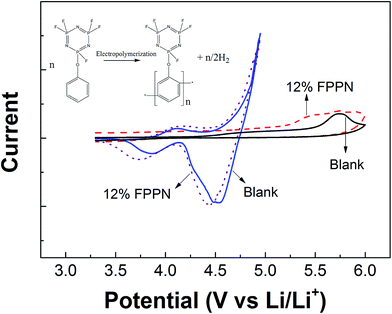
To examine the potential window of FPPN, CV was performed. Fig. 2 shows the CV curves for the 1 M LiPF6/EC–DMC–DEC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) electrolyte with (red dash solid and gray dot lines) or without (black and blue solid lines) the addition of 12% FPPN. In the presence of FPPN, a new oxidation peak appears at 5.05 V (vs. Li/Li+), which may be attributed to the electrochemical oxidation of FPPN. The higher base current of FPPN contained electrolyte may result from impurities in the FPPN (98% purity, TCI). The oxidation potential (5.05 V) of FPPN was higher than the complete charging potential of most 5 V-class cathodes such as LiNi0.5Mn1.5O4, and lower than decomposition potentials of carbonate electrolytes currently used (about 5.3 V). The CV curves of LiNi0.5Mn1.5O4 in the same electrolyte with and without the addition of 12% FPPN were also presented in the CV in the scan range of (3.3–4.95 V), the CV was performed between 3.3 and 4.9 V (vs. Li/Li+) at a scan rate of 0.1 mV s−1. From the curves we can see that LiNi0.5Mn1.5O4 functioned well in the HFPN-added electrolyte, indicating that FPPN could be used as a suitable overcharge protection additive for 5 V-class Li-ion batteries.16 Once the Li-ion batteries were overcharged to 5.05 V, the FPPN was electropolymerized on the cathode electrode (see inset graph in Fig. 2), as it was reported in the case of electrochemical polymerization of other aryl compounds.17 The process could consume the overcharge capacity and form H2 to initiate the pressure vent to halt the charging reaction.14 Moreover, the resulting polymer could alleviate the contact reaction between the cathode and the electrolyte.24
1 v/v) electrolyte with (red dash solid and gray dot lines) or without (black and blue solid lines) the addition of 12% FPPN. In the presence of FPPN, a new oxidation peak appears at 5.05 V (vs. Li/Li+), which may be attributed to the electrochemical oxidation of FPPN. The higher base current of FPPN contained electrolyte may result from impurities in the FPPN (98% purity, TCI). The oxidation potential (5.05 V) of FPPN was higher than the complete charging potential of most 5 V-class cathodes such as LiNi0.5Mn1.5O4, and lower than decomposition potentials of carbonate electrolytes currently used (about 5.3 V). The CV curves of LiNi0.5Mn1.5O4 in the same electrolyte with and without the addition of 12% FPPN were also presented in the CV in the scan range of (3.3–4.95 V), the CV was performed between 3.3 and 4.9 V (vs. Li/Li+) at a scan rate of 0.1 mV s−1. From the curves we can see that LiNi0.5Mn1.5O4 functioned well in the HFPN-added electrolyte, indicating that FPPN could be used as a suitable overcharge protection additive for 5 V-class Li-ion batteries.16 Once the Li-ion batteries were overcharged to 5.05 V, the FPPN was electropolymerized on the cathode electrode (see inset graph in Fig. 2), as it was reported in the case of electrochemical polymerization of other aryl compounds.17 The process could consume the overcharge capacity and form H2 to initiate the pressure vent to halt the charging reaction.14 Moreover, the resulting polymer could alleviate the contact reaction between the cathode and the electrolyte.24
Fig. 3 compares the overcharge behavior of LiNi0.5Mn1.5O4–Li cells with blank and 12% FPPN added electrolyte. The cells were cycled at the rate of 50 mA g−1. It can be seen that there is not much difference between these cells during the normal charge process, indicating that the 12% FPPN addition has little effect on normal performance. However, when overcharged, the potential of cells with the blank electrolyte increases until the electrolyte decomposition at 5.3 V.18 Since electrolyte oxidation is exothermic and can trigger a chain reaction in the cell,14 the overcharge may cause hazardous behaviors.14–16 In contrast, for the cells with 12% FPPN added electrolyte, the charge voltage stabilized at about 5.05 V and did not increase even after 200% overcharge capacity, suggesting the effective overcharge protection of FPPN.18 Since the plateau voltage is in accordance with the CV oxidation peak of FPPN, it is reasonable to attribute such protection to the FPPN electrochemical reaction.36–38
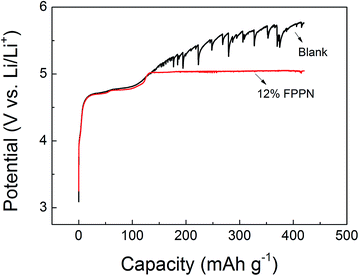 | ||
Fig. 3 Overcharge curves of LiNi0.5Mn1.5O4–Li coin cells. Electrolyte was 1 M LiPF6 EC–DMC–DEC (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1 v/v) containing no additive (blank solid line), 12% FPPN (red solid line), respectively. 1 v/v) containing no additive (blank solid line), 12% FPPN (red solid line), respectively. | ||
Ideal overcharge protection additives for lithium batteries should have no side effects on the normal charge–discharge process. Fig. 4(a) and (c) compare the initial two cycles for LiNi0.5Mn1.5O4–Li cells with the electrolytes with and without FPPN, respectively. As seen in Fig. 4, that FPPN content increases, the first coulombic efficiency slightly increases, which may be ascribed to formation of a stable solid-state interface (SEI) film on the electrode surface due to the high F content in FPPN.37–39 The cycling performance of LiNi0.5Mn1.5O4–Li cells with the FPPN addition is also better than the blank electrolyte. The low initial coulombic efficiency may be ascribed to the high cutoff voltage and the trace impurity in the cathode. Moreover, the FPPN addition also has positive effects on the charge–discharge curves and cycling performance of graphite–Li cells (Fig. 4(b) and (d)). Therefore, we can conclude that the addition of 12% FPPN does not cause side effects in the electrochemical performance of the LiNi0.5Mn1.5O4–Li and graphite–Li cells.16
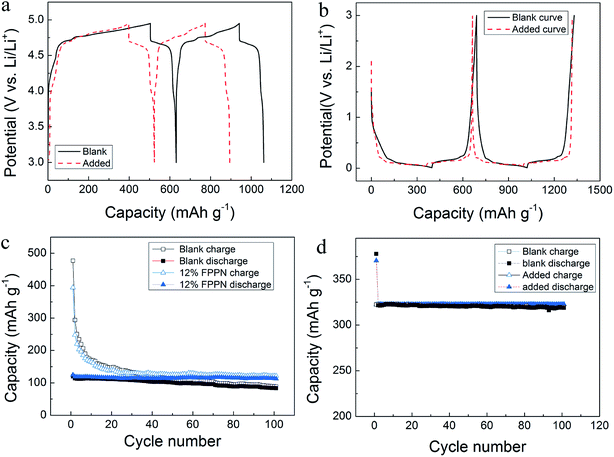 | ||
| Fig. 4 Initial cycles and cycling performance of LiNi0.5Mn1.5O4 (a and c) and graphite–Li (b andd) cells with and without addition of FPPN. | ||
To further understand the overcharge protection mechanism of FPPN, the surface morphology of the cathodes after overcharge was investigated via ex situ SEM. As shown in Fig. 5, for the cathode with 12% FPPN electrolyte additive, the electrode was covered with thick polymer (Fig. 5(b)) compared to the electrode with blank electrolyte (Fig. 5(a)), which obviously resulted from polymerization of FPPN.17 Polymerization could consume the overcharge capacity and prohibit the cell from overcharge runaway.14 Moreover, the H2 byproduct could increase the inner pressure of the cell and trigger the safety vent. Additionally, the separator (Fig. 5(c)) taken from the overcharged cell with 12% FPPN was covered with thick yellow polymer, which further proved the electropolymerization of FPPN. In contrast, no polymer is observed on the separator from the overcharged cell with blank electrolyte.
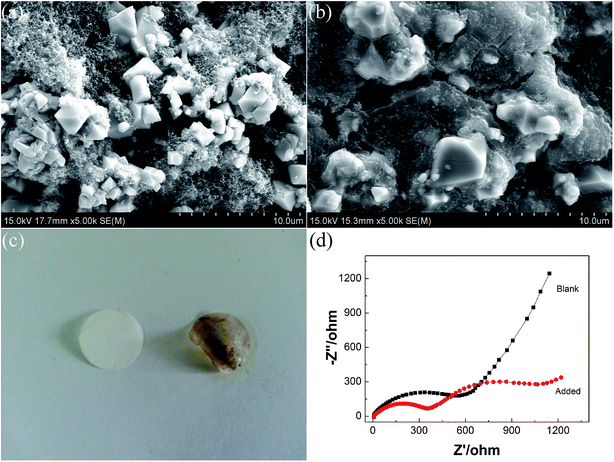 | ||
| Fig. 5 SEM images of LiNi0.5Mn1.5O4 electrodes (a andb), photo images of the separator (c) and Nyquist plots (d) of overcharged cells with and without 12% FPPN electrolyte. | ||
To further examine the actual overcharge protection effect of FPPN, electrochemical impedance spectroscopy (EIS) was also performed. Impedance results of the cells with or without the 10% FPPN additive were measured after being overcharged 1 h. As seen in Fig. 5(d), the overcharged LiNi0.5Mn1.5O4–Li cell showed two compressed cycles. The first cycle is well-known due to SEI films (RSEI) and the second resulted from the charge transfer resistance (RCT).40 The RSEI was about 350 Ohm and Rct about 900 Ohm. In contrast, the RSEI of the cell with the blank electrolyte was negligible and the Rct was about 600 Ohm. From the comparison, we can conclude that the sharp increase in the interfacial impedance (RSEI) should be ascribed to the new polymer layer formed on the electrode via FPPN polymerization.40,41
4. Conclusions
A bifunctional additive, FPPN, is characterized as a safety additive for lithium-ion batteries. Results demonstrated that the additive can also be electro-polymerized at 5.05 V (vs. Li/Li+) and used as an effective overcharge protection additive for 5 V class, lithium ion batteries. Moreover, FPPN could also be used as an efficient flammable retarding additive. The addition of FPPM has positive effects on the electrochemical performance of LiNi0.5Mn1.5O4–Li and graphite–Li cells, which suggest that it may be a promising additive for lithium ion batteries.Acknowledgements
This work was supported by the National Natural Science Foundation of China (nos 21371108 and 21203110), Key Research Plan of Shandong Province (2015GGE27286) and China Postdoctoral Science Foundation (no. 2013M541903), Shandong Provincial Natural Science Foundation for Distinguished Young Scholars (JQ201304), Independent Innovation Foundations of Shandong University (nos 2012ZD008 and 2012TB001), National Science Foundation of Shandong Province (no. ZR2012BM018), start-up funding for New Faculty at Shandong University, Opening Project of CAS Key Laboratory of Materials for Energy Conversion (no. KF2014002), and National Basic Science Personnel Training (no. J1103314).Notes and references
- J. H. Kim, N. P. W. Pieczonka and L. Yang, ChemPhysChem, 2014, 15, 1940–1954 CrossRef CAS PubMed.
- A. Kraytsberg and Y. Ein-Eli, Adv. Energy Mater., 2012, 2, 922–939 CrossRef CAS.
- L. Li, K. S. Lee and L. Lu, Funct. Mater. Lett., 2014, 7, 1430002 CrossRef.
- J. Kalhoff, G. G. Eshetu, D. Bresser and S. Passerini, ChemSusChem, 2015, 8, 2154–2175 CrossRef CAS PubMed.
- N. S. Choi, J. G. Han, S. Y. Ha, I. Park and C. K. Back, RSC Adv., 2015, 5, 2732–2748 RSC.
- S. Tan, Y. J. Ji, Z. R. Zhang and Y. Yang, ChemPhysChem, 2014, 15, 1956–1969 CrossRef CAS PubMed.
- M. Hu, X. L. Pang and Z. Zhou, J. Power Sources, 2013, 237, 229–242 CrossRef CAS.
- N. S. Choi, Z. H. Chen, S. A. Freunberger, X. L. Ji, Y. K. Sun, K. Amine, G. Yushin, L. F. Nazar, J. Cho and P. G. Bruce, Angew. Chem., Int. Ed., 2012, 51, 9994–10024 CrossRef CAS PubMed.
- J. Lu, Y. L. Chang, B. H. Song, H. Xia, J. R. Yang, K. S. Lee and L. Lu, J. Power Sources, 2014, 271, 604–613 CrossRef CAS.
- J. Zou and L. Lu, Mater. Technol., 2015, 30, A1 CrossRef.
- X. Tang, S. S. Jan, Y. Y. Qian, H. Xia, J. F. Ni, S. V. Savilov and S. M. Aldoshin, Sci. Rep., 2015, 5, 11958 CrossRef PubMed.
- J. F. Ni, W. Liu, J. Z. Liu, L. J. Gao and J. T. Chen, Electrochem. Commun., 2013, 35, 1–4 CrossRef CAS.
- H. M. Cho, M. V. Chen, A. C. MacRae and Y. S. Meng, ACS Appl. Mater. Interfaces, 2015, 7, 16231–16239 CAS.
- P. G. Balakrishnan, R. Ramesh and T. Prem Kumar, J. Power Sources, 2006, 155, 401–411 CrossRef CAS.
- S. S. Zhang, K. Xu and T. R. Jow, J. Power Sources, 2003, 113, 166–172 CrossRef CAS.
- K. Xu, Chem. Rev., 2004, 104, 4303–4417 CrossRef CAS PubMed.
- K. Shima, K. Shizuka, M. Ue, H. Ota, T. Hatozaki and J. Yamaki, J. Power Sources, 2006, 161, 1264–1274 CrossRef CAS.
- L. F. Xiao, X. P. Ai, Y. L. Cao and H. X. Yang, Electrochim. Acta, 2004, 49, 4189–4196 CrossRef CAS.
- S. L. Li, X. P. Ai, J. K. Feng, Y. L. Cao and H. X. Yang, J. Power Sources, 2008, 184, 553–556 CrossRef CAS.
- M. Q. Xu, L. D. Xing, W. S. Li, X. X. Zuo, D. Shu and G. L. Li, J. Power Sources, 2008, 184, 427–431 CrossRef CAS.
- N. Iwayasu, H. Honboua and T. Horiba, J. Power Sources, 2011, 196, 3881–3886 CrossRef CAS.
- Y. G. Lee and J. Cho, Electrochim. Acta, 2007, 52, 7404–7408 CrossRef CAS.
- B. Wang, Q. Xia, P. Zhang, G. C. Li, Y. P. Wu, H. J. Luo, S. Y. Zhao and T. van Ren, Electrochem. Commun., 2008, 10, 727–730 CrossRef CAS.
- X. M. Feng, X. P. Ai and H. X. Yang, J. Appl. Electrochem., 2004, 34, 1199–1203 CrossRef CAS.
- L. M. Moshuchak, M. Bulinski, W. M. Lamanna, R. L. Wang and J. R. Dahn, Electrochem. Commun., 2007, 9, 1497–1501 CrossRef CAS.
- J. H. Huang, I. A. Shkrob, P. Q. Wang, L. Cheng, B. F. Pan, M. N. He, C. Liao, Z. C. Zhang, L. A. Curtiss and L. Zhang, J. Mater. Chem. A, 2015, 3, 7332–7337 CAS.
- J. K. Feng, X. P. Ai, Y. L. Cao and H. X. Yang, Electrochem. Commun., 2007, 9, 25–30 CrossRef CAS.
- C. M. Ionica-Bousquet, D. Muñoz-Rojas, W. J. Casteel, R. M. Pearlstein, G. GirishKumar, G. P. Pez and M. R. Palacín, J. Power Sources, 2010, 195, 1479–1485 CrossRef CAS.
- J. W. Wen, D. W. Zhang, C. H. Chen, C. X. Ding, Y. Yu and J. Maier, J. Power Sources, 2014, 264, 155–160 CrossRef CAS.
- J. Lamb, C. J. Orendorff, K. Amine, G. Krumdick, Z. C. Zhang, L. Zhang and A. S. Gozdz, J. Power Sources, 2014, 247, 1011–1017 CrossRef CAS.
- L. F. Xiao, X. P. Ai, Y. L. Cao, Y. D. Wang and H. X. Yang, Electrochem. Commun., 2005, 7, 589–592 CrossRef CAS.
- H. Y. Zhang, Y. L. Cao, H. X. Yang, S. G. Lu and X. P. Ai, Electrochim. Acta, 2013, 108, 191–195 CrossRef CAS.
- J. K. Feng, X. P. Ai, Y. L. Cao and H. X. Yang, J. Power Sources, 2006, 161, 545–549 CrossRef CAS.
- G. Y. Chen, K. E. Thomas-Alyea, J. Newman and T. J. Richardson, Electrochim. Acta, 2005, 50, 4666–4673 CrossRef CAS.
- J. K. Feng, Y. L. Cao, X. P. Ai and H. X. Yang, Electrochim. Acta, 2008, 53, 8265–8268 CrossRef CAS.
- J. K. Feng and L. Lu, J. Power Sources, 2013, 243, 29–32 CrossRef CAS.
- J. K. Feng, Y. L. An, L. J. Ci and S. L. Xiong, J. Mater. Chem. A, 2015, 3, 14539–14544 CAS.
- L. Xia, Y. G. Xia and Z. P. Liu, J. Power Sources, 2015, 278, 190–196 CrossRef CAS.
- M. K. Harrup, H. W. Rollins, D. K. Jamison, E. J. Dufek, K. L. Gering and T. A. Luther, J. Power Sources, 2015, 278, 794–801 CrossRef CAS.
- T. Placke, V. Siozios, R. Schmitz, S. F. Lux, P. Bieker, C. Colle, H. W. Meyer, S. Passerini and M. Winter, J. Power Sources, 2012, 200, 83–91 CrossRef CAS.
- S. S. Zhang, K. Xu and T. R. Jow, J. Power Sources, 2006, 160, 1403–1409 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |