Ru coordinated with BINAP in knitting aryl network polymers for heterogeneous asymmetric hydrogenation of methyl acetoacetate†
Tao Wangac,
Yuan Lyu*a,
Xingkun Chenac,
Cunyao Liac,
Miao Jianga,
Xiangen Songa and
Yunjie Ding*ab
aDalian National Laboratory for Clean Energy, Dalian, 116023, P. R. China. E-mail: dyj@dicp.ac.cn; luyuan@dicp.ac.cn; Fax: +86-411-84379143; Tel: +86-411-84379143
bState Key Laboratory of Catalysis, Dalian Institute of Chemical Physics, Chinese Academy of Sciences, Dalian, 116023, P. R. China
cUniversity of Chinese Academy of Sciences, Beijing 100039, P. R. China
First published on 14th March 2016
Abstract
A facile method for the preparation of heterogeneous asymmetric hydrogenation catalysts was presented. BINAP was knitted with aryl compounds using formaldehyde dimethyl acetal (FDA) as a cross-linker by Friedel–Crafts reaction without any pre-modification. The prepared catalysts showed different catalytic activities, and excellent recyclablilty results could be achieved in asymmetric hydrogenation.
Asymmetric hydrogenation catalysis is an important method for the synthesis of pharmaceuticals and other fine chemicals. Although homogeneous catalysts have good reactivity and high TON (turnover number), the losses of noble metals and chiral ligands have limited their further application in industry.1 Thus considerable effort has been devoted to look for strategies to develop heterogeneous asymmetric catalysts, such as covalently and non-covalently immobilized catalysts.2 As one of the most successful chiral ligands, BINAP [2,2′-bis(diphenylphosphino)-1,1′-binaphthyl] is frequently used to explore new ways of developing heterogeneous asymmetric catalysis.3 Wherein, chiral BINAP that was immobilized in organic polymers, has exhibited extraordinary stability and activity.4 Chan et al. synthesized several soluble polymer-supported catalysts by the polycondensation of the amine group substituted BINAP, terephthaloyl chloride and several diols, such as (2S,4S)-pentanediol, poly-(ethylene glycol) or MEO-PEG-OH (Mw = 5000) for asymmetric hydrogenation, which showed a comparable activity to corresponding homogeneous catalyst.5 Xiao et al. presented a chiral cross-linked mesoporous polymer by copolymerization of the diacryloylamino substituted BINAP with divinylbenzene. After loading a ruthenium species, the catalyst exhibited a high activity and satisfactory recyclability in asymmetric hydrogenation of methyl acetoacetate.6 According to previous works, complicated modifications of BINAP were generally essential to attach this chiral ligand to a polymer, which made the traditional strategies of preparing chiral organic polymers costly and time consuming. Inspired by Tan's work, who used a simple Friedel–Crafts reaction of a low-cost cross-linker (FDA) with aromatic compounds to produce microporous polymers, a facile strategy was presented here to address this problem.7 BINAP was directly cross-linked with aromatic compounds, such as benzene, biphenyl and 1,3,5-triphenylbenzene, followed by loading a ruthenium species, and methyl acetoacetate was selected as a classic substrate to test asymmetric hydrogenation activities of these varied polymer-supported catalysts. To the best of our knowledge, this is the first time to incorporate the un-modified BINAP in the polymer backbone, and apply it for asymmetric hydrogenation.
During the preparation of materials, FDA was used to link (S)-BINAP and aromatic compounds. Typically, (S)-BINAP (0.4 mmol, 0.249 g), benzene (0.781 g, 10 mmol), and FDA (2.283 g, 30 mmol) were dissolved in 20 mL dichloromethane and reacted to form BINAP–benzene cross-linked aromatic polymers (KAP-1) under the catalysis of FeCl3 (4.486 g, 30 mmol) (Scheme 1). Using biphenyl and 1,3,5-triphenylbenzene to replace benzene comonomer, BINAP–biphenyl based knitted aromatic polymers (KAP-2) and BINAP–1,3,5-triphenylbenzene based knitted aromatic polymers (KAP-3) were obtained according to the above strategies, respectively.
The pore properties of three materials are analysed by N2 physisorption analysis (Fig. 2A). The polymer materials are insoluble and porous, having a large surface area at 1100–1200 m2 g−1 (Table S1, ESI†). However, the pore volume of KAP-1 is as high as 1.941 cm3 g−1, which is much higher than the pore volume sum of KAP-2 and KAP-3. A sharp N2 uptake at low relative pressure (P/P0 < 0.001) of KAP-2 and KAP-3 was observed due to the filling of micropores, which was confirmed by the pore size distribution curves calculated by DFT method (Fig. S1†). KAP-2 and KAP-3 were basically all made of micropores. This was in sharp contrast to the pore size distribution of KAP-1, the isotherms of which showed a hysteresis loop at 0.6–0.95 (P/P0). The scanning electron microscope (SEM) images of those materials (Fig. S2†) are in good agreement with the results of N2 sorption isotherms, showing porous knitting networks. The transmission electron microscope (TEM) images of polymer supported catalysts show that the materials still have abundant pores after loading with ruthenium species (Fig. S3–S5†). The EDS on TEM confirms the presence of Ru metal in catalysts, and its content is generally consistent with the amount of added [RuCl2(benzene)]2, which approve that the ruthenium species are highly dispersed in the catalysts. Notably, the particles of Ru cannot be observed in the high resolution TEM images of the three catalysts.
The 13C magic angle spinning (MAS) NMR spectrums of three polymers (Fig. 1B) show a broad resonance peaks near 139 and 131 ppm assigned to the substituted aromatic carbon and non-substituted aromatic carbon respectively. And the resonance peak at 20 ppm can be attributed to the carbon in the methylene linker formed from the Friedel–Crafts reaction.7 The 31P MAS NMR spectrum of KAP-1 (Fig. 1C) gives a resonance signal at δ = −14.3 ppm due to the unprotected phosphine and another signal at δ = 23.5 ppm due to phosphorous oxide. The other two materials, KAP-2 and KAP-3, also give two resonance peaks at the same location approximately. Both signals are strong and also confirm the presence of element P in the samples. Since the Friedel–Crafts reaction was catalysed by Lewis acid FeCl3, which could accelerate the oxidation of BINAP even though only trace oxygen remained in the reaction system.8 As the figure shows, the molar ratio of the unprotected phosphine and phosphorous oxide in the materials decreased in the order KAP-3 > KAP-1 > KAP-2. To test the catalytic properties of materials obtained by this method, we did not manage to reduce the phosphorous oxide in the polymers artificially. As a representative catalyst, the 31P MAS NMR spectrum of Ru/KAP-1 shows a relatively broad peak at 49.6 ppm, which can be assigned to the unprotected phosphine coordinated with Ru species (Fig. S6†).9 It should be noted that the presence of element Fe in the catalyst can produce a magnetic field interference in the NMR instrument, which may result in the peak broadening. This may be the reason of the peaks at 49.6 ppm and 25.9 ppm have partially overlapping.
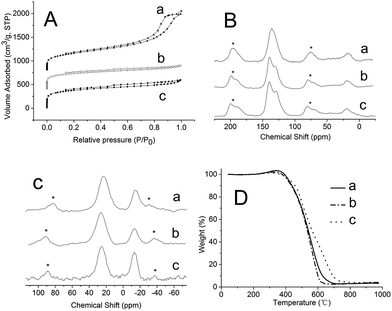 | ||
| Fig. 1 (A) N2 isotherms, (B) 13C MAS NMR, (C) 31P MAS NMR, (D) TG analysis of (a) KAP-1, (b) KAP-2, (c) KAP-3. Asterisks denote spinning sidebands. | ||
Excellent thermal stability is a crucial requirement for the polymer materials used as catalyst supports. The curves of thermogravimetric analysis (TG) under air (Fig. 1D) show that these materials have superior thermal stability without any decomposition at the temperature of 400 °C. Compared with aryl-based knitting networks that Tan et al. reported, which decomposition temperature were before 300 °C, the results demonstrate that the BINAP-based aryl networks are even more stable than aryl-based knitted networks.7 Interestingly, the curves slightly rise at 350 °C, and the phenomenon might be attributed to the oxidation of unprotected phosphine in the materials.
The MW dispersity of materials also has a great impact on their catalytic application. We attempt to determine the character by using several means, like gel permeation chromatography (GPC) and matrix-assisted laser desorption/ionization time of flight mass spectrometry (MALDI-TOF-MS). However, the solubility of our polymer materials are very poor, so no satisfactory results are obtained.
To determine the asymmetric hydrogenation activities of these series polymer-supported catalysts, we chose methyl acetoacetate as a classic substrate. After stirred for 24 hours, the ruthenium precursor, [RuCl2(benzene)]2, was successfully coordinated with polymers in mixed solution of N,N-dimethylformamide and dichloromethane (v![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) v = 1
v = 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 2) at room temperature (Fig. S6†). By optimizing the reaction conditions, like temperature and pressure, Ru/KAP-1 catalyst exhibited excellent activity (yield > 99.5%, ee = 96.7%) at 80 °C under low H2 pressure of 0.5 MPa (Table 1, entry 1). Ru/KAP-2 and Ru/KAP-3 catalysts obtained low product yield (45.1% and 45.7% respectively) under the same reaction conditions (entries 2 and 3). When the reaction pressure was increased to 2 MPa, all the catalysts exhibited excellent activity (yield > 99.5%, ee > 94%) (entries 4–6). It was also notably that the Ru/KAP-1 catalyst demonstrated a satisfactory activity at very high substrate/catalyst molar ratios (S/C = 6000) for 10 hours. Different pore structures may be one of the reasons that these catalysts showed different catalytic activities. Abundant micropores are favourable for the complete contact between substrate and the catalytically active sites, and the mesopores improved mass transfer efficiency in heterogeneous catalysis.8b,10 It also showed that the Ru/KAP-1 has more channels than the other two catalysts through the TEM images. So, Ru/KAP-1 showed higher catalytic activity than the other catalysts. The other reason for the high activity and enantioselectivity of the catalyst could be attributed to the incorporated BINAP in the polymer backbones, which offered a uniform catalytic activity sites. Interestingly, when a series of β-keto esters were catalysed by Ru/KAP-1 for asymmetric hydrogenation, their corresponding alcohols were used as the solvent rather than methanol. Otherwise transesterification reaction could easily take place (entries 9–12). This maybe because the catalyst still contains trace amounts of Lewis acid FeCl3, which may accelerate the side reaction, although the material have been thoroughly washed during its preparation process. Meanwhile, the reuse property of Ru/KAP-1 catalyst was preliminary explored, and it could be reused for four times with the high yield (higher than 99.5%) and a slight decrease on ee value (from 96.0% to 94.7%) (Fig. 2).
2) at room temperature (Fig. S6†). By optimizing the reaction conditions, like temperature and pressure, Ru/KAP-1 catalyst exhibited excellent activity (yield > 99.5%, ee = 96.7%) at 80 °C under low H2 pressure of 0.5 MPa (Table 1, entry 1). Ru/KAP-2 and Ru/KAP-3 catalysts obtained low product yield (45.1% and 45.7% respectively) under the same reaction conditions (entries 2 and 3). When the reaction pressure was increased to 2 MPa, all the catalysts exhibited excellent activity (yield > 99.5%, ee > 94%) (entries 4–6). It was also notably that the Ru/KAP-1 catalyst demonstrated a satisfactory activity at very high substrate/catalyst molar ratios (S/C = 6000) for 10 hours. Different pore structures may be one of the reasons that these catalysts showed different catalytic activities. Abundant micropores are favourable for the complete contact between substrate and the catalytically active sites, and the mesopores improved mass transfer efficiency in heterogeneous catalysis.8b,10 It also showed that the Ru/KAP-1 has more channels than the other two catalysts through the TEM images. So, Ru/KAP-1 showed higher catalytic activity than the other catalysts. The other reason for the high activity and enantioselectivity of the catalyst could be attributed to the incorporated BINAP in the polymer backbones, which offered a uniform catalytic activity sites. Interestingly, when a series of β-keto esters were catalysed by Ru/KAP-1 for asymmetric hydrogenation, their corresponding alcohols were used as the solvent rather than methanol. Otherwise transesterification reaction could easily take place (entries 9–12). This maybe because the catalyst still contains trace amounts of Lewis acid FeCl3, which may accelerate the side reaction, although the material have been thoroughly washed during its preparation process. Meanwhile, the reuse property of Ru/KAP-1 catalyst was preliminary explored, and it could be reused for four times with the high yield (higher than 99.5%) and a slight decrease on ee value (from 96.0% to 94.7%) (Fig. 2).
| Entry | Catalyst | R | S/Cb | Yieldc (%) | eec (%) |
|---|---|---|---|---|---|
| a Reaction conditions: 2 mL methanol, initial H2 pressure 2 MPa, 0.00086 mmol Ru with BINAP/Ru at 10, 80 °C, 10 h.b Molar ratio of substrate to catalyst.c Determined by GC on a Cyclosil-B capillary column.d 0.5 MPa H2.e 4 mL methanol.f 2 mL ethanol.g 2 mL isopropanol.h 2 mL t-butanol. | |||||
| 1d | Ru/KAP-1 | R1 = R2 = Me | 1000 | >99.5 | 96.4 |
| 2d | Ru/KAP-2 | R1 = R2 = Me | 1000 | 45.1 | 95.7 |
| 3d | Ru/KAP-3 | R1 = R2 = Me | 1000 | 45.7 | 94.8 |
| 4a | Ru/KAP-1 | R1 = R2 = Me | 1000 | >99.5 | 97.0 |
| 5a | Ru/KAP-2 | R1 = R2 = Me | 1000 | >99.5 | 94.1 |
| 6a | Ru/KAP-3 | R1 = R2 = Me | 1000 | >99.5 | 96.8 |
| 7a | Ru/KAP-1 | R1 = R2 = Me | 4000 | >99.5 | 97.0 |
| 8e | Ru/KAP-1 | R1 = R2 = Me | 6000 | >99.5 | 97.9 |
| 9a | Ru/KAP-1 | R1 = iPr, R2 = Me | 1000 | 91.9 | 96.4 |
| 10f | Ru/KAP-1 | R1 = Me, R2 = et | 1000 | >99.5 | 98.7 |
| 11g | Ru/KAP-1 | R1 = Me, R2 = iPr | 1000 | 96.5 | 94.0 |
| 12h | Ru/KAP-1 | R1 = Me, R2 = tBu | 1000 | 90.9 | 94.8 |
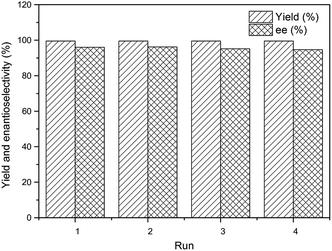 | ||
| Fig. 2 Recycle of the Ru/KAPs-1 catalyst for the hydrogenation of methyl acetoacetate. Reaction conditions: 4 mL methanol, initial H2 pressure 2 MPa, 0.00172 mmol Ru (S/C = 1000), 80 °C, 10 h. | ||
Conclusions
In summary, a series of novel porous polymers (KAP-1, KAP-2, KAP-3) with no-modified BINAP were successfully synthesized by Friedel–Crafts reaction for the first time. These polymer-supported catalysts demonstrated excellent activities and enantioselectivities on the asymmetric hydrogenation of β-keto esters. Further efforts are underway with a focus on reduction of partly oxidized BINAP in knitting networks to improve the stability of catalysts.Acknowledgements
This work was supported by the National Natural Science Foundation of China (21303190).Notes and references
- (a) B. Schäffner, J. Holz, S. P. Verevkin and A. Börner, Tetrahedron Lett., 2008, 49, 768 CrossRef; (b) J. Deng, X. Sun, S. Wang, S. Sun and J. Zhu, Synlett, 2005, 37, 2776 CrossRef; (c) R. M. Bullock, Science, 2013, 342, 1054 CrossRef CAS PubMed; (d) W. W. Zuo, A. J. Lough, Y. F. Li and R. H. Morris, Science, 2013, 342, 1080 CrossRef CAS PubMed; (e) E. Tomás-Mendivil, L. Menéndez-Rodríguez, J. Francos, P. Crochet and V. Cadierno, RSC Adv., 2014, 4, 63466 RSC; (f) M. Yoshimura, S. Tanaka and M. Kitamura, Tetrahedron Lett., 2014, 55, 3635 CrossRef CAS; (g) H. Zhang, D. D. Feng, H. B. Sheng, X. B. Ma, J. W. Wan and Q. Tang, RSC Adv, 2014, 4, 6417 RSC.
- (a) F. M. Bautista, V. Caballero, J. M. Campelo, D. Luna, J. M. Marinas, A. A. Romero, I. Romero, I. Serrano and A. Llobet, Top. Catal., 2006, 40, 193 CrossRef CAS; (b) J. Wang, H. Feng, R. X. Qin, H. Fu, M. Yuan, H. Chen and X. L. Li, Tetrahedron: Asymmetry, 2007, 18, 1643 CrossRef CAS; (c) C. Li, L. Zhang, C. Zheng, X. Zheng, H. Fu, H. Chen and R. Li, Tetrahedron: Asymmetry, 2014, 25, 821 CrossRef CAS; (d) D. Zhang, X. Gao, T. Cheng and G. Liu, Sci. Rep., 2014, 4, 5091 Search PubMed; (e) A. Ghosh and R. Kumar, J. Catal., 2004, 228, 386 CrossRef CAS; (f) P.-N. Liu, P.-M. Gu, J.-G. Deng, Y.-Q. Tu and Y.-P. Ma, Eur. J. Org. Chem., 2005, 2005, 3221 CrossRef; (g) C. Bianchini and P. Barbaro, Top. Catal., 2002, 19, 17 CrossRef CAS.
- (a) X. Wang, S. M. Lu, J. Li, Y. Liu and C. Li, Catal. Sci. Technol., 2015, 5, 2585 RSC; (b) J. Peng, X. Wang, X. Zhang, S. Bai, Y. Zhao, C. Li and Q. Yang, Catal. Sci. Technol., 2015, 5, 666 RSC; (c) M. Berthod, G. Mignani, G. Woodward and M. Lemaire, Chem. Rev., 2005, 105, 1801 CrossRef CAS PubMed; (d) A. G. Hu, H. L. Ngo and W. B. Lin, J. Am. Chem. Soc., 2003, 125, 11490 CrossRef CAS PubMed; (e) A. R. McDonald, C. Muller, D. Vogt, G. P. M. van Klinka and G. van Koten, Green Chem., 2008, 10, 424 RSC.
- (a) X. Xu, R. Wang, J. W. Wan, X. B. Ma and J. D. Peng, RSC Adv., 2013, 3, 6747 RSC; (b) Y. Wang, N. Su, L. Ye, Y. Ren, X. Chen, Y. Du, Z. Li, B. Yue, S. C. E. Tsang, Q. Chen and H. He, J. Catal., 2014, 313, 113 CrossRef CAS; (c) H. B. Yu, Q. S. Hu and L. Pu, Tetrahedron Lett., 2000, 41, 1681 CrossRef CAS; (d) Q. H. Fan, G. H. Liu, G. J. Deng, X. M. Chen and A. S. C. Chan, Tetrahedron Lett., 2001, 42, 9047 CrossRef CAS; (e) D. J. Bayston, J. L. Fraser, M. R. Ashton, A. D. Baxter, M. E. C. Polywka and E. Moses, J. Org. Chem., 1998, 63, 3137 CrossRef CAS.
- (a) Q. H. Fan, C. Y. Ren, C. H. Yeung, W. H. Hu and A. S. C. Chan, J. Am. Chem. Soc., 1999, 121, 7407 CrossRef CAS; (b) Q. H. Fan, G. J. Deng, X. M. Chen, W. C. Xie, D. Z. Jiang, D. S. Liu and A. S. C. Chan, J. Mol. Catal. A: Chem., 2000, 159, 37 CrossRef CAS; (c) Q. H. Fan, G. J. Deng, C. C. Lin and A. S. C. Chan, Tetrahedron: Asymmetry, 2001, 12, 1241 CrossRef CAS.
- Q. Sun, X. Meng, X. Liu, X. Zhang, Y. Yang, Q. Yang and F. S. Xiao, Chem. Commun., 2012, 48, 10505 RSC.
- B. Li, R. Gong, W. Wang, X. Huang, W. Zhang, H. Li, C. Hu and B. Tan, Macromolecules, 2011, 44, 2410 CrossRef CAS.
- (a) B. Li, Z. Guan, W. Wang, X. Yang, J. Hu, B. Tan and T. Li, Adv. Mater., 2012, 24, 3390 CrossRef CAS PubMed; (b) M. Jiang, Y. J. Ding, L. Yan, X. G. Song and R. H. Lin, Chin. J. Catal., 2014, 35, 1456 CrossRef CAS.
- M. Kitamura, M. Tokunaga, T. Ohkuma and R. Noyori, Tetrahedron Lett., 1991, 32, 4163 CrossRef CAS.
- (a) P. Kaur, J. T. Hupp and S. T. Nguyen, ACS Catal., 2011, 1, 819 CrossRef CAS; (b) A. Thomas, Angew. Chem., Int. Ed., 2010, 49, 8328 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available: Experimental procedures, characterization data and activities. See DOI: 10.1039/c5ra23597a |
| This journal is © The Royal Society of Chemistry 2016 |