Adsorption of amoxicillin by Mn-impregnated activated carbons: performance and mechanisms
Huaqing Liua,
Zhen Hua,
Hai Liua,
Huijun Xieb,
Shaoyong Luc,
Qingsong Wangd and
Jian Zhang*a
aShandong Key Laboratory of Water Pollution Control and Resource Reuse, School of Environmental Science & Engineering, Shandong University, Jinan 250100, China. E-mail: zhangjian00@sdu.edu.cn; liuhuaqings@163.com; Fax: +86 531 88364513
bEnvironment Research Institute, Shandong University, Jinan 250100, China
cResearch Centre of Lake Environment, Chinese Research Academy of Environmental Sciences, Beijing 100012, China
dSchool of Energy and Power Engineering, Shandong University, Jinan 250100, PR China
First published on 22nd January 2016
Abstract
Phragmites australis carbon (PAC) was produced from Phragmites australis by phosphoric acid (H3PO4) activation. To achieve a better amoxicillin (AMX) adsorption performance, manganous dihydrogen phosphate (Mn(H2PO4)2) and manganese chloride (MnCl2) were impregnated, producing PAC-MP and PAC-MC, respectively. Adsorption studies were carried out in different initial AMX concentration (15–100 mg L−1), pH (2–10) and contact time (0–60 h) to evaluate AMX adsorption performance and mechanisms. The adsorption isotherms well agreed with the Langmuir model for the three adsorbents. The maximum AMX adsorption capacity on PAC, PAC-MP and PAC-MC was 110, 132 and 122 mg g−1, respectively. Solution pH had a strong influence on AMX adsorption. For kinetic results, a significant desorption phenomenon was observed. Several possible mechanisms were elaborated which include electrostatic interaction, complexation and degradation of AMX molecules. On the whole, Mn-impregnated PAC showed superior AMX removal efficiency, which presents a promising modification method for activated carbon in the treatment of AMX containing wastewater.
1. Introduction
Antibiotics are extensively used pharmaceutical compounds to prevent or treat bacterial infections of human, animals and plants. By providing effective access to reduce common infectious diseases, antibiotics have been playing an indispensable role in modern medicine.1 It has been concluded that with the aging population and the increased quality of life, global antibiotic consumption increased by 36% between 2000 and 2010.2 However, a large volume of wastewater containing high antibiotic load is generated during antibiotic production. Studies showed that antibiotics were poorly removed in some cases by the treatment of activated sludge systems and membrane biological reactors.3 As a consequence, more and more antibiotics and their degradation products (DPs) migrated into surface water, groundwater, seawater, etc.4–6 In the ecosystems, antibiotics can not only influence community succession, but also promote the formation of antibiotic resistance genes and bacteria.9,10 The evolved bacteria may never be constrained by the former antibiotics and once again become a threat to humans.Amoxicillin (AMX), a kind of beta-lactam-containing antibiotic, has a widespread use in both human and veterinary medicine to treat infections caused by Gram-positive or Gram-negative bacteria.7 Though this semi-synthetic penicillin-type compound exhibits short half-lives, studies found that the solutions containing AMX DPs remained toxic and the formation of degradation intermediates may become more dangerous.8–10 Therefore, abatement of the risk induced by antibiotic contamination, including the risk posed by AMX became an emerging challenge. It has been concluded that urban wastewater treatment plants were regarded as the main sources of antibiotics in environment and the utilization of advanced treatment technologies was deemed as a major process to reduce antibiotic emissions.11 The available technologies include membrane filtration, ozonation, photocatalysis, adsorption, etc. Thereinto, adsorption is considered as the optimum method in the treatment of synthetic wastewater containing low concentration of antibiotic.12–14
As the most commonly used absorbent, activated carbon has been widely studied to promote its adsorption capacity through physical or chemical modifications. Among these modification methods, metal-salts impregnation can obtain a better heavy metal and dyestuff adsorption effects by the added metal complexation as well as the modified acid functional groups.15,16 Zhang et al. researched the characteristics of activated carbons which were modified by Mn(NO3)2, MnCl2 and MnSO4, respectively.15 The results indicated that the amounts of impregnated manganese and acid functional groups varied with the anion species. Research also indicated that phosphate anion can introduce phosphorus-containing functional groups to activated carbon.17 However, Mn(H2PO4)2, a well-known environmental friendly manganese salt, has never been tried on activated carbon modification. Though antibiotic pollutants were usually exist in the forms of DPs in nature water, there has been few studies paid attention on their DPs removal by activated carbon. To figure out this problem, adsorption mechanisms based on DPs analysis is necessary.
This research is conducted to explore the feasibility of modifying Phragmites australis carbon (PAC) utilizing Mn(H2PO4)2 and MnCl2 by comparing their physical–chemical and AMX adsorption characteristics with original PAC. We have evaluated the properties of the adsorbents by N2 adsorption/desorption, scanning electron microscope (SEM), X-ray diffraction (XRD), Fourier transform infrared (FTIR), Boehm's titration, etc. A series adsorption experiments were carried out and the AMX solutions after adsorption were also analysed by mass spectrum (MS) to have a better understanding of the adsorption mechanisms.
2. Experimental section
2.1. Preparation of the adsorbents
The dry Phragmites australis (PA) was harvested from Baiyun Lake in Shandong, China. After the removal of impurities, PA was smashed and screened though mesh size of 16. Elements composition of the smashed PA was analysed in our previous study, and the results were C (45.1%), H (5.9%), O (48.3%), N (0.5%) and S (0.2%).18 The carbon content showed the applicability of PA being the precursor of activated carbon. The smashed PA and was then adequately mixed with H3PO4 solution at the ratio of 10 g PA/0.2 mol H3PO4. After 8 h of impregnation, the mixture was heated to 450 °C in muffle at the rate of 10 °C min−1 and kept 1 h at this temperature. The product was then washed with hot distill water until the pH was steady. The carbon, after being separated by suction filtration, was dried at 105 °C for 12 h and sieved through the 120 mesh sieve. Finally, PAC was produced.To prepare Mn-impregnated activated carbon, 1 g of PAC was added into 50 mL of Mn(H2PO4)2/MnCl2 solution at the concentration of 0.02 mol L−1. After being stirred at 160 rpm for 12 h, modified carbon was filtered and washed with 500 mL distill water in a vacuum filtration device. Through the last process of drying at 60 °C for 12 h, PAC-MP/PAC-MC was obtained.
2.2. Determination of characteristics of the adsorbents
Pore structure parameters of the three adsorbents were derived by N2 adsorption/desorption at 77 K using a surface area analyser (Quantachrome Corporation, USA). Based on gauged data from N2 adsorption/desorption isotherms, pore size distribution was determined by density functional theory method (DFT) and specific surface area (SBET) was calculated by Brunauer–Emmett–Teller method (BET). Total pore volume (Vt) was obtained at P/P0 = 0.99. Detailed information such as micropore surface area (Smic), external surface area (Sext) and micropore volume (Vmic) were calculated by t-plot method. The mean pore diameter (Dp) was calculated by Dp = 4Vt/SBET. The surface texture was observed by SEM (Hitachi S4800, Japan). The morphology of Mn was characterized by XRD patterns, which were recorded on the Rigaku D/MAX-YA diffractometer with Ni-filtered Cu Kα radiation as the X-ray source. Surface functional group characteristics of the adsorbents were identified using a FTIR spectrometer (Fourier-380 FTIR, USA). The differences of acidic and basic functional groups content were determined by the method of Boehm's titration.19 The analysis based on the assumptions that HCl neutralizes all the basic groups; NaOH neutralizes carboxyl, phenolic and lactonic groups; Na2CO3 neutralizes carboxyl and lactonic groups; and NaHCO3 only neutralizes carboxyl groups. Each of the adsorbents (0.25 g) was fully reacted with 50 mL of 0.05 M HCl, NaOH, Na2CO3 and NaHCO3 solutions, respectively. The remained acid or alkali was titrated and quantified, and the contents of relevant functional groups were obtained based on these calculations. The point of zero charge (pHpzc) was obtained by batch pH titration procedures described by Babić et al.20 Solutions with 0.01 M NaCl were adjusted to a pH value between 2 and 11. A certain amount of each adsorbent (0.15 g) was added into 50 mL of NaCl solutions with various pH values. After 48 h of balance, the final pH of the solutions was measured. The value of intersection point of pHinitial vs. pHfinal curve and pHinitial = pHfinal line was the desired pHpzc of the adsorbent.2.3. Adsorption experiments
To understand AMX (99.8%, standard grade, Beijing Huamaike biotechnology Co., LTD.) adsorption behaviors and mechanisms of the adsorbents, the effects of initial AMX concentration, initial pH and contact time were investigated. In order to guarantee the accuracy of the results, triplicate experiments were carried out and the average values (RSD < 5%) were adopted in the result. In initial AMX concentration study, the three adsorbents (0.02 g) were added into 50 mL of AMX solution (15–100 mg L−1). While in initial pH study, adsorbents dose were 0.06 g and AMX concentration were 30 mg L−1. Initial pH (2–10) of AMX solutions (adjusted by diluted HCl or NaOH solutions) were measured by a pH meter (Model pHS-3C, Shanghai). All the batch adsorption experiments were shaken at 20 ± 1 °C and 160 rpm for 48 h in a water bath shaker (SHZ-88). AMX adsorption amount qe (mg g−1) and removal rate (%) at equilibrium can be calculated by the following equations:
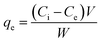 | (1) |
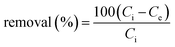 | (2) |
In contact time study, 1.2 g of each adsorbent was added into 1000 mL of AMX solutions with the concentration of 30.0 mg L−1. The mixed solutions were agitated at 160 rpm on an electromagnetic stirrer (Model 78-1) to guarantee sufficient dispersion of adsorptions. Samples (about 10 mL) were taken out and filtered through 0.45 μm membrane filter at setting times (0–60 h). AMX concentrations of the filtrates were analysed using a UV/visible spectrophotometer (UV-754, Shanghai) at wave length of 230 nm.14 AMX adsorption amount qt (mg g−1) can be calculated by the equation:
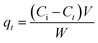 | (3) |
In addition, extra samples were taken out at contact time of 10 h and 48 h, respectively. These samples were analysed by MS (J&W Scientific, USA) to investigate the species of produced AMX DPs in the adsorption processes.
3. Results
3.1. Physical characteristics of adsorbents
Pore size distributions and N2 adsorption/desorption isotherms (inset) are shown in Fig. 1. No remarkable difference of the three adsorbents was found here. According to the International Union of Pure and Applied Chemistry (IUPAC) classification, N2 adsorption/desorption isotherms of the adsorbents all fit the type IV curve. The inflection point in isotherm at low P/P0 indicated the accomplished monolayer adsorption of N2. Multilayer adsorption began to carry out in the next stage. The adsorption hysteresis loop corresponds to capillary condensation phenomenon of mesopore. Pore size distributions showed that micropore (<2 nm) and mesopore (2–50 nm) were dominated structures of the adsorbents, which was consistent with N2 adsorption/desorption isotherms. As listed in Table 1, both the SBET and Vt of PAC-MP and PAC-MC were larger than PAC, which is conductive to AMX adsorption. In addition, all the adsorbents showed their superiority in Sext (>85%) and Vext (>90%). It was reported that activated carbon with manganese modification exhibit polymorphic structures and large surface area.21,22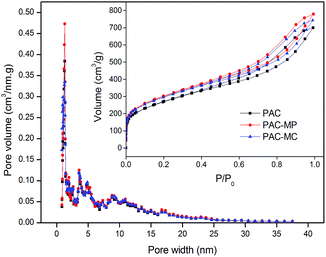 | ||
| Fig. 1 Pore size distribution curves and N2 adsorption/desorption isotherms (inset) of the adsorbents. | ||
| Adsorbents | PAC | PAC-MP | PAC-MC |
|---|---|---|---|
| SBET (m2 g−1) | 944.01 | 1053.36 | 1032.94 |
| Sext (m2 g−1) | 816.42 | 898.12 | 882.49 |
| Sext (%) | 86.48 | 85.26 | 85.43 |
| Vt (m3 g−1) | 1.03 | 1.14 | 1.09 |
| Vext (m3 g−1) | 0.97 | 1.07 | 1.02 |
| Vext (%) | 95.06 | 93.52 | 93.51 |
| Dp (nm) | 4.35 | 4.34 | 4.24 |
| Carboxyl (mmol g−1) | 0.25 | 0.49 | 0.36 |
| Lactone (mmol g−1) | 0.10 | 0.12 | 0.34 |
| Phenolic (mmol g−1) | 0.70 | 1.27 | 0.97 |
| Total acidic (mmol g−1) | 1.05 | 1.88 | 1.67 |
| Total alkaline (mmol g−1) | 0.24 | 0.13 | 0.26 |
| pHpzc | 6.57 | 4.37 | 5.89 |
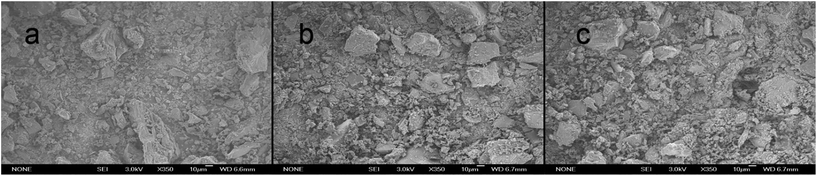
The SEM images of PAC, PAC-MC and PAC-MP were shown in Fig. 2. Compared with PAC (Fig. 2a), we can clearly find the surface morphology changes of Mn-impregnated activated carbons (Fig. 2b and c). The structures of PAC-MC and PAC-MP were corroded severely. The shapes of these activated carbon particles became more irregular. The rough surfaces explained their large surface area and high porosity.
The XRD patterns (not shown) of the three adsorbents were similar, and no apparent diffraction peaks were found. These results indicated the Mn was distributed amorphously and randomly in PAC-MP and PAC-MC.
3.2. Chemical characteristics of adsorbents
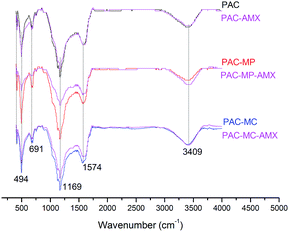
The infrared spectra, ranging from 400–4000 cm−1, of adsorbents were analysed to investigate the surface functional groups. Several intense peaks were formed in the transmitted spectrum on the regions of 3409, 1574, 1169, 691 and 494 cm−1 as shown in Fig. 3. Adsorption peak at 3409 cm−1 is attributed to stretching vibration of –OH and –NH2.23 The peak at 1574 cm−1 is assigned to C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C stretching vibrations.24 The bands in the range of 1000–1300 cm−1 are corresponding to stretching vibration of –C–O–C–, O–H and P–O–C.25,26 The peak at 691 cm−1 may be assigned to C–H out-of-plane vibration.27 The peak at 494 cm−1 was probably due to the vibration of Mn–O.28 The spectra peaks for the adsorbents appeared in similar positions on the whole, demonstrating that species of main surface functional groups on the three adsorbents were not changed. Characteristics of infrared spectra of blank and AMX-loaded adsorbents are also contrasted in Fig. 3. No new peak was discovered after adsorbing AMX. However, almost all of the peaks become weaker after AMX adsorption, indicating the interactions between AMX and surface functional group. The degrees of discrepancy for the three adsorbents before and after AMX adsorption were on the whole as follows: PAC-MP > PAC-MC > PAC. The greater the difference was, the more interactions were proceeded. To sum up, surface functional groups played important role on AMX adsorption.
C stretching vibrations.24 The bands in the range of 1000–1300 cm−1 are corresponding to stretching vibration of –C–O–C–, O–H and P–O–C.25,26 The peak at 691 cm−1 may be assigned to C–H out-of-plane vibration.27 The peak at 494 cm−1 was probably due to the vibration of Mn–O.28 The spectra peaks for the adsorbents appeared in similar positions on the whole, demonstrating that species of main surface functional groups on the three adsorbents were not changed. Characteristics of infrared spectra of blank and AMX-loaded adsorbents are also contrasted in Fig. 3. No new peak was discovered after adsorbing AMX. However, almost all of the peaks become weaker after AMX adsorption, indicating the interactions between AMX and surface functional group. The degrees of discrepancy for the three adsorbents before and after AMX adsorption were on the whole as follows: PAC-MP > PAC-MC > PAC. The greater the difference was, the more interactions were proceeded. To sum up, surface functional groups played important role on AMX adsorption.
The amounts of surface functional groups of the adsorbents can further be quantified through Boehm's titration as listed in Table 1. The detected acidic functional groups include carboxyl, lactone and phenolic, which are coincidence with the results of infrared spectra. Of all the three adsorbents, the amounts of surface functional groups were regularly as follows: phenolic > lactone > carboxyl. Although alkaline functional groups also detected on the adsorbents surface, their amounts were much lower than acidic functional groups. In comparison with PAC, all acidic functional groups were higher in manganese salt modified PAC. On the other hand, the amounts of alkaline functional groups increased a little in PAC-MC but reduced in PAC-MP.
The pHpzc of an adsorbent is a pH point where net charge of the adsorbent is zero. As shown in Table 1, pHpzc of PAC, PAC-MP and PAC-MC were 6.57, 4.37 and 5.89, respectively. This result is consistent with the amounts of acidic functional groups on the adsorbents surfaces. If the pH of external environment is below pHpzc, the adsorbent will be protonated and positively charged. Conversely, if the pH of external environment is above pHpzc, the adsorbent will be deprotonated and negatively charged.
3.3. Effects of initial concentration
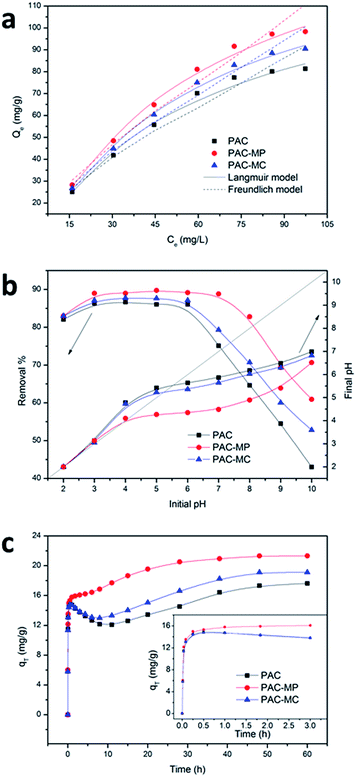
As shown in Fig. 4a, AMX adsorption amounts of the adsorbents increased with the initial AMX concentration, and the increased trend induced by initial AMX concentration was slowed down gradually. Results of AMX adsorption with different initial concentration were fitted by Langmuir29 and Freundlich30 models to describe the adsorption behaviors of the three adsorbents. The parameters of the two models were calculated and listed in Table 2, where qm (mg g−1) is theoretical maximum AMX adsorption amount predicted by Langmuir model; KL (L mg−1) is Langmuir constant related to the energy of adsorption; RL is dimensionless constant to estimate the favorable or unfavorable adsorption process; KF ((mg g−1) (L mg−1)1/n) is Freundlich constant of adsorption capacity; n is a constant related to surface heterogeneity; R2 is correlation coefficient between the simulated results and measured ones. The adsorption isotherms, including experimental and simulated results, were depicted in Fig. 4a. The values of Langmuir parameter RL (0 < RL < 1) and Freundlich parameter n (1 < n < 10) indicated AMX adsorption onto the three adsorbents were favorable adsorption in the concentration range studied.31,32 The correlation coefficient (R2) in Table 2 demonstrated that both of the two models can represent the experimental results well, but Langmuir model showed higher fitting than Freundlich models. The maximum AMX adsorption capacity for PAC, PAC-MP and PAC-MC, as predicted by Langmuir model, was 109.9, 131.6 and 122.0 mg g−1 respectively.| Adsorbent | Langmuir | Freundlich | |||||
|---|---|---|---|---|---|---|---|
| qm (mg g−1) | KL (L mg−1) | R2 | RL | KF | n | R2 | |
| PAC | 109.8901 | 0.0492 | 0.9907 | 0.1727 | 10.7381 | 1.9455 | 0.9669 |
| PAC-MP | 131.5789 | 0.0565 | 0.9940 | 0.1538 | 13.7344 | 1.9455 | 0.9740 |
| PAC-MC | 121.9512 | 0.0505 | 0.9958 | 0.1690 | 11.9915 | 1.9435 | 0.9736 |
3.4. Effects of initial pH
AMX adsorption performance on three adsorbents with different initial solution pH was investigated and the results were shown in Fig. 4b. It can be found that solution pH had a significant influence on AMX adsorption process. Nevertheless, the removal rate curves for three adsorbents showed similar variation tendencies. The AMX removal rates were more than 85% in initial pH range of 3–6 for the three adsorbents. Beyond this initial pH range, AMX adsorption capacities for the adsorbents declined gradually. The PAC-MP showed its superiority in AMX removal on the whole, followed by PAC-MC and PAC. To have a better understand of the pH conditions of the solution, the final pH were measured and the results were depicted in Fig. 4b.3.5. Effects of contact time
As can be seen in Fig. 4c, all the three adsorbents took a long time to achieve adsorption equilibrium. AMX was rapidly adsorbed on the adsorbents at the early stage. During this initial stage, PAC-MP showed better adsorption performance but not obvious. Afterwards, a significant desorption phenomenon appeared both in PAC and PAC-MC, and the former showed a more serious AMX desorption. In addition, the adsorption rate of AMX on PAC-MP slowed down apparently. These abnormal trends continued for almost 10 h, and then AMX adsorption amounts for PAC and PAC-MC started to increase again. AMX adsorption rate for PAC-MP also became faster in this stage. After 60 h of adsorption, the adsorption amounts stopped rising, indicating the adsorption saturation of PAC-MP (21.3 mg g−1), PAC-MC (19.1 mg g−1) and PAC (17.6 mg g−1). It was worth mentioning that similar phenomenon of AMX desorption was also obtained by Adriano et al.33 However, the reason of this particular phenomenon was not explained.To further study the desorption mechanisms, several samples were taken out and then analysed by MS. As molecule weight (MW) of AMX DPs can be measured by MS, the molecule structure and degradation processes may further be determined by related studies. The MS data (Table 3) showed that MW of the generated AMX DPs to be 339 ([MH]+ = 340) and 383 ([MH]+ = 384) at 10 h of adsorption on PAC and PAC-MC, and relative abundance of the former became the base peak. In the situation of PAC-MP, the base peak remained corresponding to AMX sample ([MH]+ = 366). While at the contact time of 48 h, another AMX DPs at m/z 179 was detected.
| Sample | Main detected mass to charge ratio (m/z) | Base peak (m/z) |
|---|---|---|
| Original AMX solution | 366 | 366 |
| AMX–PAC-10 h | 340, 366, 384 | 340 |
| AMX–PAC-MP-10 h | 340, 366 | 366 |
| AMX–PAC-MC-10 h | 340, 366, 384 | 340 |
| AMX–PAC-48 h | 179, 340 | 340 |
| AMX–PAC-MP-48 h | 179, 366 | 176 |
| AMX–PAC-MC-48 h | 179, 210, 340 | 340 |
4. Discussion
Based on the characteristics of the adsorbents and AMX and related researches, several possible mechanisms can be suggested as the explanations for the adsorption of AMX onto the adsorbents. The first mechanism is electrostatic interaction. As both of the carbons and AMX molecule can be protonated and deprotonated, AMX and the adsorbents with opposite charges are attracted. The second mechanism is complexation. Alekseev et al. found the complexation between AMX and manganese ions could take place in neutral and weak alkaline state (6.2 < pH < 8.5).34 Therefore, the impregnated manganese iron could interact with AMX through complexing reactions in proper pH condition. As AMX adsorption onto all the adsorbents were strongly influenced by initial pH of the solution, electrostatic interaction was most likely the predominate mechanism for AMX adsorption.35The maximum AMX adsorption was obtained in the initial pH range of 3.0–6.0. This optimum pH range of was around the pHpzc of the three adsorbents, where acidic and basic functional groups on the carbon surface were weakly protonated or deprotonated. On the other hand, the dissociation constant (pKa) of carboxyl, amino and hydroxyl in AMX molecule are 2.68 (pKa1), 7.49 (pKa2) and 9.63 (pKa3), respectively.36 For AMX molecule, active groups such as carboxyl, amino and hydroxyl in pH 3.0–6.0 presented in the forms of –COO−, –NH3+ and –OH, respectively.37,38 In this condition, adsorption affinity was strong since AMX is possibly combined with both acid and basic functional groups on the surface of the carbons. In the pH range of 6.0–10.0, AMX adsorption capacity decreased as initial pH increased. Fig. 4b showed that the final pH were no more than 7.49 (pKa2) in the initial pH range of 6.0–10.0. In another words, active groups of AMX may still exist as the forms in initial pH 3.0–6.0. Nevertheless, surface functional groups on the adsorbents were negatively charged in high pH value, which lead to a decline of the electrostatic affinity between AMX and the adsorbents.35 The excellent performance of PAC-MP and PAC-MC may owing to their strong electrostatic force brought by the more acidic functional groups as well as the complexation induced by Mn(II). The declined AMX adsorption capacity at initial pH 2.0 can be related to the electrostatic repulsion between AMX and the adsorbents, which were both highly protonated.35
However, the AMX desorption phenomenon in contact time study can hardly be explained by the mechanisms of electrostatic interaction and complexation. Since similar phenomenon has appeared in AMX adsorption onto chitosan beads, we conjecture that this phenomenon is mainly in consequence of the special nature of AMX.33 Table 3 showed the degradation properties of AMX, we suppose that the degradation process could separate AMX DPs from the adsorbents. Furthermore, if AMX molecule were degraded into many pieces, adsorption competitions between AMX DPs would be generated.
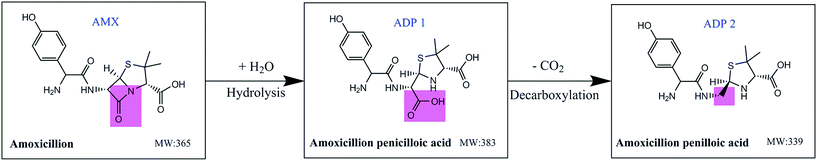
The confirmation and identification of AMX DPs have been carried out by Nägele et al.39 Four main DPs were detected and explained. They were amoxicillin penicilloic acid (ADP 1), amoxicillin penilloic acid (ADP 2), diketopiperazine amoxicillin and 4-hydroxyphenylglyl amoxicillin, respectively. Similar results have been found in other studies.9,40,41 Based on the above researches and MS results in Table 3, the main AMX degradation pathways in the present study were suggested and depicted in Fig. 5. ADP 1 and ADP 2 were corresponding to the detected AMX DPs with MW of 383 and 339 (Table 3). ADP 1 was produced from the hydrolysis of AMX and ADP 2 was produced from the decarboxylation of ADP 1. The significant desorption phenomenon may thus be explained as follows: on the one hand, as AMX can be hydrolysed in aqueous, another carboxyl (the red area of ADP 1) will be produced and combined with alkaline functional groups on carbon surface by electrostatic interaction. Since this carboxyl group is segregative, the degradation product ADP 2 will be separated from the adsorbents and detected in the solution again. On the other hand, the amino group of AMX can be combined with acid functional groups on carbon surface. Since this amino group is relative stable, the main body of AMX molecule can hardly be desorbed from the adsorbents. Comparatively, PAC-MP had the maximum acid functional groups and minimum alkaline functional groups. Therefore, AMX desorption phenomenon of PAC-MP was lightest among the three adsorbents. Though PAC had less alkaline functional groups than PAC-MC, it also possessed the minimum acid functional groups. As a result, desorption phenomenon of PAC was the most serious one.
The discussions above suggested AMX adsorption mechanisms mainly include three steps: firstly, AMX were adsorbed on adsorbents surface by electrostatic interaction and complexation; secondly, some of the adsorbed AMX were degraded and released into the solution; finally, AMX DPs were adsorbed onto the adsorbents again. Besides, from the prospective of space, intraparticle diffusion is possible as the Dp (>40 Å) of the adsorbents is much larger than the size of AMX molecule (about 13 Å).14 The size of AMX DPs will become smaller than AMX, which is more conductive to the function of intraparticle diffusion. The adsorption of AMX existed throughout the whole process, and the degradation process slowed down gradually as AMX concentration became lower over time. As a result, the adsorption process was predominant in the later stage.
5. Conclusion
In this study, adsorption of AMX onto PAC, PAC-MP and PAC-MC, were investigated. The surface areas of PAC, PAC-MP and PAC-MC were 944, 1053 and 1033 m2 g−1 respectively, and their total pore volumes were 1.03, 1.14 and 1.09 cm3 g−1, respectively. The amounts of acidic functional groups of PAC-MP and PAC-MC were largely increased compared with PAC. The maximum adsorption abilities of PAC, PAC-MP and PAC-MC were 110, 132 and 122 mg g−1, respectively as calculated by the well-agreed Langmuir model. Possible mechanisms like electrostatic interaction, complexation and degradation of AMX molecule were elaborated to explain the adsorption performance.Acknowledgements
This work was supported by Fundamental Research Funds of Shandong University (No. 2014TB003&2015JC056) and National Natural Science Foundation of China (No. 21307076& No.51578321).References
- R. Laxminarayan, A. Duse, C. Wattal, A. K. Zaidi, H. F. Wertheim, N. Sumpradit, E. Vlieghe, G. L. Hara, I. M. Gould and H. Goossens, Lancet Infect. Dis., 2013, 13, 1057–1098 CrossRef PubMed.
- T. P. Van Boeckel, S. Gandra, A. Ashok, Q. Caudron, B. T. Grenfell, S. A. Levin and R. Laxminarayan, Lancet Infect. Dis., 2014, 14, 742–750 CrossRef PubMed.
- P. Verlicchi, M. Al Aukidy and E. Zambello, Sci. Total Environ., 2012, 429, 123–155 CrossRef CAS PubMed.
- D. W. Kolpin, E. T. Furlong, M. T. Meyer, E. M. Thurman, S. D. Zaugg, L. B. Barber and H. T. Buxton, Environ. Sci. Technol., 2002, 36, 1202–1211 CrossRef CAS PubMed.
- D. Avisar, G. Levin and I. Gozlan, Environ. Earth Sci., 2009, 59, 939–945 CrossRef CAS.
- T. B. Minh, H. W. Leung, I. H. Loi, W. H. Chan, M. K. So, J. Mao, D. Choi, J. C. Lam, G. Zheng and M. Martin, Mar. Pollut. Bull., 2009, 58, 1052–1062 CrossRef CAS PubMed.
- I. Gozlan, A. Rotstein and D. Avisar, Environ. Chem., 2010, 7, 435–442 CrossRef CAS.
- L. Rizzo, S. Meric, M. Guida, D. Kassinos and V. Belgiorno, Water Res., 2009, 43, 4070–4078 CrossRef CAS PubMed.
- I. Gozlan, A. Rotstein and D. Avisar, Chemosphere, 2013, 91, 985–992 CrossRef CAS PubMed.
- J. Radjenović, C. Sirtori, M. Petrović, D. Barcelo and S. Malato, Appl. Catal., B, 2009, 89, 255–264 CrossRef.
- L. Rizzo, C. Manaia, C. Merlin, T. Schwartz, C. Dagot, M. Ploy, I. Michael and D. Fatta-Kassinos, Sci. Total Environ., 2013, 447, 345–360 CrossRef CAS PubMed.
- S.-Z. Li, X.-Y. Li and D.-Z. Wang, Sep. Purif. Technol., 2004, 34, 109–114 CrossRef CAS.
- K. Ikehata, N. Jodeiri Naghashkar and M. Gamal El-Din, Ozone: Sci. Eng., 2006, 28, 353–414 CrossRef CAS.
- E. K. Putra, R. Pranowo, J. Sunarso, N. Indraswati and S. Ismadji, Water Res., 2009, 43, 2419–2430 CrossRef CAS PubMed.
- J. Zhang, Q. Shi, C. Zhang, J. Xu, B. Zhai and B. Zhang, Bioresour. Technol., 2008, 99, 8974–8980 CrossRef CAS PubMed.
- H. Liu, W. Liu, J. Zhang, C. Zhang, L. Ren and Y. Li, J. Hazard. Mater., 2011, 185, 1528–1535 CrossRef CAS PubMed.
- F. Suárez-García, A. Martínez-Alonso and J. M. Tascón, Carbon, 2004, 42, 1419–1426 CrossRef.
- Z. Guo, L. Xu, C. Liu, F. Sun, Y. Kang and S. Liang, Desalin. Water Treat., 2015, 1–11 Search PubMed.
- H. Boehm, Carbon, 1994, 32, 759–769 CrossRef CAS.
- B. Babić, S. Milonjić, M. Polovina and B. Kaludierović, Carbon, 1999, 37, 477–481 CrossRef.
- L. Huang, J. Kong, W. Wang, C. Zhang, S. Niu and B. Gao, Desalination, 2012, 286, 268–276 CrossRef CAS.
- H.-Q. Wang, G.-f. Yang, Q.-Y. Li, X.-X. Zhong, F.-P. Wang, Z.-S. Li and Y.-h. Li, New J. Chem., 2011, 35, 469–475 RSC.
- J. Zawadzki, Chem. Phys. Carbon, 1989, 21, 147–380 CAS.
- J. M. Devi, P. Tharmaraj, S. Ramakrishnan and K. Ramachandran, Mater. Lett., 2008, 62, 852–856 CrossRef CAS.
- S. Bourbigot, M. Le Bras, R. Delobel, P. Bréant and J.-m. Trémillon, Carbon, 1995, 33, 283–294 CrossRef CAS.
- H. Boehm, Carbon, 2002, 40, 145–149 CrossRef CAS.
- A.-N. A. El-Hendawy, J. Anal. Appl. Pyrolysis, 2006, 75, 159–166 CrossRef CAS.
- H. Ma, Z. Zhu, L. Dong, Y. Qiu and J. Zhao, Sep. Sci. Technol., 2010, 46, 130–136 CrossRef.
- I. Langmuir, J. Am. Chem. Soc., 1916, 38, 2221–2295 CrossRef CAS.
- B. Hameed, A. M. Din and A. Ahmad, J. Hazard. Mater., 2007, 141, 819–825 CrossRef CAS PubMed.
- Ş. Kubilay, R. Gürkan, A. Savran and T. Şahan, Adsorption, 2007, 13, 41–51 CrossRef.
- D. D. Do, Adsorption analysis: equilibria and kinetics, Imperial College Press, 1998 Search PubMed.
- W. Adriano, V. Veredas, C. Santana and L. Gonçalves, Biochem. Eng. J., 2005, 27, 132–137 CrossRef CAS.
- V. Alekseev, O. Lyamtseva and I. Samuilova, Russ. J. Inorg. Chem., 2007, 52, 383–385 CrossRef.
- G. Moussavi, A. Alahabadi, K. Yaghmaeian and M. Eskandari, Chem. Eng. J., 2013, 217, 119–128 CrossRef CAS.
- A. F. Goddard, M. J. Jessa, D. A. Barrett, P. N. Shaw, J. Idstrom, C. Cederberg and R. C. Spiller, Gastroenterology, 1996, 111, 358–367 CrossRef CAS.
- V. Homem, A. Alves and L. Santos, Int. J. Environ. Anal. Chem., 2010, 90, 1063–1084 CrossRef CAS.
- R. Andreozzi, M. Canterino, R. Marotta and N. Paxeus, J. Hazard. Mater., 2005, 122, 243–250 CrossRef CAS PubMed.
- E. Nägele and R. Moritz, J. Am. Soc. Mass Spectrom., 2005, 16, 1670–1676 CrossRef PubMed.
- A. G. Trovó, R. F. P. Nogueira, A. Agüera, A. R. Fernandez-Alba and S. Malato, Water Res., 2011, 45, 1394–1402 CrossRef PubMed.
- X. Li, T. Shen, D. Wang, X. Yue, X. Liu, Q. Yang, J. Cao, W. Zheng and G. Zeng, J. Environ. Sci., 2012, 24, 269–275 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |