Comparative cell adhesion properties of cysteine extended peptide architectures†
Saniye Soylemez‡
a,
Bilal Demirb,
Gizem Oyman Eyrilmezb,
Seçkin Kesicia,
Aytül Saylama,
Dilek Odaci Demirkolb,
Salih Özçubukçua,
Suna Timur*bc and
Levent Toppare*adef
aDepartment of Chemistry, Middle East Technical University, Ankara 06800, Turkey. E-mail: toppare@metu.edu.tr
bDepartment of Biochemistry, Ege University, Izmir 35100, Turkey. E-mail: suna.timur@ege.edu.tr
cInstitute of Drug Abuse Toxicology & Pharmaceutical Sciences, Izmir 35100, Turkey
dDepartment of Biotechnology, Middle East Technical University, Ankara 06800, Turkey
eDepartment of Polymer Science and Technology, Middle East Technical University, Ankara 06800, Turkey
fThe Center for Solar Energy Research and Application (GUNAM), Middle East Technical University, Ankara 06800, Turkey
First published on 23rd December 2015
Abstract
This study presents the comparative cell attachment investigation of TAT and well-known RGD peptide modified surfaces. Initially, cysteine containing arginine–glycine–aspartic acid (RGD) and TAT peptides, a class of cell penetration peptides, were synthesized. Gold film coated indium tin oxide (gold/ITO) surfaces were coated with RGD and TAT peptides and used for cell culture applications. Thiol groups on the peptides provide post-modification of the surface. The efficient bonding of the peptides with the modified surface brings proper attachment of the cells. The peptide modified surfaces were tested for adhesion of several cell lines such as monkey kidney epithelial cell (Vero), human cervical carcinoma cell (HeLa), human glioblastoma cell (U87-MG) and human immortalized skin keratinocyte cell (HaCaT) lines. These cells were cultured on RGD and TAT modified gold/ITO surfaces. Cell imaging studies were performed on these surfaces using fluorescence microscopy technique. Scanning electron microscopy (SEM), atomic force microscopy (AFM), cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS), X-ray photoelectron spectroscopy (XPS) and contact angle measurements were carried out for the surface characterization. The results indicate that the RGD and TAT modified surfaces exhibited better cell adhesion. Therefore, besides RGD as a well-known adhesion peptide, TAT functionalized substrates were found to be efficient bio-sensing candidates for further studies.
Introduction
Recently much attention has been given to biomimetic systems using peptides since they represent a promising way of functionalizing surfaces due to their good incorporating cell binding ability. Extracellular matrix (ECM) proteins, poly-L-lysine, and collagen have been used to develop cell-adhesion surfaces in order to display their cell adhesion properties.1,2 Although ECM proteins have been widely used in cell culture studies, their expensive and uncontrollable thickness cause a decrease in the electrochemical sensitivity of electrodes.3 Hence, other materials that can enhance cell-adhesion property are necessary. Surface modification with the RGD (R: arginine, G: glycine and D: aspartic acid) has attracted great attention in material science and used to improve the biocompatibility of bio-functional surfaces. RGD is a tripeptide which is used commonly in recognition system for cell adhesion since it has a better cell adhesion ability to various cells including mammalian and human cell lines. A number of different types of integrins recognize RGD peptide and this binding activity is used to study some certain cells which are rich in integrins especially integrin alpha V beta 3.4 Cysteine extended RGD peptide, RGDC, is also commonly used to immobilize the RGD peptide on a surface with thiol affinity such as gold or maleimide functionalized nanoparticles. For instance Spencer et al. reported successful immobilization of RGDC on maleimide grafted titanium substrate.5 Similarly gold surfaces can also be modified with RGDC to examine the cell adhesion and detachment.6 Moreover; cell-penetrating peptides (CPPs) are very important class of peptides which are commonly used to carry molecular cargos into the cells since they have unique property of crossing cellular membrane unlike most other peptides.7TAT derivative peptide is an arginine rich peptide which is commonly used for cell penetrating purposes. It has been used to overcome the lipophilic barrier of the cellular membranes to deliver large molecules, drugs and even nanoparticles to perform certain biological activities.8 De la Fuente et al. reported synthesis of a TAT peptide anchored to water soluble gold nanoparticles to transfer them across the cell membrane and locate in the nucleus.9 In vitro studies with a human fibroblast cell line showed that these nanoparticles are penetrating into the cell membrane and reach the nucleus. In vivo imaging studies are also possible by TAT peptide. Nie et al. reported TAT peptide conjugated quantum dots for in vivo imaging in living cells. It is also possible to have a dual effect of TAT peptide by functionalizing it with two different groups.10 Weissleder et al. showed that extending the peptide sequence of the C-terminal with particular amino acids is useful in attaching super paramagnetic iron nanoparticles and fluorescein-5-isothiocyanate (FITC).11 They synthesized the TAT peptide with a sequence of GRKKRRQRRRGYKC and cysteine residue was used for the bio-conjugation of the nanoparticles and lysine residue for attaching FITC on the TAT peptide. As a modifier agent on gold substrate, there is one study related to TAT peptide. It compares TAT coating with other alkylthiol modifications using various surface characterization and modification techniques such as AFM, XPS and FT-IR.12 Hereby, according to the best of our knowledge, unlike RGD peptide, TAT peptide has not been used for cell surface adhesion purposes.
Several strategies for immobilizing peptides onto a wide variety of substrate have been studied previously. Among them self-assembled monolayers (SAMs) have been mostly preferred. Those formed on gold surfaces have attracted great attention to obtain stable interfaces and well-oriented biomolecule on the surface.13,14 In addition, the use of ITO for cell adhesion applications can be significantly enhanced by modifying it with gold. This brings many desirable properties such as enabling fast electron transfer, good film forming ability and biocompatibility.15 Functionalized surfaces generated by SAM technique also enable biosensing applications for several small molecules, proteins and cell attachments by using amperometric, cyclic and differential pulse voltammetry and electrochemical impedance spectroscopy.16,17
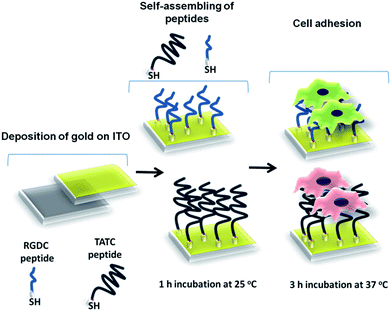
The focus of the present study is to investigate cell adhesion properties of monkey kidney epithelial (Vero), human cervical carcinoma (HeLa), human glioblastoma (U87-MG) and human immortalized skin keratinocyte (HaCaT) cells on RGD and TAT peptide modified gold/ITO surfaces. TAT peptide has been used extensively for drug delivery where efficient cell penetration features are required. However, it will be the first time for the application of TAT peptide in cell adhesion and sensing on the biosensor surfaces. For this purpose in order to immobilize peptides on gold/ITO surfaces, cysteine extended RGD (RGDC) and TATC peptides with a sequence of GRKKRRQRRRGYKC–NH2 were synthesized. Cysteine is a functional amino acid where the thiol side chain is capable of forming gold–thiolate bond with gold surfaces. This property gives the cysteine residues to play an important role in bio-molecular anchoring to surfaces. Gold/ITO substrate was used as the working electrode since ITO is non-toxic to cells and gold has a well-known biocompatible property and it is easy to modify by thiolated structures.
Thus, this combination leads to enhancement of cell adhesion on the surface and allows observing the surface with an optical microscope due to its transparent nature. Schematic overview of surface modification of RGDC and TAT was illustrated in Scheme 1. Surface properties were examined by contact angle measurement, cyclic voltammetry (CV), electrochemical impedance spectroscopy (EIS) and X-ray photoelectron spectroscopy (XPS). Vero, HeLa, U87-MG and HaCaT cell lines were cultivated and monitored by fluorescence microscopy (FM) to test cell adhesion on modified surfaces. In addition, scanning electron microscopy (SEM) and atomic force microscopy (AFM) techniques were used to examine cell morphology of modified surfaces. Good adhesion ability of RGD and TAT peptide makes these peptides promising candidates for different applications.
Experimental
Materials and apparatus
All amino acids and reagents for peptide synthesis were obtained from Chem-Impex, IL, USA. Dimethylformamide (DMF), CH3CN, CH2Cl2 were purchased from Sigma-Aldrich. Rink amide resin for peptide synthesis was provided by Merck. Phosphate buffered saline (pH 7.4, PBS) was prepared using 8.0 g L−1 NaCl, 0.2 g L−1 KCl, 1.44 g L−1 Na2HPO4·2H2O and 0.2 g KH2PO4 (Merck).Dulbecco's Modified Eagle Medium (DMEM), Eagle's Minimum Essential Medium (EMEM), fetal bovine serum (FBS), penicillin/streptomycin (P/S) (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000/10
000/10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 units) and 200 mM L-glutamine were purchased from Lonza. CytoPainter Phalloidin-iFluor 555 reagent, Anti-Integrin alpha V beta 3 (αvβ3) antibody (ab78289), and Goat Anti-Mouse IgG H&L (Alexa Fluor® 488) (ab150117) were purchased from Abcam.
000 units) and 200 mM L-glutamine were purchased from Lonza. CytoPainter Phalloidin-iFluor 555 reagent, Anti-Integrin alpha V beta 3 (αvβ3) antibody (ab78289), and Goat Anti-Mouse IgG H&L (Alexa Fluor® 488) (ab150117) were purchased from Abcam.
Cyclic voltammetry experiments were carried out with a Palm Sens electrochemical measurement system (Palm Instruments, Houten, The Netherlands) in a three-electrode cell consisting of a working electrode (gold/ITO glasses), a platinum electrode (Metrohm, Switzerland) as the counter electrode, and a Ag/AgCl (with 3.0 M KCl saturated with AgCl as the internal solution, Metrohm Analytical, CH-9101) as the reference electrode. The electrodes were inserted into a conventional electrochemical cell (10 mL). Electrochemical impedance studies (EIS) were performed with a CHI Instruments (6005C, electrochemical analyzer). The EIS measurements were recorded within the frequency range between 0.03 Hz and 1000 Hz by applying 0.18 V. Atomic force microscopy (AFM) studies for modified surfaces were carried out on Veeco Multimode V AS-130 (“J”). For surface imaging of the modified electrodes, scanning electron microscope (SEM) (JEOL JSM-6400 model) was used. Contact angle measurements of RGDC and TATC films were performed with a KSV CAM 200 (Finland) Goniometer. For each measurement, a drop of water (5.0 μL) was delivered to the surface using a syringe equipped with a micrometer. At least 4 measurements were done for a given surface and average values were calculated. X-ray photoelectron spectroscopy (XPS) studies were carried out on a PHI 5000 Versa Probe (F ULVACPHI, Inc., Japan/USA) model X-ray photoelectron spectrometer instrument with monochromatic Al Kα radiation (1486.6 eV) as the X-ray anode at 24.9 W. Analytical LC-MS-QTOF analyses of peptides were recorded on an Agilent Technologies High Resolution Mass Quadrupole Time-of-Flight (TOF) LC/MS 1200 series and Zorbax Eclipse XDB-C18 analytical 4.6 × 150 mm 5-micron column was employed.
Synthesis of the peptides
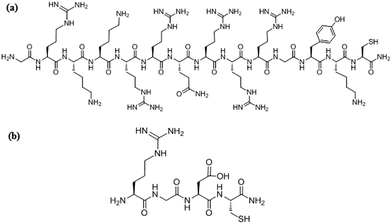
The synthesis of cysteine and lysine modified TAT derivative (GRKKRRQRRRGYKC) and RGDC peptides were performed using standard solid phase peptide synthesis with Fmoc protocol on Rink amide resin. The purification of the crude peptides was performed with Dionex Ultimate 3000 Series equipped with a variable wavelength absorbance detector using a reverse phase C18 column (Hypersil Gold, 12 μm, 250 × 10 mm). A binary gradient of water (0.1% TFA) and acetonitrile (0.1% TFA) were used with a flow rate of 3.0 mL min−1 and the eluent was monitored by UV absorbance at 210 and 280 nm. Fractions were collected and lyophilized after their purity was confirmed by analytical HPLC performed using a RP-C18 column (Acclaim 120, 3.0 μm, 4.6 × 150 mm) with a flow rate of 0.5 mL min−1. The mass spectra of the peptides are shown in Fig. S1 (ESI†). The structures of the peptides are illustrated in Fig. 1.Construction of bio-functional electrode surfaces
Individual ITO glasses were cut into small pieces of 1.0 × 1.0 cm (the electrode area: 1 cm2) and cleaned using detergent, water, acetone, and isopropyl alcohol sequentially in an ultrasonic bath for 15 min. Subsequently oxygen plasma cleaning was performed in Harrick Plasma Cleaner. After the cleaning steps, gold (thickness, 5 nm) was coated on cleaned ITO surfaces using sputter coater. Then 100 μL of RGDC (0.05 mg mL−1) and TATC (0.05 mg mL−1) peptides were immobilized on the gold coated ITO surface (gold/ITO), separately and left for 1 h for proper attachment of the peptides on modified surfaces. Peptide modified gold/ITO substrates were rinsed twice with ultrapure water to remove unbound molecules and dried under nitrogen. The presence of the free thiol groups on the peptides backbone was utilized for the attachment of RGDC and TATC peptides on the electrode surface. The modified gold/ITO surfaces were subjected to 15 min UV sterilization before immobilization of cells. The stock solutions of cell suspensions in cell culture medium were replaced with PBS, pH 7.4 before adhesion onto modified gold/ITO surfaces by centrifugation of cells. Finally, 75 μL of cell solutions with a certain concentration in the range of 10 to 106 cells per mL (Vero, HeLa, U87-MG and HaCaT) were allowed to adhere to the peptide coated surfaces via 3 h of incubation at 37 °C. Unattached cells were removed from the surfaces by immersing in ultrapure water for 1–2 seconds. The formation of each species on gold/ITO electrodes is presented in Scheme 1. The redox characteristics at the modified electrodes were assessed by CV and EIS techniques. The experiments were conducted at ambient temperature (25 °C).Cell culture and imaging studies
HaCaT (human keratinocyte, CLS), HeLa (human cervical carcinoma cell, ATCC), and Vero (monkey kidney fibroblast like epithelial cell, ATCC) cell lines were maintained in DMEM. U87-MG (human glioblastoma, ATCC) was maintained in EMEM. All of them supplemented with 10.0% FBS, and 1.0% P/S at 37 °C in a humidified incubator with 5.0% CO2 in air. All cells were sub-cultured at 80% confluence by trypsinization for every two or three days. To compare cell adhesion behaviors, different cell lines were incubated for 24 h on gold coated ITO surface (gold/ITO), RGDC functionalized gold coated ITO surface (RGDC/gold/ITO), and also TATC functionalized (TATC/gold/ITO) surfaces.For immunofluorescence staining, cells were fixed with 4.0% formaldehyde in PBS for 45 min at 4 °C after 24 h incubation times on modified surfaces. Permeability of cells was satisfied by treatment with 0.1% Triton X-100 for 4 min. Then cells were stained for actin and integrin αvβ3 using CytoPainter Phalloidin-iFluor 555 Reagent (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1000) and anti-integrin αvβ3 antibody (1
1000) and anti-integrin αvβ3 antibody (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500), followed by Alexa 488 fluorophore conjugated secondary antibody (1
500), followed by Alexa 488 fluorophore conjugated secondary antibody (1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500). Also, nucleus (1.0 mg mL−1) staining with DAPI was performed at ambient temperature for 5 min. After extensive washing with PBS, cell images were taken by Olympus CKX41 fluorescence microscope with a 40× objective. The cell numbers were processed and quantified for each surface by using Image J (NIH) software.
500). Also, nucleus (1.0 mg mL−1) staining with DAPI was performed at ambient temperature for 5 min. After extensive washing with PBS, cell images were taken by Olympus CKX41 fluorescence microscope with a 40× objective. The cell numbers were processed and quantified for each surface by using Image J (NIH) software.
Statistical analysis
GraphPad Prism version 5.03 software (GraphPad Software, San Diego, CA) was used to obtain graphs and statistical analyses. The non-parametric Mann–Whitney U-test was used to compare relative cell numbers per surface area among different surfaces. Statistical significance was denoted with *, **, and *** for p ≤ 0.05, p ≤ 0.01, and p ≤ 0.001, respectively.Results and discussion
Surface characterization of the modified surfaces
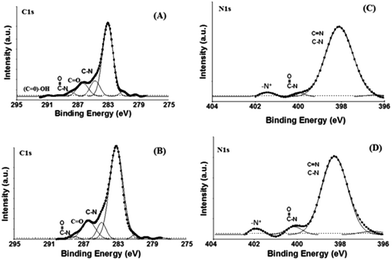
X-ray photoelectron spectroscopy (XPS) experiments were carried out to characterize the modified layers of RGDC/gold/ITO and TATC/gold/ITO. The carbon and nitrogen signals were resolved by a fitting program as depicted in Fig. 2A–D. Specific chemical bonding of peptides were readily detected through XPS analysis. In the C 1s spectra of RGDC/gold/ITO, the peak at 283.1 eV was observed which is consistent with the presence of hydrocarbon chain containing mainly C–C and C–H bonds. For TATC/gold/ITO surface, presence of hydrocarbon chains was observed at 283.3 eV.12,18–20 The C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O peaks for RGDC/gold/ITO (286.3 eV) and TATC/gold/ITO (286.4 eV) in the C 1s spectrum (Fig. 2A) were observed. The peaks at higher energies (for RGDC immobilized surface; 287.9 eV and for TATC immobilized surface; 288.0 eV) can be assigned to the carbons atoms of O
O peaks for RGDC/gold/ITO (286.3 eV) and TATC/gold/ITO (286.4 eV) in the C 1s spectrum (Fig. 2A) were observed. The peaks at higher energies (for RGDC immobilized surface; 287.9 eV and for TATC immobilized surface; 288.0 eV) can be assigned to the carbons atoms of O![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) C–N in the peptide structure.12,21–23 In addition, the RGDC modified surface exhibited a characteristic signal at 291.0 eV representing the (C
C–N in the peptide structure.12,21–23 In addition, the RGDC modified surface exhibited a characteristic signal at 291.0 eV representing the (C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O)–OH group of RGDC.21,22 Moreover, the perfect attachment of peptides to the surface can also be detected from the N 1s spectra (Fig. 2C and D).
O)–OH group of RGDC.21,22 Moreover, the perfect attachment of peptides to the surface can also be detected from the N 1s spectra (Fig. 2C and D).
 | ||
| Fig. 2 XPS C 1s spectra for (A) RGDC/gold/ITO and (B) TATC/gold/ITO and N 1s spectra for (C) RGDC/gold/ITO and (D) TATC/gold/ITO electrodes. | ||
In Fig. 2C, intensity of the –N–(C![[double bond, length as m-dash]](https://www.rsc.org/images/entities/char_e001.gif) O) peak (400.1 eV) was increased for the TATC modified surface due to the increasing terminal groups in TATC peptide. The same peak was observed at 400.0 eV for RGDC modified surface.19,24,25 Moreover, the peaks at 401.5 eV (RGDC/gold/ITO) and 401.8 eV (TATC/gold/ITO) can also be due to the presence of protonated amine groups (NH3+) in the peptides.12,26,27 Hence, the XPS data confirms that RGDC and TATC peptides were formed onto the gold/ITO surfaces.
O) peak (400.1 eV) was increased for the TATC modified surface due to the increasing terminal groups in TATC peptide. The same peak was observed at 400.0 eV for RGDC modified surface.19,24,25 Moreover, the peaks at 401.5 eV (RGDC/gold/ITO) and 401.8 eV (TATC/gold/ITO) can also be due to the presence of protonated amine groups (NH3+) in the peptides.12,26,27 Hence, the XPS data confirms that RGDC and TATC peptides were formed onto the gold/ITO surfaces.
Contact angle measurement is a simple and alternative tool to examine the surface property after each modification. To verify whether the surface functionalization altered the hydrophilicity of the modified surface, the water contact angles at the surface were measured after RGDC and TATC grafting. Also, the formation of SAMs on gold/ITO surfaces has been qualitatively assessed by measuring contact angles for water. Therefore, it is possible to investigate the deposition processes by following the changes in surface wettability, which can be qualitative by measuring the contact angle of the substrate.28 For this purpose, electrodes with gold/ITO, RGDC/gold/ITO and TATC/gold/ITO were prepared. Ability of a drop of water to spread over the surface was studied by averaging the left angles of drops with the same volume. The electrode with gold/ITO was highly hydrophobic with a contact angle of 75.20 ± 3.6°. This value decreases dramatically to 37.2 ± 2.5° when RGD was immobilized to gold/ITO surface which illustrates successful immobilization on the electrode surface. TAT peptide modifying on the gold/ITO electrode reveals a decrease in the contact angle (27.0 ± 1.9°) owing to more hydrophilic character of the TAT peptide. These changes in contact angles suggest that both RGDC and TATC modified layers have hydrophilic surface; thus providing a fascinating platform for cell applications. The trends in these contact angle measurements are consistent with those observed for peptides.12,18,29
Atomic force microscopy (AFM) was used as an alternative technique to investigate morphology of the surfaces. Fig. 3 shows the characteristic AFM image of the surface topography. Modified electrodes at various deposition steps of gold/ITO, RGDC and TATC modified gold/ITO were prepared. The gold/ITO surface is fairly smooth according to 2D (Fig. 3A) and 3D (Fig. 3D) images. After the immobilization of the RGD peptides, a slightly heterogeneous surface was obtained. Root mean square (RMS) value was determined as 1.6 nm for the RGDC modified surface. Moreover, when gold/ITO electrode was modified with TATC peptide, surface roughness reached the maximum value and a non-uniform surface was obtained (Fig. 3C). For the TATC modified surface, RMS value was determined as 3.4 nm. Moreover, AFM images of fixed U87-MG cells and SEM images of fixed U87-MG and Vero cells on the RGDC and TATC modified surfaces are shown in Fig. S2 and S3 (ESI†). Depending on the smaller structure of RGDC, roughness of the surface is relatively smaller compare to that of TATC modified surfaces.
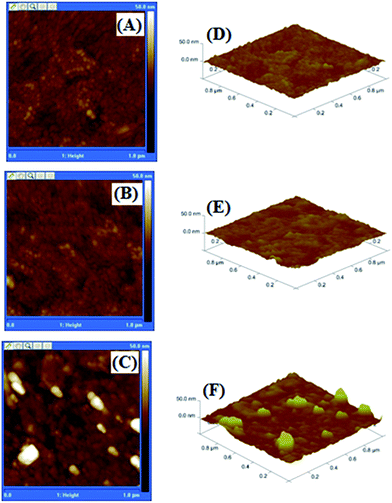 | ||
| Fig. 3 2-D (A–C) and 3-D (D–F) topographic AFM height images of (A and D) gold/ITO, (B and E) RGDC/gold/ITO and (C and F) TATC/gold/ITO modified electrode surface. | ||
Electrochemical characterization of the modified surfaces
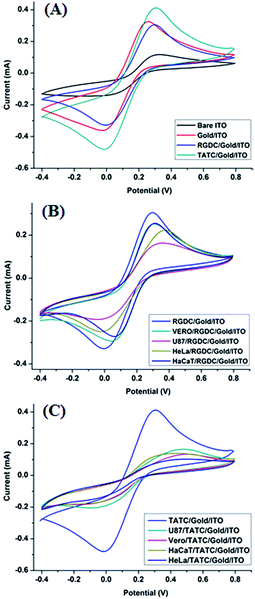
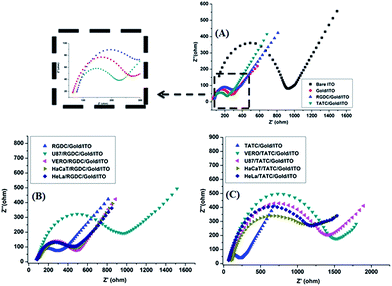
Fig. 4A–C show comparative CV evolutions of the bare ITO, gold/ITO, RGDC/gold/ITO, TATC/gold/ITO and several cell lines (Vero, HeLa, HaCaT and U87-MG) adhered RGDC and TATC surfaces in 5.0 mM [Fe(CN)6]4−/3− (50 mM pH 7.4 PBS) at 50 mV s−1. Each step is expected to form a different surface structure due to the modification of surfaces. CV of [Fe(CN)6]4−/3− is a powerful redox marker to follow the barrier of the modified electrode surface after each layer construction. In Fig. 4A, well-defined redox peaks were detected for modified surfaces. As shown in Fig. 4A, the electrochemical study demonstrates that compare to the bare ITO electrode (115 μA), the relevant peak current of [Fe(CN)6]3−/4− on the gold/ITO electrode (332 μA) increased remarkably. This result clearly shows that electron transfer rate and film forming ability of the modified electrode were improved.3,30 However, after the addition of the RGDC peptide on the surface of gold/ITO the peak current (308 μA) was decreased, confirming that the surface of the electrode was successfully modified. On the contrary when TATC peptide was introduced on the gold/ITO surface, the peak current (416 μA) was increased. This is an expected result since TATC has a cationic nature because of the presence of increased number of 6 arginine and 2 lysine residues in the peptide sequence thereby making the peptide highly cationic.31,32 Hence, TATC peptide provides a conductive pathway for electron transfer and attracts the negatively charged [Fe(CN)6]4−/3− causing an increase in the peak current. In addition, the capture of cells on different electrodes was investigated and results were shown in Fig. 4B and C. The peak currents were significantly diminished since the additional layer blocks electron transfer on the surface.33,34 On such surfaces the decrease of peak current confirmed the effective attachment of the cells on the modified electrode surfaces.Electrochemical impedance spectroscopy (EIS) is a powerful non-destructive tool to study interfacial properties of the molecules on surfaces during modification.35–37 Fig. 5A–C show examples of the Nyquist plots obtained for the modified electrodes. In a typical Nyquist plot, the semi-circle portion corresponds to the electron-transfer resistance at the higher frequency range which refers to the electron transfer kinetics of the redox probe at the electrode surface. Linear part of the plot at lower frequency range represents the diffusion limited process. Increase in semicircle diameter proved the hindrance of the electron flow. On the basis of the EIS results, immobilization of peptides was achieved successfully on the gold/ITO surface. In addition, the attachment of the cells on the RGDC/gold/ITO and TATC/gold/ITO electrodes generated an insulating layer on the electrode that acted as a barrier for the interfacial electron transfer. As expected, cell attached surfaces exhibit more blocking behaviour towards the RGDC/gold/ITO and TATC/gold/ITO electrodes compare to others.
Moreover, this observation suggests that cells on the electrode surface act as a blocking film for the diffusion of [Fe(CN)6]4−/3− toward the electrode surface thereby increasing the charge transfer resistance significantly (Fig. 5B and C). Hence the matrix in concern is a well-established candidate for peptide matrix generation. Also, the EIS results are in a good agreement with CV observations. This revealed that the change in the peak current refers to the surface modification of the electrodes.
After the construction and electrochemical characterization steps for the peptide coated gold/ITO surfaces, a calibration curve was generated for U87-MG, HeLa and Vero cell lines. A cell concentration between 10 and 105 cells per mL was used during the construction of linear curves for each cell line. The linearity was generated by calculating the logarithm of both x (cell concentration) and y (differences in semi-circle) axis results. Fig. 6A and B show the calibration curves of RGDC/gold/ITO and TATC/gold/ITO surfaces for HeLa, Vero and U87-MG cells. In the case of RGDC modified gold/ITO surfaces U87-MG cells demonstrated greater resistance in impedance measurements. Hence, it can be claimed that RGDC modification provided higher adhesion for U87-MG cells. Moreover, TATC modified surfaces which have longer amino acid residues have created layers for different cell lines. Vero and U87-MG cells demonstrated similar linearity characteristics with HeLa cells. The linearity characteristics relevant to each tested cell lines were also given in Table S1.†
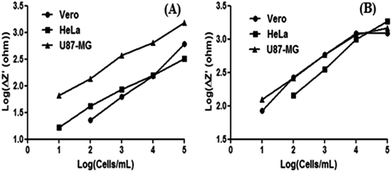 | ||
| Fig. 6 Calibration curves for the RGDC/gold/ITO (A) and TATC/gold/ITO (B) surfaces for the detection of different cell lines. | ||
Cell adhesion on the modified surfaces
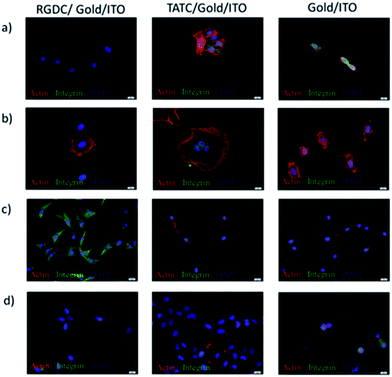
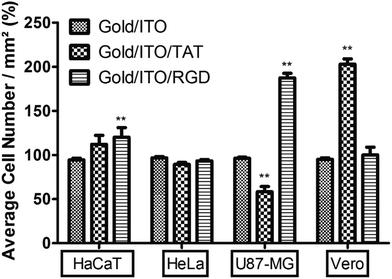
The effects of peptide modification on the cell adhesion behaviours of different cell lines were investigated on gold/ITO, RGDC/gold/ITO, and also TATC/gold/ITO surfaces. Herein, actin and integrin targeted staining were carried out to illustrate the cell adhesion to verify the electrochemical detection techniques. Fig. 7 shows that all cell lines were attached and spread on the RGDC and TATC functionalized surfaces to a greater extent than the one on unmodified gold/ITO surface.Besides, the relationship between the average number of cells per area (mm2) and the cell lines is shown in Fig. 8. The integrin αvβ3 overexpression at U87-MG cells was shown in our previous results.38
Also, the highest number of U87-MG cells was observed due to the overexpression of integrin αvβ3 which interacts with RGD peptide on RGDC functionalized surface.39–41 However, Vero cell adhesion was higher than the other cell lines on the TAT peptide functionalized surface. The increased interaction can be mediated by various signaling receptors in cells, including the vascular endothelial growth factor (VEGF) receptor 2,42 chemokine receptors,43 and also integrins.44–46 On the other hand, there is no significant difference observed between unmodified and modified surfaces in case of HeLa cell adhesion and HaCaT cells. The reason of the lower adhesion of HaCaT cells might be lower expression of integrin αvβ3.38
Conclusions
In conclusion, cysteine extended TAT peptide can be used in the surface modification for both cell attachment and biosensing applications in comparison to RGD which is a well-characterized peptide in many previous studies. Herein, the RGDC/gold/ITO and TATC/gold/ITO were easily fabricated by self-assembly technique that enhanced attachment of cells on the electrodes. The constructed surfaces are suitable for electrical and optical measurements. In addition, RGDC and TATC modified surfaces are cost effective, easily prepared and good platform for using as a cell culture material.Acknowledgements
We acknowledge the OYP grant.References
- D. Liu, C. A. C. Abdullah, R. P. Sear and J. L. Keddie, Soft Matter, 2010, 6, 5408 RSC.
- K. Ziolkowska, R. Kwapiszewski and Z. Brzozka, New J. Chem., 2011, 35, 979 RSC.
- T.-H. Kim, W. Ahmed El-Said, J. Hee An and J.-W. Choi, Nanomedicine: Nanotechnology, Biology and Medicine, 2013, 9, 336 CrossRef CAS PubMed.
- E. Ruoslahti, Annu. Rev. Cell Dev. Biol., 1996, 12, 697 CrossRef CAS PubMed.
- S. J. Xiao, M. D. Textor, N. Spencer, M. Wieland, B. Keller and H. Sigrist, J. Mater. Sci.: Mater. Med., 1997, 8, 867 CrossRef CAS PubMed.
- S. H. Yoon and M. R. Mofrad, Biomaterials, 2011, 32, 7286 CrossRef CAS PubMed.
- M. Francesca, Drug Discovery Today, 2012, 17, 850 CrossRef PubMed.
- F. Madani, S. Lindberg, Ü. Langel, S. Futaki and A. Gräslund, J. Biophys., 2011, 2011, 1 CrossRef PubMed.
- J. M. de la Fuente and C. C. Berry, Bioconjugate Chem., 2005, 16, 1176 CrossRef CAS PubMed.
- G. Ruan, A. Agrawal, A. I. Marcus and S. Nie, J. Am. Chem. Soc., 2007, 129, 14759 CrossRef CAS PubMed.
- M. Lewin, N. Carlesso, C. H. Tung, X. W. Tang, D. Cory, D. T. T. Scadden and R. Weissleder, Nat. Biotechnol., 2000, 18, 410 CrossRef CAS PubMed.
- Y. Cho and A. Ivanisevic, J. Phys. Chem. B, 2005, 109, 6225 CrossRef CAS PubMed.
- E. R. de Jong, N. Deloch, W. Knoll, C.-O. Turrin, J.-P. Majoral, A.-M. Caminade and I. Koper, New J. Chem., 2015, 39, 7194 RSC.
- M. Holzinger, A. le Goff and S. Cosnier, New J. Chem., 2014, 38, 5173 RSC.
- G. Scari, F. Porta, U. Fascio, S. Avvakumova, V. Dal Santo, M. de Simone, M. Saviano, M. Leone, A. Del Gatto, C. Pedone and L. Zaccaro, Bioconjugate Chem., 2012, 23, 340 CrossRef CAS PubMed.
- M. Akin, M. Yuksel, C. Geyik, D. Odaci, A. Bluma, T. Höpfner, S. Beutel, T. Scheper and S. Timur, Biotechnol. Prog., 2010, 26, 896 CrossRef CAS PubMed.
- Y. Tepeli, B. Demir, S. Timur and U. Anik, RSC Adv., 2015, 5, 53973 RSC.
- Y. Cho and A. Ivanisevic, J. Phys. Chem. B, 2005, 109, 12731 CrossRef CAS PubMed.
- S. Jewett, D. Zemlyanov and A. Ivanisevic, Langmuir, 2011, 27, 3774 CrossRef CAS PubMed.
- H. S. Seo, Y. M. Ko, J. M. Shim, Y. K. Lim, J.-K. Kook, D.-L. Cho and B. H. Kim, Appl. Surf. Sci., 2010, 257, 596 CrossRef CAS.
- S. Demirci, F. B. Emre, F. Ekiz, F. Oğuzkaya, S. Timur, C. Tanyeli and L. Toppare, Analyst, 2012, 137, 4254 RSC.
- F. Ekiz, F. Oğuzkaya, M. Akin, S. Timur, C. Tanyeli and L. Toppare, J. Mater. Chem., 2011, 21, 12337 RSC.
- Y. Zheng, C. Xiong, X. Li and L. Zhang, Appl. Surf. Sci., 2014, 320, 93 CrossRef CAS.
- W. Liu, K. L. Koh, J. Lu, L. Yang, S. Phua, J. Kong, Z. Chen, G. L. Xuehong and G. Bhargava, J. Mater. Chem., 2012, 22, 18395 RSC.
- T. A. Ramanarayanan and S. L. Bernasek, Langmuir, 2010, 26, 215 CrossRef PubMed.
- S.-J. Xiao, M. Textor and N. D. Spencer, Langmuir, 1998, 14, 5507 CrossRef CAS.
- K. Benson, E. A. Prasetyanto, H.-J. Galla and N. S. Kehr, Soft Matter, 2012, 8, 10845 RSC.
- L. Wu, X. Zhang and H. Ju, Anal. Chem., 2007, 79, 453 CrossRef CAS PubMed.
- S.-H. Yoon and M. R. K. Mofrad, Biomaterials, 2011, 32, 7286 CrossRef CAS PubMed.
- X. Lv, W. Ge, Q. Li, Y. Wu, H. Jiang and X. Wang, ACS Appl. Mater. Interfaces, 2014, 6, 11025 CAS.
- Z. Mao, L. Wan, L. Hu, L. Ma and C. Gao, Colloids Surf., B, 2010, 75, 432 CrossRef CAS PubMed.
- C. Peetla, K. S. Rao and V. Labhasetwar, Mol. Pharm., 2009, 6, 1311 CrossRef CAS PubMed.
- B. Huang and N. Jia, Anal. Chem., 2014, 86, 6940 CrossRef CAS PubMed.
- B. Ballarin, M. C. Cassani, E. Scavetta and D. Tonelli, Electrochim. Acta, 2008, 53, 8034 CrossRef CAS.
- M. E. Orazem and B. Tribollet, Electrochemical Impedance Spectroscopy, John Wiley & Sons Inc., Hoboken, NJ, 2008 Search PubMed.
- J. Pillay, B. O. Agboola and K. I. Ozoemena, Electrochem. Commun., 2009, 11, 1292 CrossRef CAS.
- E. Barsoukov and J. R. Macdonald, Impedance Spectroscopy Theory, Experiment, and Applications, Wiley, Hoboken, New Jersey, 2nd edn, 2005, ch. 1–4 Search PubMed.
- G. O. Eyrilmez, S. Doran, E. Murtezi, B. Demir, D. O. Demirkol, H. Coskunol, S. Timur and Y. Yagci, Macromol. Biosci., 2015, 15, 1233 CrossRef PubMed.
- H. Wu, H. Chen, Y. Sun, Y. Wan, F. Wang, B. Jia and X. Su, Cancer Lett., 2013, 335, 75 CrossRef CAS PubMed.
- Y. Wu, X. Zhang, Z. Xiong, Z. Cheng, D. R. Fisher, S. Liu, S. S. Gambhir and X. Chen, J. Nucl. Med., 2005, 46, 1707 CAS.
- H. Wang, K. Chen, W. Cai, Z. Li, L. He, A. Kashefi and X. Chen, Mol. Cancer Ther., 2008, 7, 1044 CrossRef CAS PubMed.
- A. Albini, R. Soldi, D. Giunciuglio, E. Giraudo, R. Benelli, L. Primo, D. Noonan, M. Salio, G. Camussi and W. Rockl, Nat. Med., 1996, 2, 1371 CrossRef CAS PubMed.
- A. Albini, S. Ferrini, R. Benelli, S. Sforzini, D. Giunciuglio, M. G. Aluigi, A. E. Proudfoot, S. Alouani, T. N. Wells and G. Mariani, Proc. Natl. Acad. Sci. U. S. A., 1998, 95, 13153 CrossRef CAS.
- G. Barillari, C. Sgadari, C. Palladino, R. Gendelman, A. Caputo, C. B. Morris, B. C. Nair, P. Markham, A. Nel and M. Sturzl, J. Immunol., 1999, 163, 1929 CAS.
- V. Fiorelli, G. Barillari, E. Toschi, C. Sgadari, P. Monini, M. Sturzl and B. Ensoli, J. Immunol., 1999, 162, 1165 CAS.
- S. Mitola, R. Soldi, I. Zanon, L. Barra, M. I. Gutierrez, B. Berkhout, M. Giacca and F. Bussolino, J. Virol., 2000, 74, 344 CrossRef CAS PubMed.
Footnotes |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra23352f |
| ‡ On leave from Ordu University. |
| This journal is © The Royal Society of Chemistry 2016 |