In situ growth behavior of boron nitride nanotubes on the surface of silicon carbide fibers as hierarchical reinforcements†
Guangxiang Zhuabc,
Shaoming Dong*ab,
Jianbao Huab,
Yanmei Kanab,
Ping Heab,
Le Gaoab,
Xiangyu Zhangab and
Haijun Zhouab
aState Key Laboratory of High Performance Ceramics and Superfine Microstructure, Shanghai Institute of Ceramics, Chinese Academy of Sciences, Shanghai 200050, China. E-mail: composites@mail.sic.ac.cn; Tel: +86-21-52414324
bStructural Ceramics and Composites Engineering Research Center, Shanghai Institute of Ceramics, Chinese Academy of Sciences, Shanghai 200050, China
cUniversity of Chinese Academy of Sciences, Beijing 100049, China
First published on 27th January 2016
Abstract
BNNTs grown in situ on the surface of silicon carbide fibers via a simplified ball milling, impregnation and annealing method using boron powder as the raw material were synthesized. The morphology, microstructure and chemical composition of as-grown BNNTs were well characterized by field-emission scanning electron microscopy, field emission transmission electron microscopy, electron energy loss spectroscopy and Raman spectroscopy. The analysis results indicate that the nanostructures are BNNTs with the diameter of 50–130 nm, exhibiting a multi-walled and bamboo-like structure and possessing two distinct morphologies of the tube walls: flat walls and bubble-chain walls, respectively. Based on the experimental characterization of BNNTs and as-milled powders, the possible growth process of BNNTs has been described and the formation mechanisms of two types of bamboo-like BNNTs have been proposed, including the tip growth mode and the base growth mode.
Introduction
Ceramic matrix composites (CMCs) have been known to be the most promising candidates for aero-engine hot section components due to their excellent properties, such as outstanding high-temperature strength, fracture toughness, thermal stability and low density.1 With the existence of continuous fibers, such as carbon and silicon carbide (SiC) fibers, embedded in the monolithic ceramic matrix, CMCs exhibit a non-catastrophic failure mode.2 Nonetheless, there still remains a major problem that the inter-fiber-bundle and inter-laminar matrix still show a brittle behavior at the micron scale, which will definitely weaken the overall performance of CMCs. Introducing one dimensional nanostructures, such as carbon nanotubes (CNTs) with extraordinary mechanical properties, into the matrix to produce hierarchical (or multi-scale or hybrid) composites has been proved to be an effective way to solve the above-mentioned problem. So far, much work has been done with respect to fiber/CNTs hierarchical composites and the anticipation that CNTs as one dimensional nanoscale reinforcements could strengthen and toughen inter-fiber-bundle and inter-laminar matrix has been realized.3–7In recent years, with the same one dimensional nanostructure as CNTs, boron nitride nanotubes (BNNTs) also have attracted tremendous attention from frontier researchers due to their excellent properties such as electrical insulation, distinctive violet or ultraviolet (UV) luminescence emission and super-hydrophobicity, which promote potential applications in semiconductor devices, UV lasing devises, and self-cleaning systems.8 By virtue of the structural similarity to CNTs, BNNTs also possess high elastic modulus, high tensile strength and high thermal conductivity,8–10 which demonstrates a promising application in composites as nanoscale reinforcements like CNTs. Additionally, BNNTs are superior to CNTs in chemical and thermal stability on account of a more pronounced resistance to oxidation. BNNTs are stable in air from 900 °C to over 1000 °C as compared to CNTs with only 400 °C to 600 °C.9,11 Hence, this superiority may enable BNNTs to replace CNTs in many structural applications for enduring service at high temperature. In order to avoid the agglomeration of BNNTs resulting from directly dispersion in the precursor like CNTs,3,12 in situ growing BNNTs directly on the surface of fibers to build fiber/BNNTs hierarchical reinforcements is a favorable method to introduce BNNTs into matrix of composites. Therefore, in situ growth of BNNTs on the surface of fibers is the prerequisite to fabricate fiber/BNNTs hierarchical reinforcements.
To date, various synthesis methods have been developed for BNNTs growth, such as arc discharge,13 laser ablation,14 ball milling–annealing,15–18 substitution reaction with CNTs template19 and chemical vapor deposition (CVD).20,21 However, no study on in situ growth behavior of BNNTs on the surface of fibers has been reported. In the present study, SiC fiber cloths are used as the substrates for BNNTs growth while in our further study of hierarchical composites three dimensional (3D) fiber preforms will be employed. Compared with other methods as mentioned above, the ball milling–annealing method can avoid the high synthesis temperature in arc discharge and laser ablation methods, the impurity of carbon atoms in the lattice of BNNTs in the substitution reaction with CNTs template method and the poor diffusion of gas precursors from outside into 3D fiber preforms in CVD method. Hence, it is deemed as an effective and suitable approach for in situ growth of BNNTs uniformly on the surface of fibers with high yield in our study. In the ball milling–annealing method, boron powder is used as raw material. The boron source for BNNTs growth comes from the powder by solid phase diffusion and the growth of BNNTs follows the solid–liquid–solid (SLS) mechanism.22 So the diffusion of boron atoms from the powder to catalysts is an essential step for BNNTs growth. However, the solid diffusion is very slow, which definitely limits the growth of nanotubes. After high-energy ball milling, the average particle size of boron powder declines dramatically and a highly chemical activity is gained. The existence of massive defects on the surface of boron powder induced by mechanically ball milling impacts will accelerate the surface diffusion rate of boron atoms from boron powder to catalysts, promoting the growth of BNNTs.15,23 So the pre-ball milling step is extremely indispensable before subsequent thermal annealing. Nonetheless, in order to gain such a highly chemical activity, boron powder should be ball-milled for over 100 hours until the average particle size is less than 100 nm.24 Additionally, boron powder should be protected from oxidation during ball milling by dry and inert gas atmosphere such as ammonia (NH3). Such a process as described above is a little difficult to fulfill and hard on the equipment such as ball-milling machine and sealed milling vessels.
In this paper, a simplified ball milling, impregnation and annealing method is developed, consisting of ball milling of boron powder, impregnation of fiber cloths with as-milled boron powder slurry and thermal annealing in high pressure nitrogen gas (N2), to synthesize BNNTs in situ grown on the surface of fibers. To the best of our knowledge, this fiber/BNNTs hierarchical reinforcements has not yet been reported, which will be employed to produce multi-scale composites in further study. This paper emphatically elucidates the features and in situ growth of BNNTs on the SiC fibers. The morphology, microstructure and chemical composition of BNNTs were investigated. On the basis of structural observations and possible chemical reactions, the growth process and formation mechanism of BNNTs were also discussed.
Experimental
Synthetic procedures
Elemental boron powder with a high purity (>99.9%) was used as the boron source and nitrogen gas (N2) as the nitrogen source for BNNTs growth. 8 g boron powder and 500 ml ethanol were loaded into the simple polyurethane (PU) milling vessels (1 L) together with some zirconia balls (the weight ratio of milling balls: ϕ 10 mm![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ϕ 6 mm balls = 1) with a ball to powder weight ratio of 60
ϕ 6 mm balls = 1) with a ball to powder weight ratio of 60![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
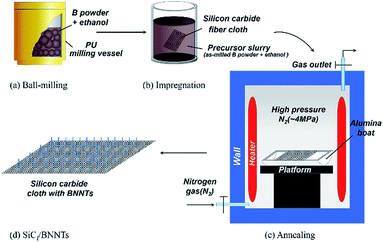
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1. Ball milling treatment was conducted using a vertical planetary ball mill and lasted for 120 h at ambient temperature at a speed of 350 rpm. Then, zirconia balls were replaced by stainless steel balls (ϕ 10 mm, ASTM-S30400) with the same ball to powder weight ratio and ball milling was continued for another 6 h at 350 rpm. Herein, ball-milling treatment with stainless steel balls is aiming at introducing catalysts necessary for the growth of one dimensional nanostructures into boron powder. The as-milled powder with 500 ml ethanol was prepared as the precursor slurry. A piece of SiC fiber cloth (0/90°) with the size of 7 cm × 7 cm was under ultrasonic treatment in ethanol for 1 h to clean impurities absorbed on the surface of fibers, then impregnated in the precursor slurry for 1 h under ultrasonic vibration. After dried in vacuum oven at 85 °C for 1 h, SiC fiber cloth was put in the furnace and heat-treated at 1300 °C for 4 h under N2 atmosphere with a pressure of 4 MPa. The schematic diagram of above-mentioned process is shown in Fig. 1.
1. Ball milling treatment was conducted using a vertical planetary ball mill and lasted for 120 h at ambient temperature at a speed of 350 rpm. Then, zirconia balls were replaced by stainless steel balls (ϕ 10 mm, ASTM-S30400) with the same ball to powder weight ratio and ball milling was continued for another 6 h at 350 rpm. Herein, ball-milling treatment with stainless steel balls is aiming at introducing catalysts necessary for the growth of one dimensional nanostructures into boron powder. The as-milled powder with 500 ml ethanol was prepared as the precursor slurry. A piece of SiC fiber cloth (0/90°) with the size of 7 cm × 7 cm was under ultrasonic treatment in ethanol for 1 h to clean impurities absorbed on the surface of fibers, then impregnated in the precursor slurry for 1 h under ultrasonic vibration. After dried in vacuum oven at 85 °C for 1 h, SiC fiber cloth was put in the furnace and heat-treated at 1300 °C for 4 h under N2 atmosphere with a pressure of 4 MPa. The schematic diagram of above-mentioned process is shown in Fig. 1.
Characterization
The phases and particle size distributions of as-received and as-milled boron powder were analyzed respectively by a Bruker D8-ADVANCE X-ray diffraction (XRD) and a Mastersizer-3000 laser diffraction particle size analyzer. In particle size distribution analysis, Dv10, Dv50 and Dv90 are defined as the size values, below which there are 10, 50 and 90 vol% of particles, respectively. Dv50 is also regarded as mass median diameter. The surface chemical state of as-milled boron powder was examined using ESCALAB 250Xi X-ray photoelectron spectrometry (XPS). A Hitachi SU8220 field-emission scanning electron microscopy (FESEM) was employed to characterize the morphology of BNNTs in situ grown on the fiber surface. A FEI Tecnai G2 F20 field emission transmission electron microscopy (FETEM) was used to examine the microstructure of nanotubes. For structure analysis, a piece of the fiber cloth with BNNTs grown was ultrasonically vibrated in ethanol for 1–2 h to collect adequate quantity of BNNTs from fibers first. Then the dispersion solution was dripped onto copper grids with a holey carbon film for TEM investigations. A X-ray energy dispersive spectroscopy (EDS) and a Gatan Enfina electron energy loss spectroscopy (EELS) attached to FEI Tecnai G2 F20 FETEM were used to determine chemical contents of nanotubes and catalytic particles. The as-grown nanotubes were also characterized by a Nicolet DXR Raman spectroscopy (RS) to check the phase of nanostructures.Results and discussion
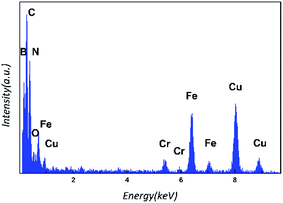
The SEM image (Fig. 2(a)) taken at a low magnification from fibers before the growth of BNNTs shows that SiC fibers have a smooth surface after ultrasonic cleaning in ethanol. After the growth of BNNTs, the color of SiC fiber cloth changes from black to gray or white, which is ascribed to BNNTs covering on the outside surface. The typical morphology of BNNTs in situ grown on the surface of fibers is exhibited in Fig. 2(b) and (c). It can be seen that the surface of fibers is utterly and uniformly besieged by BNNTs. The length of BNNTs can't been measured accurately because they entangle with each other. However, it is obvious that the diameter of fibers increases from about 15 μm to 35 μm after the growth of BNNTs. High magnification SEM images (Fig. 2(d)–(f)) of as-grown BNNTs, clearly reveal that as-grown BNNTs possess two different morphologies of tube walls: bubble-chain walls (Fig. 2(e)) and flat walls (Fig. 2(f)). From Fig. 2(d), it can be estimated that BNNTs have the outer diameter in the range of 50–130 nm. Additionally, it needs to be especially described in Fig. 2(d) that the majority of BNNTs with bubble-chain walls are shorter than the ones with flat walls, which makes them look like to be under the ones with flat walls. So the tips of BNNTs with bubble-chain walls are closer to the fiber surface where the growth initiates.TEM observations of as-grown BNNTs, separated by ultrasonic treatment from fibers were conducted. Representative TEM images of several investigated areas are displayed in Fig. 3. It can be noticed from Fig. 3(a) that, both of different morphologies of BNNTs exhibit a multi-walled and bamboo-like structure in which the inside of nanotubes is separated by regular compartment layers. From the enlarged image inserted on the left, in bamboo-like BNNTs with flat walls, the compartment layers are much closer to each other than those in bamboo-like BNNTs with bubble-chain walls, which is a typical result of all nanotubes investigation. Some nanoparticles are observed inside the nanotubes for bamboo-like BNNTs with bubble-chain walls, as displayed in Fig. 3(b). These nanoparticles were characterized by EDS to determine the composition. The typical EDS spectra shown in Fig. 4 confirms that these nanoparticles are identified as steel particles with the composition of Fe and a little Cr. The Cu and C peaks in Fig. 4 are attributed to the Cu grid and C film for TEM sample preparation. A low level of O element may be due to the surrounding air absorbed on the surface of nanotubes. The strong B and N peaks detected in EDS spectra result from the nanotube outside the steel nanoparticles. These steel nanoparticles, which are responsible for catalytic growth of BNNTs,21–23 are introduced into as-milled boron powder by ball-milling treatment with stainless steel balls. During ball-milling treatment, some steel particles are peeled down from stainless steel balls due to mechanical impacts, then ball-milled with boron powder for long time. Some of these steel particles are downsized to be at the nanoscale, as affirmed in Fig. 3(b). What should be pointed out is that those nanoparticles inside the nanotubes are always located in the hemisphere-like end of one bamboo unit, as confirmed in Fig. 3(b), which is in good accordance with the observation results reported by Zhong et al.,25,26 Tang et al.27 and Huo et al.28 In addition, the diameters of bamboo units filled with nanoparticles (labeled in TEM image) decline gradually against the direction of the bamboo, as depicted in Fig. 3(b), which is also consistent with the reported results from Tang et al.27 The tips of bamboo-like BNNTs with bubble-chain walls observed in Fig. 3(b) are also filled with a small size catalytic nanoparticles. The above results give the evidence that catalytic nanoparticles move from the original bamboo unit to the last one and sometimes a part of the catalyst nanoparticle due to the occurrence of splitting remains in the hemisphere-like end of one bamboo unit during the growth. It also reveals that the growth direction could be against the direction of the bamboo, as indicated by white arrows in Fig. 3(b).28 On the other hand, according to the observation of BNNTs by TEM, no encapsulated catalytic nanoparticles are found inside or at the tips of the tubes for bamboo-like BNNTs with flat walls. According to the results reported by Chen et al.,22 Wang et al.29 and Bi et al.,30 catalytic nanoparticles are necessary for the growth of bamboo-like BNNTs and for such bamboo-like BNNTs with flat walls catalytic nanoparticles may locate at the bottoms of BNNTs, i.e. on the fiber surface in the present study.
A TEM picture (Fig. 5(a)) taken from one bubble-like joint of BNNTs with bubble-chain walls, shows that the tube wall of the joint at the head of one bamboo unit is much thicker than that at the foot, as indicated by white arrows. The measurements of wall thicknesses were conducted on several nanotubes, showing that the thicknesses of the thinnest portions range from 5 nm to 7 nm in different measured nanotubes while from 21 nm to 24 nm for the thickest ones. To deeply investigate the fine structure of as-grown BNNTs, high-resolution TEM (HRTEM) observation was employed. A typical HRTEM image of the joint in Fig. 5(b) obviously reveals that the nanotube has perfect crystalline structure with clear parallel fringes. The spacing between the parallel fringes is ∼0.33 nm, which is in perfect accordance with d002 inter-planar distance of bulk h-BN. Further observation of parallel fringes exhibits that there are some structural defects as indicated by white arrow in Fig. 5(c) such as edge dislocations (clearly depicted by white dotted lines in the inset) in the compartment layers due to inherent strains, which gives the evidence that stresses indeed exist in the compartment layers.25 Fig. 5(d) displays a typical TEM image of the catalytic nanoparticle encapsulated inside the joint of BNNTs with bubble-chain walls, showing that the shape of nanoparticle fits tightly to the inner wall of the joint. This fact indicates that the steel nanoparticle should be in a liquid or quasi-liquid state during the growth process of BNNTs. The range of the contact angle between the nanoparticle and the tube wall in different measured nanotubes is 126–133 degrees (>90 degrees), as indicated in Fig. 5(d), demonstrating poor compatibility between the steel nanoparticle and the inner wall of BNNTs.
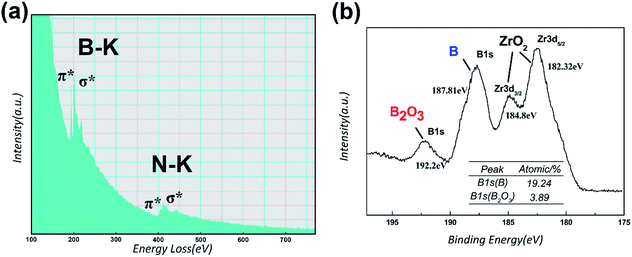
The EELS spectra taken from the tubular part of bamboo-like nanotube, as shown in Fig. 6(a), presents two distinct absorption peaks of B at ∼188 eV and N at ∼401 eV, which correspond to the K-shell ionization edges of B and N, respectively. It can be illustrated that B and N atoms of the nanotube are in sp2-bonded state with the proof of the evident π* peaks and σ* peaks of B–K and N–K signals. Quantitative analysis with an estimation error of 20% can be done to calculate out that the B/N ratio is estimated to be ∼0.9, close to stoichiometric ratio of BN. Hence, EELS analysis demonstrates that bamboo-like nanotubes are BNNTs, which is in line with the results of EDS. The as-grown BNNTs covered on the fiber cloth were also examined by Raman spectroscopy (with a laser wavelength of 532 nm). The Raman spectra of BNNTs depicts only one obvious Raman peak at ∼1365.33 cm−1, as shown in Fig. S1,† which is attributed to the well-known E2g vibrational mode of h-BN.31,32 This Raman active mode is caused by in-plane atomic movement of B and N atoms towards each other. This Raman result also suggests that the nanotubes on the fiber cloth are BNNTs.
To pin down the growth process and possible formation mechanisms of BNNTs in the present study, the features of boron powder were specially examined using XRD, laser diffraction particle size analyzer and XPS. The analysis of XRD patterns taken from as-received and as-milled boron powder, as depicted in Fig. S2(a),† indicates that the structure of boron powder changes into amorphous structure after ball milling due to mechanical energy from the collision between milling balls and particles. It is believed that this disordered structure of as-milled boron powder is unstable and may improve the chemical reactivity because of extra mechanical energy gained after ball milling.17 Additionally, one sharp diffraction peak which is indexed as Fe–Cr–Ni phase (steel, JCPDS card no. 35-1375), is detected in XRD pattern of boron powder after ball-milling treatment with stainless steel balls. This XRD analysis result sheds light on the fact that steel catalytic nanoparticles encapsulated inside the nanotubes, which are observed and determined by TEM images and EDS spectra, originate from the abrasion of stainless steel milling balls during ball milling. Fig. S2(b)† shows the particle size distribution of as-received and as-milled boron powder. The as-received boron powder exhibits a broad particle size distribution, for which the values of Dv10, Dv50 and Dv90 are 0.800 μm, 3.010 μm and 65.400 μm, respectively. After ball milling treatment, the distribution becomes relatively narrow. The mass median diameter (Dv50) is 0.515 μm and 10 vol% of boron powder have the size values below 0.134 μm. It can be concluded that the size of boron powder declines and chemical activity of boron powder is improved by ball milling to some extent. Nevertheless, chemical activity of boron powder in the present study is still extremely inferior to that of high-energy ball milled boron powder with the average particle size of 0.010–0.100 μm in traditional ball milling–annealing method.24 Hence, the solid-state diffusion of boron atoms from boron powder to catalysts in the present study can be ignored. The solid–liquid–solid (SLS) mechanism for BNNTs growth in traditional ball milling–annealing method using high-energy ball milled boron powder as the precursor is not dominant in the present study. For further investigation, the surface chemical state of as-milled boron powder was determined by XPS analysis. Fig. 6(b) is the high resolution scans in B1s and Zr3d regions of as-milled boron. It is apparent that two distinct peaks in B1s region are observed at binding energies of 192.2 eV and 187.81 eV. The peak at 187.81 eV corresponds to element boron and the other one at 192.20 eV is ascribed to B2O3,33 implying that boron powder was oxidized during the milling process. It should be clarified that, in Fig. 6(b), two Zr3d peaks at 184.80 eV and 182.32 eV are also detected, which are attributed to the contaminant ZrO2 from zirconia milling balls. The oxidation layer outside the boron particle could be far from thick, as estimated from the atomic percentage of B2O3 and element B signals in the table inserted in Fig. 6(b). According to the results reported by Singhal et al.34 and Chen et al.,35 B2O3 can react with B to generate the vapor B2O2, which is a kind of boron source widely employed for BNNTs growth. Therefore, vapor–liquid–solid (VLS) mechanism is preferable to well interpret the growth of BNNTs in the present study.
Based on the analysis results described above, the growth process and possible formation mechanisms of bamboo-like BNNTs with bubble-chain and flat walls can be proposed now, as schematically illustrated in Fig. 7. With regard to VLS mechanism, several steps are involved: solid metallic particles firstly transform to liquid or quasi-liquid state, and the vapor sources needed for growth diffuse and dissolve into the molten catalytic particles, which causes the liquid catalyst to become supersaturated, and then the dissolved atoms precipitate to grow one dimensional nanostructure.26 In VLS mechanism, three main growth modes are included, i.e. tip growth, base growth and base–tip growth.36 Given that catalytic nanoparticles are observed inside and at the tips of the nanotubes, the tip growth mode is preferred to comprehend the growth process of bamboo-like BNNTs with bubble-chain walls. The possible chemical reactions during the growth process are illustrated as follows:
| B(s) + B2O3(l) → B2O2(g) | (1) |
| B2O2(g) + N2(g) → BN(s) + O2(g) | (2) |
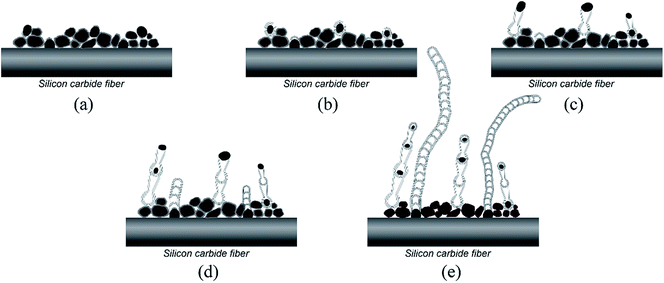 | ||
| Fig. 7 Schematic presentation of the growth process of bamboo-like BNNTs with bubble-chain walls and flat walls. | ||
At first, when annealed at 1300 °C, catalytic steel nanoparticles, with a lower melting point than bulk steel due to the nanoscale effect,22 become to be in a liquid or quasi-liquid state, as affirmed in TEM characterization. Then the oxidation layer outside the as-milled boron particle react with the inner core B, producing the vapor B2O2 (eqn (1)). The vapor B2O2 and the gas N2 in the furnace chamber in situ diffuse and dissolve into the surrounding molten catalytic nanoparticles, meanwhile B2O2 reacts with N2 to generate the BN species (eqn (2)). It should be admitted that the amount of the vapor B2O2 generated from the reaction between B2O3 and B is small because oxidation layers are not thick as shown in XPS analysis result. When the growth process is in nitrogen gas with the negative or atmospheric pressure, BNNTs barely can be found on the fiber surface. Hence, high nitrogen pressure is employed in the present study. Under this circumstance, BNNTs can grow prosperously because the existence of more nitrogen molecules can lower the movement of B2O2 molecules, which hinders the vapor B2O2 from spreading uniformly in the furnace chamber. Consequently, to some degree the concentration of B2O2 close to the fiber surface is kept at a high level, which is beneficial for BNNTs growth. What also can be concluded is that the relatively slow diffusion of B atoms from the solid powder to the liquid catalytic nanoparticles makes almost no contribution to the growth of BNNTs according to the fact that BNNTs can't grow when the vapor B2O2 is out of function in N2 gas with the negative or atmospheric pressure, verifying the above-mentioned speculation that SLS mechanism is not dominant in the present study. This result is divergent from the analysis reported by Mo et al.37
The elaborate formation mechanism of bamboo-like BNNTs with bubble-chain walls has been well explained with the improved stress-induced sequential growth mode.28 When the concentration of BN species in the liquid catalytic nanoparticle reaches the super-saturation value, BN layers begin to precipitate and surround the catalytic nanoparticle, as presented in Fig. 7(a) and (b). With the continuous precipitation of BN layers between the catalytic nanoparticle and the formerly grew BN layers, the stress appears and accumulates between BN layers and the inner nanoparticle because of the increasing curvature of newly grew BN layers. The liquid catalytic nanoparticle is expulsed from the defects of BN layers when the stress reaches a certain value and then due to a low formation energy, tubular structure with the large diameter and thin walls is formed via epitaxial growth from the joint point of lifted particle and formerly grew walls, as inferred in Fig. 5(a). So far one bamboo unit is done, as presented in Fig. 7(c). The above process is repeated for many times until the vapor B2O2 is exhausted and the bamboo-like BNNTs with bubble-chain walls are finally formed, as illustrated in Fig. 7(d) and (e). According to the improved stress-induced sequential growth mode,28 a splitting of the liquid catalytic nanoparticle may occur and a part of the nanoparticle remains in the hemisphere-like end of one bamboo unit, as confirmed in Fig. 3(b), as well as depicted in Fig. 7(e). The reason why the diameters of bamboo units filled with nanoparticles decline gradually along the growth direction also can be revealed during this above process, i.e. the size of the liquid catalytic nanoparticle decreases due to partially splitting.
For the bamboo-like BNNTs with flat walls, catalytic nanoparticles may locate at the bottoms of BNNTs on the fiber surface, as discussed before. So the growth of this type BNNTs follows base growth mode, as well explained in Fig. 7. A possible interpretation for the reason why it is distinct from the growth mode of bamboo-like BNNTs with bubble-chain walls, is that the strength of the interaction between the catalytic nanoparticle and the fiber surface determines the growth mode. The strong interaction between the catalytic nanoparticle and the fiber surface obstructs the liquid catalytic nanoparticle from departing from the fiber surface and moving along the growth direction.36 Therefore, the liquid catalytic nanoparticle is always immured on the fiber surface during the growth process. The initially precipitated BN layers just form a cap on the surface of the nanoparticle instead of encircling the nanoparticle due to the strong interaction between the nanoparticle and the fiber surface, as revealed in Fig. 7(a) and (b). The base growth mode is elaborated as follows36,37 and well comprehended via the process in Fig. 7. The BN layers precipitate on the nanoparticle and form a cap. The cap is lifted from the nanoparticle due to the stress accumulated between BN layers and the nanoparticle. In the following process, the walls of the nanotube grow, meanwhile, the compartment layers also begin to form as a result of precipitation of BN species inside the tube. Then the compartment layers connect with the walls and also depart from the nanoparticle due to the stress accumulated between the compartment layers and the nanoparticle. The growth of the compartment layers and the walls are repeated, and thus bamboo-like BNNTs with flat walls are eventually formed. In this case, the liquid catalytic nanoparticle is much closer to the accumulated vapor B2O2 in the vicinity of the fiber surface while the nanoparticle lifted from the fiber surface during the growth process of bamboo-like nanotube with bubble-chain walls is a little far away from B2O2. As a consequence, bamboo-like nanotubes with bubble-chain walls are shorter than ones with flat walls, as inferred in SEM images and also indicated in Fig. 7(e), due to the failure in assimilating adequate vapor source over the existed distance between the nanoparticle and B2O2 and then earlier cease of the growth.
Conclusions
In summary, a simplified ball milling, impregnation and annealing method to synthesize BNNTs in situ grown on the surface of fibers has been developed, consisting of ball milling of boron powder, impregnation of fiber cloths with as-milled boron powder slurry and thermal annealing in high pressure nitrogen gas (N2). The as-grown BNNTs utterly and uniformly besiege the fibers. The high nitrogen pressure is adopted in order to retard the uniformly diffusion of B2O2 molecules in the furnace chamber, which is beneficial for BNNTs growth. The as-grown BNNTs with the diameter of 50–130 nm exhibit a multi-walled and bamboo-like structure and possess two distinct morphologies of tube walls: flat walls and bubble-chain walls, respectively. The growth process and formation mechanism for two types of bamboo-like BNNTs both are well interpreted by stress-induced sequential growth mode in VLS mechanism. The distinction of formation mechanism for two types of bamboo-like BNNTs is that BNNTS with bubble-chain walls are formed following the tip growth mode while the base growth mode for BNNTS with flat walls, owing to the different strength of the interaction between catalytic nanoparticles and the fiber surface.Acknowledgements
This work is supported by the National Natural Science Foundation of China (Grant No. 51502323 and 51172256), Shanghai Key Project of Basic Research (Grant No. 14JC1406200) and Shanghai Scientific Research Project (Grant No. 13521101203).Notes and references
- R. Naslain, Compos. Sci. Technol., 2004, 64, 155–170 CrossRef CAS.
- R. R. Naslain, Composites, Part A, 1998, 29, 1145–1155 CrossRef.
- J. B. Hu, S. M. Dong, X. Y. Zhang, H. J. Zhou, B. Wu, Z. Wang, P. He and L. Gao, Composites, Part A, 2013, 48, 73–81 CrossRef CAS.
- Z. H. Hu, S. M. Dong, J. B. Hu and B. Lu, Ceram. Int., 2013, 39, 2147–2152 CrossRef CAS.
- J. B. Hu, S. M. Dong, B. Wu, X. Y. Zhang, H. J. Zhou, Z. Wang and Y. M. Kan, Int. J. Appl. Ceram. Technol., 2014, 11, 207–217 CrossRef CAS.
- F. Zhao and Y. D. Huang, J. Mater. Chem., 2011, 21, 2867–2870 RSC.
- E. T. Thostenson, W. Z. Li, D. Z. Wang, Z. F. Ren and T. W. Chou, J. Appl. Phys., 2002, 91, 6034–6037 CrossRef CAS.
- A. Pakdel, C. Y. Zhi, Y. Bando and D. Golberg, Mater. Today, 2012, 15, 256–265 CrossRef CAS.
- Y. Chen, J. Zou, S. J. Campbell and G. Le Caer, Appl. Phys. Lett., 2004, 84, 2430–2432 CrossRef CAS.
- C. Y. Su, W. Y. Chu, Z. Y. Juang, K. F. Chen, B. M. Cheng, F. R. Chen, K. C. Leou and C. H. Tsai, J. Phys. Chem. C, 2009, 113, 14732–14738 CAS.
- J. Hurst, in Nanotube Superfiber Materials: Changing Engineering Design, ed. M. J. Schulz, V. N. Shanov and Z. Z. Yin, William Andrew, Oxford, 2014, vol. 9, pp. 267–287 Search PubMed.
- H. Qian, E. S. Greenhalgh, M. S. Shaffer and A. Bismarck, J. Mater. Chem., 2010, 20, 4751–4762 RSC.
- N. G. Chopra, R. J. Luyken, K. Cherrey, V. H. Crespi, M. L. Cohen, S. G. Louie and A. Zettl, Science, 1995, 269, 966–967 CrossRef CAS PubMed.
- D. Golberg, Y. Bando, M. Eremets, K. Takemura, K. Kurashima and H. Yusa, Appl. Phys. Lett., 1996, 69, 2045–2047 CrossRef CAS.
- Y. Chen, J. F. Gerald, J. S. Williams and P. Willis, J. Metastable Nanocryst. Mater., 1999, 2, 173–178 CrossRef.
- Y. Chen, J. F. Gerald, J. S. Williams and S. Bulcock, Chem. Phys. Lett., 1999, 299, 260–264 CrossRef CAS.
- J. Kim, S. Lee, Y. R. Uhm, J. Jun, C. K. Rhee and G. M. Kim, Acta Mater., 2011, 59, 2807–2813 CrossRef CAS.
- S. Y. Bae, H. W. Seo, J. Park, Y. S. Choi, J. C. Park and S. Y. Lee, Chem. Phys. Lett., 2003, 374, 534–541 CrossRef CAS.
- W. Q. Han, Y. Bando, K. Kurashima and T. Sato, Appl. Phys. Lett., 1998, 73, 3085–3087 CrossRef CAS.
- P. Ahmad, M. U. Khandaker, Z. R. Khan and Y. M. Amin, RSC Adv., 2015, 5, 35116–35137 RSC.
- C. H. Lee, J. Wang, V. K. Kayatsha, J. Y. Huang and Y. K. Yap, Nanotechnology, 2008, 9, 455605 CrossRef PubMed.
- H. Chen, Y. Chen, Y. Liu, L. Fu, C. Huang and D. Llewellyn, Chem. Phys. Lett., 2009, 463, 130–133 CrossRef.
- Y. Chen, M. Conway, J. S. Williams and J. Zou, J. Mater. Res., 2002, 17, 1896–1899 CrossRef CAS.
- Y. Chen and L. H. Li, US Pat., Appl. 20130029131, 2010.
- B. Zhong, X. X. Huang, G. W. Wen, H. M. Yu, X. D. Zhang, T. Zhang and H. W. Bai, Nanoscale Res. Lett., 2011, 6, 36–43 CrossRef PubMed.
- B. Zhong, X. X. Huang, G. W. Wen, L. Xia, H. M. Yu and H. W. Bai, J. Phys. Chem. C, 2010, 114, 21165–21172 CAS.
- D. M. Tang, C. Liu and H. M. Cheng, J. Mater. Res., 2007, 22, 2809–2816 CrossRef CAS.
- K. F. Huo, Z. Hu, J. J. Fu, H. Xu, X. Z. Wang, Y. Chen and Y. N. Lü, J. Phys. Chem. B, 2003, 107, 11316–11320 CrossRef CAS.
- X. B. Wang, W. P. Hu, Y. Q. Liu, C. F. Long, Y. Xu, S. Q. Zhou, D. B. Zhu and L. M. Dai, Carbon, 2001, 39, 1533–1536 CrossRef CAS.
- X. F. Bi, Y. C. Yin, J. B. Li, Y. J. Chen, J. Li and Q. Q. Su, Solid State Sci., 2013, 25, 39–44 CrossRef CAS.
- R. Arenal, A. C. Ferrari, S. Reich, L. Wirtz, J. Y. Mevellec, S. Lefrant and A. Loiseau, Nano Lett., 2006, 6, 1812–1816 CrossRef CAS PubMed.
- C. Y. Zhi, Y. Bando, C. C. Tang, D. Golberg, R. G. Xie and T. Sekigushi, Appl. Phys. Lett., 2005, 86, 213110 CrossRef.
- C. W. Ong, H. Huang, B. Zheng, R. W. M. Kwok, Y. Y. Hui and W. M. Lau, J. Appl. Phys., 2004, 95, 3527–3534 CrossRef CAS.
- S. K. Singhal, A. K. Srivastava, A. K. Gupta and Z. Chen, J. Nanopart. Res., 2010, 12, 2405–2413 CrossRef CAS.
- Z. G. Chen, J. Zou, F. Li, G. Liu, D. M. Tang, D. Li, C. Liu, X. Ma, H. M. Cheng, G. Q. Lu and Z. Zhang, Adv. Funct. Mater., 2007, 17, 3371–3376 CrossRef CAS.
- J. L. Wang, L. P. Zhang, G. W. Zhao, Y. L. Gu, Z. H. Zhang, F. Zhang and W. M. Wang, J. Solid State Chem., 2011, 184, 2478–2484 CrossRef CAS.
- L. B. Mo, Y. J. Chen and L. J. Luo, Appl. Phys. A, 2010, 100, 129–134 CrossRef CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra23318f |
| This journal is © The Royal Society of Chemistry 2016 |