DOI:
10.1039/C5RA24379C
(Paper)
RSC Adv., 2016,
6, 17845-17856
Design and development of a nanoemulsion system containing copper peptide by D-optimal mixture design and evaluation of its physicochemical properties
Received
18th November 2015
, Accepted 1st February 2016
First published on 2nd February 2016
Abstract
Copper peptide is an important compound used to prevent ageing; a process that leads to several unwanted aging signs such as wrinkles, sagging, pigmented spots and dryness. The D-optimal mixture design was used for optimizing the particle size of Virgin Coconut Oil (VCO) nanoemulsions for topical delivery of copper peptide. Effects of formulation variables including VCO (10–20%, w/w), Tween 80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pluronic PF68 (10–15%, w/w), xanthan gum (0.5–1.0%, w/w) and water (64.008–79.291), towards particle size as a response were studied. D-optimal mixture design analysis demonstrated that the variation in particle size as a response could be expressed as a quadratic function of the main components of the emulsion. Analysis of variance (ANOVA) showed that the quadratic polynomial model sufficiently fits the experimental data. The best combination of the composition factors that gave the optimum particle size was found to be VCO (10%, w/w), T80
Pluronic PF68 (10–15%, w/w), xanthan gum (0.5–1.0%, w/w) and water (64.008–79.291), towards particle size as a response were studied. D-optimal mixture design analysis demonstrated that the variation in particle size as a response could be expressed as a quadratic function of the main components of the emulsion. Analysis of variance (ANOVA) showed that the quadratic polynomial model sufficiently fits the experimental data. The best combination of the composition factors that gave the optimum particle size was found to be VCO (10%, w/w), T80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PF68 (15%, w/w), xanthan gum (0.867%, w/w) and water (74.133%, w/w). Under these optimum compositions, the actual particle size showed good agreement with predicted particle size with a residual standard error (RSE) less than 2%. The formulation containing copper peptide was successfully prepared with a particle size value of 120.7 nm, which was consistent with the result obtained by TEM. The final formulation also showed good conductivity and pH values as well as stability against phase separation. A rheology study demonstrates that the VCO nanoemulsion containing copper peptide exhibited shear thinning and pseudoplastic properties and showed more elastic rather than viscous characteristics due to the presence of a large linear viscoelastic region. Permeability test results showed that copper peptide in aqueous solution with and without penetration enhancer cannot be transported through the cellulose acetate membrane after 8 h application. The nanoemulsion increased the permeability of copper peptide with 21.89 ± 0.53% active matter release after 8 h application.
PF68 (15%, w/w), xanthan gum (0.867%, w/w) and water (74.133%, w/w). Under these optimum compositions, the actual particle size showed good agreement with predicted particle size with a residual standard error (RSE) less than 2%. The formulation containing copper peptide was successfully prepared with a particle size value of 120.7 nm, which was consistent with the result obtained by TEM. The final formulation also showed good conductivity and pH values as well as stability against phase separation. A rheology study demonstrates that the VCO nanoemulsion containing copper peptide exhibited shear thinning and pseudoplastic properties and showed more elastic rather than viscous characteristics due to the presence of a large linear viscoelastic region. Permeability test results showed that copper peptide in aqueous solution with and without penetration enhancer cannot be transported through the cellulose acetate membrane after 8 h application. The nanoemulsion increased the permeability of copper peptide with 21.89 ± 0.53% active matter release after 8 h application.
1. Introduction
Ageing is characterized by the accumulation of molecular damage as well as the progressive failure of the maintenance and repair of skin,1 which leads to several signs of aging, such as wrinkles, sagging, pigmented spots and dryness. It is divided into two types, which are chronological aging (intrinsic aging) and photo-aging (extrinsic aging). The first type occurs due to passage of time,2 which causes irreversible degeneration of skin tissue, loss of skin thickness and elastic tissue and reduction in the number of dermal fibroblasts, whereas the latter happens when the skin is exposed to ultraviolet radiation,3 resulting in coarse and rough skin with deep lines and wrinkles and hyperpigmentation in the skin.
Antioxidants are reported to play a role in preventing cells from oxidative stress by scavenging free radicals or reactive oxygen species (ROS)4 and pairing them with oxygen molecules in the destabilization process, which in turns neutralizes the free radical.5 On the other hand, cosmeceutical peptides are important in many natural processes involving skin care which include modulation of cell proliferation, cell migration, and synthesis and regulation of protein. Copper peptide or copper binding tri-peptide (glycyl-L-histidyl-lysine) possesses both anti-oxidant and rejuvenating properties. As antioxidant, it acts by quenching the toxic by-products produced from fatty acids lipid peroxidation. On the other hand, the rejuvenating property of copper peptide relies on its ability to stimulate collagen synthesis, maintaining epidermal stem cells, restoring viability of damaged skin cells and many others reparative actions.1,6
The amount of peptides that can be delivered into skin through oral supplementation is limited due to the physiological processes related to active's absorption and stability.7 Therefore, topical application has the added advantage of targeting the peptides to the area of skin which requires protection. Nanoemulsion was used in this study to enhance the penetration and permeability of active ingredients through skin surface.8 Nanoemulsion is defined as emulsion with small size around 20–200 nm.9 It has become attractive for application in both pharmaceutical and cosmetic industry due to its high kinetic stability,10,11 non-toxic, non-irritant and is suitable for efficient delivery of active through the skin.11 For topical use, the small size of droplets in nanoemulsions allows them to be deposited uniformly on the skin thus enhances their penetration.12
The optimization of mixture composition to obtain product with required characteristic is one of the common issue in the pre-formulation of cosmetic products. There are several statistical techniques used for the optimization of mixture compositions and processing parameters to obtain desirable properties such as response surface methodology,13–18 Box Behnken19 and D-optimal mixture design.20–23 According to Borhan et al. (2014),21 D-optimal has been practiced in product formulation in food, pharmaceutical and cosmeceutical industries. There are several advantages of D-optimal mixture design including reduction in the number of experimental run and the ability to identify interaction statistically to overcome the shortcomings of traditional formulation method. To date, there are no reported studies on the optimization of VCO based nanoemulsions using D-optimal mixture design and only a few reported works on optimization of cosmetic products using this type of mathematical tool. In addition, there are no published works on the utilization of nanoemulsions as carrier of copper peptide for cosmeceuticals use.
Therefore, in this work, the D-optimal mixture design was used as a tool to optimize VCO nanoemulsions. The interaction effects between factors (oil, emulsifier, xanthan gum and water) were evaluated simultaneously and variables that affect the particle size were studied. Apart from that, VCO nanoemulsion containing active (copper peptide) was formulated using the optimum formulation obtained from the mathematical model and physicochemically characterized in terms of pH, conductivity, zeta potential, stability and rheological behavior. In vitro permeation study was also carried out to check the transport of copper peptide through skin barrier.
2. Experimental section
2.1. Materials
Copper peptide was obtained from GL Biochem Ltd Shanghai, China. Virgin coconut oil, xanthan gum, Tween 80 (polyoxyethylenesorbitan monostearate) and Pluronic F68 (PF68) were purchased from Sigma-Aldrich St Louis, USA. Phenonip was procured from Bramble Berry Bellingham, USA. Water was deionized using a Milli-Q filtration system.
2.2. Formation of VCO based nanoemulsions
The preparation of oil phase was carried out by mixing T80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PF68 (40
PF68 (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and VCO while for the aqueous phase, xanthan gum was added into deionized water. The oil and aqueous phase were initially heated separately until all ingredients dissolved. Using homogenizer, the aqueous phase was added drop wise into the oil phase until completed. The final mixture was homogenized for 3 hours using overhead stirrer. The emulsion obtained was further subjected to high shear homogenizer for 10 minutes at 15
1) and VCO while for the aqueous phase, xanthan gum was added into deionized water. The oil and aqueous phase were initially heated separately until all ingredients dissolved. Using homogenizer, the aqueous phase was added drop wise into the oil phase until completed. The final mixture was homogenized for 3 hours using overhead stirrer. The emulsion obtained was further subjected to high shear homogenizer for 10 minutes at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm. Phenonip (0.7% w/w) was added as anti-microbial agent.
000 rpm. Phenonip (0.7% w/w) was added as anti-microbial agent.
2.3. Formation of VCO based nanoemulsions containing copper peptide
The nanoemulsions were prepared following the method described in Section 2.2. After the addition of active matter (copper peptide 0.003% w/w) into the pre-mixed emulsion, the final mixture was homogenized for 3 hours using overhead stirrer. The emulsion obtained was further subjected to high shear homogenizer for 10 minutes at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm. Phenonip (0.7%, w/w) was added as anti-microbial agent.
000 rpm. Phenonip (0.7%, w/w) was added as anti-microbial agent.
2.4. Experimental design
A four-factor D-optimal design mixture was utilized to investigate the effect of VCO (A), T80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PF68 (B), xanthan gum (C) and water (D) on response taken as particle size. For D-optimal design, there are restrictions on the component, Xj with general form as follows:
PF68 (B), xanthan gum (C) and water (D) on response taken as particle size. For D-optimal design, there are restrictions on the component, Xj with general form as follows:
| ∑Xj = 1 and Lj ≤ Xj ≤ Uj. |
These restrictions take the form of lower (Lj) and upper (Uj) constraints which purpose is to prevent the experimenter from exploring the entire simplex region.24 The constraints of the component proportions are listed in Table 1. Design matrix with a total of 20 runs was generated and the results were statistically analyzed using Design Expert version 7.0.0 by Stat-Ease Inc. (Minneapolis, USA) as shown in Table 2. The composition of each run was carried out following the D-optimal model design in a randomized order so that the effect of the unexplained variability on the actual response caused by extraneous factor could be minimized.
Table 1 Constraint of independent variables proportion
| Independent variables, Xj |
Lower limit, Lj |
Upper limit, Uj |
| VCO, A |
10 |
20 |
T80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PF68, B PF68, B |
10 |
15 |
| Xanthan gum, C |
0.5 |
1.0 |
| Water, D |
64.008 |
79.291 |
Table 2 The D-optimal design with predicted and experimental values of particle size for VCO nanoemulsions
| Experiment No. |
Blocks |
A |
B |
C |
D |
Particle size (nm) |
| Actual |
Predicted |
| 1 |
1 |
10.003 |
10.000 |
0.706 |
79.291 |
138.95 |
143.39 |
| 2 |
1 |
10.089 |
15.000 |
0.503 |
74.408 |
137.25 |
131.80 |
| 3 |
1 |
20.000 |
10.435 |
0.610 |
68.954 |
617.50 |
626.42 |
| 4 |
1 |
20.000 |
10.435 |
0.610 |
68.954 |
628.73 |
626.42 |
| 5 |
1 |
15.168 |
13.202 |
0.757 |
70.873 |
161.23 |
149.93 |
| 6 |
1 |
19.902 |
10.729 |
1.000 |
68.369 |
427.22 |
426.13 |
| 7 |
1 |
10.614 |
14.999 |
1.000 |
73.387 |
135.78 |
121.97 |
| 8 |
1 |
10.000 |
12.301 |
0.500 |
77.199 |
135.65 |
138.26 |
| 9 |
1 |
14.779 |
10.047 |
0.53 |
73.636 |
533.53 |
525.95 |
| 10 |
1 |
15.168 |
13.202 |
0.757 |
70.873 |
143.48 |
149.93 |
| 11 |
1 |
17.696 |
14.064 |
0.500 |
67.740 |
167.40 |
168.34 |
| 12 |
1 |
19.994 |
15.000 |
0.998 |
64.008 |
299.57 |
295.88 |
| 13 |
1 |
10.089 |
15.000 |
0.503 |
74.408 |
126.60 |
131.80 |
| 17 |
1 |
19.994 |
15.000 |
0.998 |
64.008 |
295.23 |
295.88 |
| 18 |
1 |
12.773 |
14.996 |
1.000 |
71.231 |
136.00 |
154.49 |
| 19 |
1 |
10.003 |
10.000 |
0.706 |
79.291 |
145.27 |
143.39 |
| 20 |
1 |
20.000 |
14.994 |
0.502 |
64.505 |
139.37 |
138.79 |
2.5. Statistical analysis
The optimum conditions for the independent variables were obtained through D-optimal mixture design which allows the prediction of the effects of changing the ingredient compositions against particle size. The optimal compositions for the preparation of the nanoemulsions were chosen based on the condition resulting on minimum particle size. Analysis of variance (ANOVA) as well as R2 (coefficient of determination) were carried out to investigate the significant differences among the independent variables. In order to get a good final reduced model, p value must be significant (p < 0.05) and R2 higher than 0.9 is considered as model having a very high correlation.25 The design was expressed by polynomial regression equation to generate the following model:| | |
Yi = β0 + β1x1 + β2x2 + β3x3 + β4x4 − β11x12 + β22x22 + β33x32 + β44x42 + β12x1x2 + β13x1x3 + β14x1x4 + β23x2x3 + β24x2x4 + β34x3x4
| (1) |
where Yi is the predicted response; β0, βi, βii and βij are constant, linear, quadratic and interaction coefficients, respectively.
2.6. Verification of models
The experimental and theoretical prediction values were compared quantitatively by preparing a few random formulations to validate the obtained models. This is the most important step to make sure the adequacy of the final reduced models. The recommended optimum composition was also carried out to verify the predicted optimum values from the model. The response obtained was compared to the predicted values by calculating the residual standard error as follows:5| |
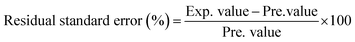 | (2) |
2.7. Characterization of the nanoemulsions
2.7.1 Particle size analysis. Particle size was determined by dynamic light scattering technique, scattered at an angle of 173° at temperature of 25 °C. The measurements were carried out using zetasizer (Nano ZS, Malvern Instrument Ltd., UK). Intensity distribution was used for the measurement of mean average (z-average) droplet size. The samples were diluted with deionized water in order to avoid multiple scattering effects. Reading was repeated three times.
2.7.2 Transmission electron microscopy (TEM). The size and morphology of VCO nanoemulsion containing copper peptide was studied using Hitachi H-7100 TEM (Japan). The sample was first diluted with deionized water and then dropped to 200-mesh formvar-coated copper grids. The sample was negatively stained with uranyl acetate for better contrast. Whatman filter paper was used to dry excess liquid and the prepared sample was dried at room temperature.
2.7.3 pH and conductivity measurement. The pH and conductivity of the formulations were tested at room temperature using Delta 320 pH meter (Mettler-Toledo, Schwerzenbach) and Conductometer (Mettler Toledo, Switzerland) respectively. The pH meter was calibrated with three pH standard buffer solutions (pH 4.01, pH 7.00 and pH 10.01) before measurements.
2.7.4 Surface charge. Zeta potential analysis was carried out using Zetasizer (Nano ZS, Malvern Instrument Ltd., UK) at room temperature. Sample was diluted with deionized water prior to measurement. The calculation of zeta potential was done based on measurement of the electrophoretic mobility of dispersed particles in a charged field.
2.7.5 Stability study. Stability test was carried out to predict the long term physical stability of the nanoemulsions. For storage stability, VCO nanoemulsions were kept at two storage temperatures (25 °C and 45 °C). The physical appearances of the formulations (no phase separation) were observed within 3 months. Sample was also subjected to centrifugal force at 4500 rpm for 15 min. The freeze–thaw cycle test was conducted by alternately keeping the formulations at 4 °C and 25 °C for 24 h for 6 cycles. Observation of phase changes was made for each cycle.
2.7.6 Rheological measurement. The rheology study was carried out using Kinexus Rotational Rheometer (Malvern Instruments, UK). The analysis was performed at 25 °C with 4°/40 mm cone plate geometry. The steady rheological behavior of the emulsions was obtained over a shear rate of 0.01–50 s−1. Samples were allowed to rest for 5 minutes after sample loading before the measurement was started. The experimental data were fitted to the power law model26 as follows:where η is the viscosity (Pa s), ỳ is the shear rate (s−1), k and n as the consistency index and flow behavior index respectively. Viscoelastic properties of the final formulation was determined using oscillatory strain sweep analysis at strain amplitudes varying from 1%–1000% at fixed frequency of 1 Hz. This test was carried out to identify storage (G′) and loss (G′′) modulus to get a linear viscoelastic region (LVE).
2.8. In vitro permeation study
The diffusion of copper tripeptide across cellulose acetate membrane was studied using Franz diffusion cells. The receptor compartments were filled with phosphate buffer solution (pH 7.4) at temperature 37 °C. The elution medium was stirred using magnetic bar. Samples (3 ml) were loaded into separate donor compartments evenly onto the membrane. One milliliter of the receptor medium was withdrawn at different time interval (1, 2, 3, 4, 5, 6, 7 and 8 h) and replaced with the same volume of phosphate buffer pH 7.4. These samples were analyzed using UV spectrophotometer at 287 nm. This was the specified wavelength that showed maximum absorbance of copper tripeptide (GHK-cu) in phosphate buffer solution. Calibration curve with linearity of r2 = 0.9803 for copper peptide in VCO nanoemulsion (concentration range from 0.06 to 0.30 μg ml−1) was obtained. All experiments were carried out in duplicate.
3. Results and discussion
3.1. Screening of the variables
A preliminary study was performed to find out the levels of independent variables. The lower and upper limit for three independent variables (VCO, T80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PF68 and xanthan gum) were determined based on the data obtained while the level of water was determined by the software. The VCO based emulsions showed the size in the range of 100 nm–350 nm with composition of VCO, T80
PF68 and xanthan gum) were determined based on the data obtained while the level of water was determined by the software. The VCO based emulsions showed the size in the range of 100 nm–350 nm with composition of VCO, T80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PF68 and xanthan gum were 10–20% w/w, 10–15% w/w and 0.5–1.0% w/w respectively. Table 1 shows the values of variable used in the formulation. The upper- and lower-bound on the component proportion are needed for the construction and analysis of mixture design.
PF68 and xanthan gum were 10–20% w/w, 10–15% w/w and 0.5–1.0% w/w respectively. Table 1 shows the values of variable used in the formulation. The upper- and lower-bound on the component proportion are needed for the construction and analysis of mixture design.
3.2. Fitting the models
Table 2 depicts the composition factors on the particle size of nanoemulsions which were obtained experimentally based on D-optimal design. The predicted values were in agreement with the values obtained experimentally in almost all cases. The prediction of the particle size values was made based on the experimental data. The final equation for the model describing the particle size can be written as eqn (4):| |
Y = 1035.81A + 2164.00B − 23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 882.69C + 314.20D − 5761.57AB + 19 882.69C + 314.20D − 5761.57AB + 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 896.16AC − 113.91AD + 46 896.16AC − 113.91AD + 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 667.83BC − 3563.50BD + 11 667.83BC − 3563.50BD + 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 497.74CD 497.74CD
| (4) |
The residual analysis was performed using the normal probability plot as given in Fig. 1(a). The plot was found to be normally distributed which resembled a straight line with no outlier points encountered. The plot of predicted value versus actual value (Fig. 1(b)) gives indication of a good agreement between actual and predicted responses.
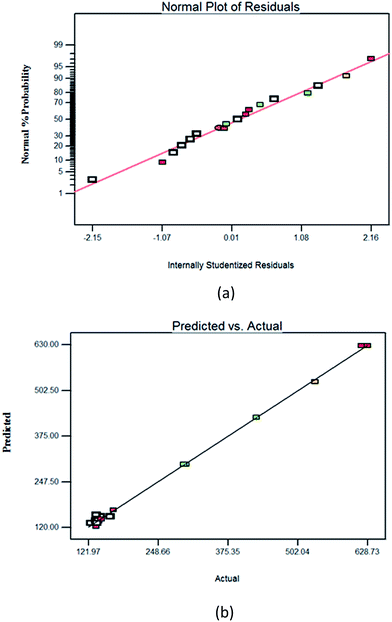 |
| | Fig. 1 (a) The normal plot of residual from D-optimal design, (b) scatter plot of predicted particle size (nm) versus actual particle size (nm) values. | |
The experimental data was statistically analyzed using analysis of variance (ANOVA) for obtaining the best fitted model for the four independent variables. Table 3 shows the analyzed statistical parameter values generated by Design Expert software which also includes the value of coefficient of determination (R2), adjusted coefficient of determination (adjusted R2), p-value and lack of fit. These data were used to obtain the best-fitting mathematical model as given in eqn (4). The assumed mathematical model has proven by the ANOVA test value to be significant and valid for the response (Y1, particle size). The R2 and adjusted R2 for the model were found out to be 0.9982 and 0.9959, respectively, indicating a good fit between the experimental values and the regression model. As for the p-value, it is considered as significant when the values are less than 0.0500.20 In this work, the final reduced model has a significant p-value (p < 0.0001). Apart from that, the model's lack of fit has no indication of significant relative to pure error (p ≥ 0.05) with a value of 0.0596. In this study, the interaction between AB and BD are significant terms since the p-values are less than 0.05. The model terms are not significant if the p-value is greater than 0.1000.
Table 3 Analysis of variance (ANOVA) for the D-optimal mixture design of the quadratic model
| Source |
Sum of Squares |
Df |
Mean square |
F value |
p-value |
Significance |
| Model |
5.217 × 105 |
9 |
57![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 962.41 962.41 |
428.18 |
<0.0001 |
Significant |
| Linear mixture |
3.643 × 105 |
3 |
1.214 × 105 |
897.16 |
<0.0001 |
|
| AB |
33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 552.04 552.04 |
1 |
33![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 552.04 552.04 |
247.86 |
<0.0001 |
|
| AC |
36.37 |
1 |
36.37 |
0.27 |
0.6202 |
|
| AD |
238.80 |
1 |
238.80 |
1.76 |
0.2258 |
|
| BC |
201.56 |
1 |
201.56 |
1.49 |
0.2619 |
|
| BD |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 131.02 131.02 |
1 |
12![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 131.02 131.02 |
89.61 |
<0.0001 |
|
| CD |
12.30 |
1 |
12.30 |
0.091 |
0.7718 |
|
| Residual |
947.58 |
7 |
135.37 |
|
|
|
| Lack of fit |
640.89 |
2 |
320.44 |
5.22 |
0.0596 |
Not significant |
| Pure error |
306.69 |
5 |
61.34 |
|
|
|
| Cor total |
5.226 × 105 |
16 |
|
|
|
|
| R2 |
0.9982 |
|
|
|
|
|
| R2 (predicted) |
0.9243 |
|
|
|
|
|
| R2 (adjusted) |
0.9959 |
|
|
|
|
|
| Regression (p-value) |
<0.0001 |
|
|
|
|
|
The regression coefficients obtained are presented in Table 4. A positive value in the regression coefficient implies that the optimization was due to a synergistic effect, whereas a negative value shows an antagonistic effect between the factor and response.27 From the final mathematical equation of particle size, the greatest influence on the response was shown by factor C (xanthan gum).
Table 4 Regression coefficient values for the final reduced model
| Source |
Coefficient estimate |
| A |
1035.81 |
| B |
2164.00 |
| C |
−23![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 883.69 883.69 |
| D |
314.20 |
| AB |
−5761.57 |
| AC |
19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 896.16 896.16 |
| AD |
−113.91 |
| BC |
46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 667.83 667.83 |
| BD |
−3563.50 |
| CD |
11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 497.74 497.74 |
3.3. D-optimal analysis
Generally, there is a high demand for the production of cosmeceutical products with a smaller droplet size (20–200 nm). The small size of nanoemulsions could enhance the permeability of actives through stratum corneum which is an effective barrier for hydrophilic and high molecular weight compounds.
For the optimization of VCO nanoemulsions, contour and three dimensional surface graphs were plotted using Design Expert software. As can be seen from Fig. 2 and 3, the particle size of the emulsion increases when the amount of oil (VCO) increases. This phenomenon can be explained by the increased in viscosity of the disperse phase as a result of the rise in VCO content. Consequently, this led to an increase in flow resistance and restriction on the droplet break-up rate.28 In addition, such occurrence may be associated with the increasing rate of collision frequency between droplets, followed by an increase of coalescence frequency which then leads to a higher probability of coalescence of the droplets.5 On the other hand, the emulsifier (T80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PF68), showed an opposite effect whereby the particle size decreased as the amount of T80
PF68), showed an opposite effect whereby the particle size decreased as the amount of T80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PF68 increased. This observation has been previously reported5,29,30 whereby the particle size decreased due to more emulsifier present to cover any new droplet surfaces formed during homogenization and leads to the reduction of the interfacial tension between oil and water. Increasing amount of emulsifier decreased the interfacial tension followed by reduction of Laplace pressure and stress required for droplet deformation. Similar trend was reported by Rebolleda et al., (2015).17 The plots demonstrated that a smaller particle size can be observed at high concentration of T80
PF68 increased. This observation has been previously reported5,29,30 whereby the particle size decreased due to more emulsifier present to cover any new droplet surfaces formed during homogenization and leads to the reduction of the interfacial tension between oil and water. Increasing amount of emulsifier decreased the interfacial tension followed by reduction of Laplace pressure and stress required for droplet deformation. Similar trend was reported by Rebolleda et al., (2015).17 The plots demonstrated that a smaller particle size can be observed at high concentration of T80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PF68 and low concentration of VCO.
PF68 and low concentration of VCO.
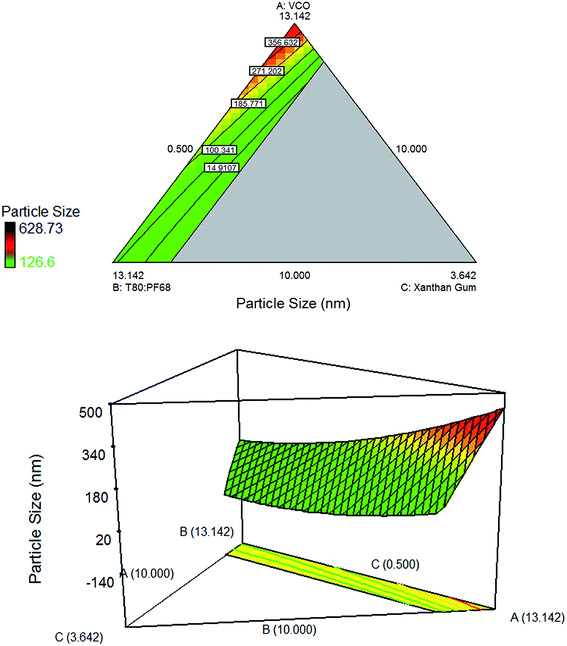 |
| | Fig. 2 Contour plot and three dimensional surface plot showing the interaction effect between three variables: (A) (VCO), (B) (T80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PF68), (C) (xanthan gum); and one variable was kept constant: (D) (water). PF68), (C) (xanthan gum); and one variable was kept constant: (D) (water). | |
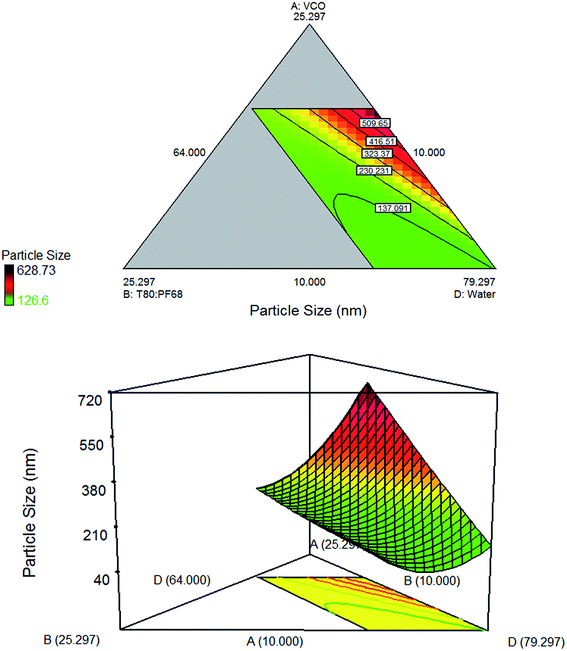 |
| | Fig. 3 Contour plot and three dimensional surface plot showing the interaction effect between three variables: (A) (VCO), (B) (T80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PF68), (D) (water); and one variable was kept constant: (C) (xanthan gum). PF68), (D) (water); and one variable was kept constant: (C) (xanthan gum). | |
The three dimensional surface and contour plots in Fig. 2 show a reduction of particle size when xanthan gum concentration increases. Decreased in particle size was due to the increased in water phase viscosity and formation of network which slow down both droplets movement and number of droplet collision.31,32 Similar observation was reported by Ngan et al. (2014)5 where they claimed that the results were due to the movement restriction of the oil droplets which prevent them from coalescing.
Fig. 3 demonstrated that the particle size reduces when the amount of water increases at constant amount of xanthan gum. As in Fig. 4, increasing concentration of xanthan gum at high amount of water shows similar effect. However, when the concentration of xanthan gum increases at low amount of water, the particle size seems to increase. According to Qian & McClements (2011),30 the phase viscosities can alter the efficiency of droplet disruption and the minimum droplet size attainable by homogenizer. Increasing the amount of xanthan gum concentration at high amount of water was enough to make the water phase more viscous for the formation of network. However, at low mount of water, increasing xanthan gum's concentration cause the emulsion to be highly viscous and therefore hard to be homogenized. As the viscosity increases, droplet disruption efficiency decreased and led to the formation of larger droplet size. The contour plot demonstrated large particle size since the amount of oil kept at constant value was quite high (19.189%, w/w).
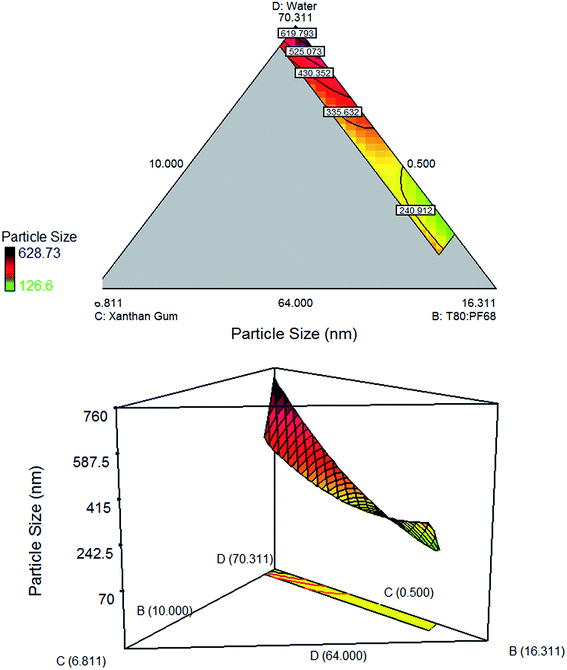 |
| | Fig. 4 Contour plot and three dimensional surface plot showing the interaction effect between three variables: (D) (water), (C) (xanthan gum), (B) (T80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PF68); and one variable was kept constant: (A) (VCO). PF68); and one variable was kept constant: (A) (VCO). | |
3.4. Verification of reduced model
In order to check the adequacy of the final model, the experimental and predicted values of several additional randomized formulations were compared as in Table 5. All results showed no significant difference between the actual and predicted values which give a good indication of the fitness of model generated.
Table 5 The predicted and actual values for randomized formulation in optimization of composition factors
| (VCO) % |
(T80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PF68) % PF68) % |
(Xanthan gum) % |
(Water) % |
Particle size (nm) |
| Actual |
Predicted |
RSE% |
| 13.5 |
12.532 |
0.706 |
73.262 |
134.10 |
134.32 |
0.16 |
| 14.0 |
14.500 |
0.570 |
70.930 |
129.20 |
128.41 |
0.62 |
| 14.5 |
14.500 |
0.570 |
70.430 |
132.00 |
131.75 |
0.19 |
| 15.5 |
14.500 |
0.570 |
69.430 |
140.65 |
139.14 |
1.09 |
3.5. Optimization of D-optimal mixture design for VCO nanoemulsions
Desirable formulation attained by preparing the VCO nanoemulsions with specific conditions is referred to as the optimum formulation. In order to obtain this optimized formulation, the Design-Expert software was used to find out the desirable composition of the emulsion. An optimum formulation should have small droplet size around 20–200 nm. D-optimal surface and contour plots were utilized to observe the interaction between the independent variables. By determining the optimization constraint and evaluating the interaction effect between independent variables, the optimum VCO nanoemulsion was prepared. The optimized formulation has the composition of VCO, T80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PF68, xanthan gum and water of 10% w/w, 15% w/w, 0.867% w/w and 74.133% w/w respectively as tabulate in Table 6. The predicted particle size given by the software for the optimum formulation was 120.882 nm. Nanoemulsions with particle size more than 100 nm (less than 200 nm) are considered good for cosmetic applications as those obtained by Ngan et al. (2014).5
PF68, xanthan gum and water of 10% w/w, 15% w/w, 0.867% w/w and 74.133% w/w respectively as tabulate in Table 6. The predicted particle size given by the software for the optimum formulation was 120.882 nm. Nanoemulsions with particle size more than 100 nm (less than 200 nm) are considered good for cosmetic applications as those obtained by Ngan et al. (2014).5
Table 6 The predicted and actual response values for optimized formulation
| (VCO) % |
(T80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) : :![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PF68) % PF68) % |
(Xanthan gum) % |
(Water) % |
Particle size (nm) |
| Actual |
Predicted |
RSE% |
| 10 |
15 |
0.867 |
74.133 |
122.70 |
120.88 |
1.50 |
3.6. Formulation of VCO nanoemulsions containing copper peptide
For the preparation of VCO nanoemulsions containing active, copper peptide was incorporated into the optimum VCO nanoemulsion (VCOB) formulation. Table 7 shows the particle size of VCO nanoemulsion containing containing 0.003% (w/w) copper peptide (VCOCP). There were no significant difference observed between the particle size of the optimum formulation and formulation containing active whereby the difference was around 2.00 nm. The resulting VCO nanoemulsions containing copper peptide could be used as cosmeceuticals for anti-aging purpose by minimum particle size value.
Table 7 Response value for optimized formulation and VCO nanoemulsion containing copper peptide
| Response |
VCOB |
VCOCP |
| Particle size (nm) |
122.70 |
120.70 |
3.7. Characterization studies
3.7.1 Particle morphology. This study was carried out in order to confirm that the nanoemulsion droplet was spherical and particle size measured is consistence with Malvern Zetasizer ZS. Fig. 5 shows the TEM image of VCO nanoemulsion containing copper peptide (VCOCP). As depicted in Fig. 5, the droplets of VCOCP were in spherical shape. Apart from that, the particle size measured using TEM is in accordance with the size procured from photon correlation spectroscopy.
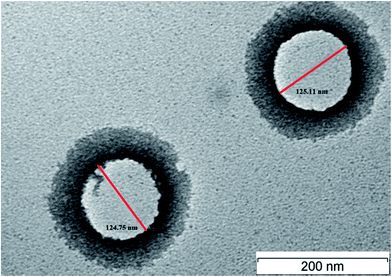 |
| | Fig. 5 TEM image of VCOCP. | |
3.7.2 pH and conductivity studies. The pH of human skin varies from 4–6 depending on individual's age and the area of skin,12 a pH value between four to seven is usually chosen for the aqueous phase of a topical formulation. The pH value of final formulation was in the range 4–6 as depicted in Table 8 showing the suitability of the final formulation to be used topically. Conductivity of the nanoemulsions was investigated to determine whether the nanoemulsions formed were oil in water (O/W) or water in oil (W/O) system. O/W system has been reported to possess high conductivity value as compared to W/O system because water is in the external phase.33 VCO nanomulsion containing copper peptides in this study was detected as O/W nanoemulsions owing to the high conductivity value as presented in Table 8.
Table 8 pH, conductivity and zeta potential of the optimum VCO nanoemulsion containing active matter
| Formulation |
pH |
Conductivity (μS cm−1) |
Zeta potential (mV) |
| VCOCP |
5.68 ± 0.06 |
727.33 ± 4.73 |
32.70 ± 0.71 |
3.7.3 Surface charge analysis. Measurement of zeta potential is important to predict the long term stability of formulations as well as to understand the state of nanoparticle surface.34 Nanoemulsions with high degree of stability are characterized by having zeta potential value higher than +25 mV and lower than −25 mV. The zeta potential value of VCOCP is as presented in Table 8. The low zeta potential (less than −25 mV) indicates the stability of the formulation.
3.7.4 Accelerated stability study. Stability study was carried out due to their predictive ability on the stability of cosmetic products when exposed to a situation which aimed to accelerate changes that may occur under market conditions.35 Therefore, VCOCP was subjected to various extreme storage conditions to evaluate the ability of sample to withstand over a period of time. Under centrifugation, the rate of creaming or sedimentation (phase separation) can be accelerated. Mat Hadzir et al. (2013)36 stated that centrifugation test can be used to predict the shelf life under normal storage condition by observing the creaming or coalescence of the disperse phase. The formulation exhibited no phase separation after centrifugation at 4500 rpm for 15 min. This gives a positive indication on the stability of the formulation, if phase separation occurs; all characteristic of the formulations will be affected. For freeze–thaw cycling test; VCOCP was found to be stable (homogenous) even when temperatures were drastically changed. Sample storage at elevated temperature will increase the movement and collision between the oil droplets thus leads to droplets coalescence. The physical stability of VCOCP at storage temperature of 25 °C and 45 °C for a period of 90 days is shown in Table 9. The nanoemulsion was able to maintain the homogeneity up to 90 days even at temperature 45 °C.
Table 9 Storage stability
| Day (s) |
1 |
30 |
60 |
90 |
| Temperature (°C) |
25 |
45 |
25 |
45 |
25 |
45 |
25 |
45 |
| VCOCP |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
√ |
The outstanding stability of the nanoemulsion could be due to the ability of the nonionic surfactant, T80, to form steric barrier at oil/water interface, preventing droplet collision.5 Apart from that, utilization of polymeric surfactant, PF68, contributed to the thickening and stabilizing properties of the nanoemulsions.37 As stated by Stoke's law, the rate of creaming or sedimentation decreases as the viscosity of continuous phase increases. Incorporation of xanthan gum can enhance the viscosity of the bulk phase and reduces the probability of droplets collision, protecting the emulsion against coalescence.36
3.7.5 Rheological characterization. Determination of rheological properties of semisolid preparations is crucial in manufacturing, pumping, filling, storage and the esthetic use of the final products. The applications as well as acceptance of pharmaceuticals and cosmetics will be dependence on the flow properties of the finished product.12 It is preferable to formulate plastic sample especially for topically applied cosmetic or pharmaceutical products since they must be applied to the skin in layers. Plastic sample has low resistance to flow when applied under high shear conditions and zero flow under stress caused by gravity. Most of pharmaceutical and cosmetic emulsions exhibits both shear thinning properties and pseudoplastic behavior. Fig. 6 shows that the viscosity of the final nanoemulsions decreases with an increase in shear rate, indicating a shear thinning behavior. Shear thinning behavior demonstrates the presence of weak attractive forces (weak elastic gel-like network) between the emulsions droplets.26 Application of shear leads to deformation or reorganization of dispersed phase droplets that had previously aggregated due to the weak attractive forces. Flow behavior index (n) can be used as indicator for emulsion characteristic, with n = 1 indicating a Newtonian fluid, n < 1 indicates a shear thinning fluid and n > 1 indicates a shear thickening fluid. The flow behavior indexes of VCOB and VCOCP (Table 10) were lower than 1 (n < 1) which further support the shear thinning behavior of the nanoemulsions. Addition of active into the optimum formulation did not affect the flow behavior of the VCO nanoemulsion. Both VCOB and VCOCP nanoemulsions exhibited non-linear relationship between shear stress and shear rate, which described their pseudoplastic behavior (Fig. 7). Apart from that, the consistency index, k values of final formulations were quite high as depicted in Table 10. A previous study reported k value of 13.84 (Pa sn) for formulation containing xanthan gum.26 High k value indicates stronger emulsion structure.
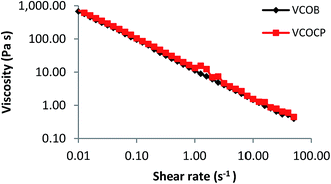 |
| | Fig. 6 Dependence of viscosity on shear rate for VCOB and VCOCP. | |
Table 10 Flow behavior indices (n), consistency coefficients (k), and regression coefficients for VCOB and VCOCP nanoemulsions
| Formulation |
k |
n |
R2 |
| VCOB |
11.625 |
0.110 |
0.9996 |
| VCOCP |
13.758 |
0.117 |
0.9981 |
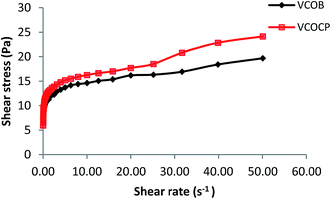 |
| | Fig. 7 Plot of shear stress as a function of shear rate for VCOB and VCOCP. | |
Nanoemulsions have elastic properties whereby the elasticity is the interfacial energy of the dispersed droplets. This elastic property of VCO nanoemulsion containing copper peptide was proven through oscillatory study as shown in Fig. 8. At the linear viscoelastic region (LVR), final formulation demonstrated higher G′ value (storage modulus) compare to G′′ value (loss modulus), indicating elastic behavior of the emulsion systems rather than viscous behavior.38 As can be seen from the Fig. 7, G′ and G′′ are constant at low deformation indicating the sample's structure is undamaged and highly structured. The end of the LVR is marked when G′ value starts to fall and G′′ starts to increase. VCOCP nanoemulsion possessed wide LVR which suggested the high rigidity and stability against phase separation of the emulsion system.39 The excellent stability of the formulation was in agreement with the results obtained from stability study where VCOCP was found to be homogeneous after 3 months storage at temperatures 25 °C and 45 °C.
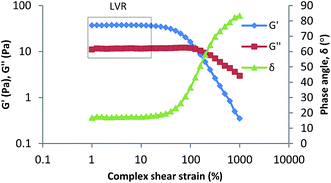 |
| | Fig. 8 Storage modulus (G′), loss modulus (G′′), and phase angle (δ) versus strain amplitude ỳ of VCOCP nanoemulsion. | |
3.8. In vitro permeation study
Utilization of Franz diffusion cell for assessing skin permeability provides the key insights into the relationship between skin, active matter and formulation. Synthetic membrane such as cellulose acetate membrane is used in Franz cell diffusion study to simulate the skin.40 Transport of active matter through this cellulose acetate membrane gives an indication of how much of the active matter is available for absorption.12 The permeation profile of VCO nanoemulsion containing copper peptide is plotted in Fig. 9. The 0.003% w/w copper peptide aqueous solution and aqueous solution containing 40% ethanol were used as controls groups. There was no drug detected at end time point (8 h) for the controls showing the difficulty of transporting the hydrophilic copper peptide through skin barrier even when using 40% ethanol as penetration enhancer. However, when nanoemulsion was used as the carrier vehicle, permeability of copper peptide was significantly improved and release of the active matter increased from 2.26 ± 0.09% at 1 h to 21.89 ± 0.53% after 8 h application as depicted in Fig. 9. Similar result was reported by Tsai et al. (2014)8 where the transport of hydrophilic ropinirole hydrochloride through skin barrier enhanced when using nanoemulsion as carrier vehicle. Tsai et al. (2014)8 added that nanoemulsions could change the surface electrical charge of the drug and enhance its' permeability. Besides the small droplet size, the surfactant used in the nanoemulsion was able to compromise with the barrier function of skin hence facilitate the passage of copper peptide.41 Results from this permeation study show that VCO nanoemulsion has the potential to be used as a carrier vehicle for delivery of copper peptide.
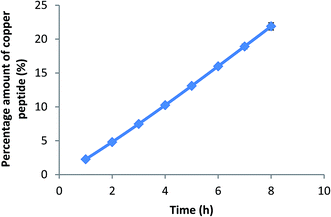 |
| | Fig. 9 In vitro permeation profile for VCO nanoemulsion containing copper peptide. | |
4. Conclusion
This study demonstrated that D-optimal mixture design is an effective tool for performing the optimization of VCO nanoemulsions formulations for topical delivery of copper peptide by combining four different variables: VCO, T80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PF68, xanthan gum and water. The effects of mixture components on particle size were investigated using D-optimal design. The analysis of variance showed the fitness of the model with a high F-value (428.18), a low p-value (<0.0001) and a non-significant lack of fit. The model also showed high coefficient of determination of R2 = 0.9982. This research provides not only the guideline on improving specific desirable characteristic using D-optimal design but also a guideline on the effects of ingredients towards the physical properties of the cosmeceutical products. Most importantly, the particle size value of the optimum formulation showed no significant difference when compared to the particle size of formulation containing copper peptide. The physicochemical properties of VCOCP showed suitability for topical application with excellent physical stability against phase separation. In addition, the permeation of copper peptide was enhanced through utilization of nanoemulsion as carrier vehicle.
PF68, xanthan gum and water. The effects of mixture components on particle size were investigated using D-optimal design. The analysis of variance showed the fitness of the model with a high F-value (428.18), a low p-value (<0.0001) and a non-significant lack of fit. The model also showed high coefficient of determination of R2 = 0.9982. This research provides not only the guideline on improving specific desirable characteristic using D-optimal design but also a guideline on the effects of ingredients towards the physical properties of the cosmeceutical products. Most importantly, the particle size value of the optimum formulation showed no significant difference when compared to the particle size of formulation containing copper peptide. The physicochemical properties of VCOCP showed suitability for topical application with excellent physical stability against phase separation. In addition, the permeation of copper peptide was enhanced through utilization of nanoemulsion as carrier vehicle.
Acknowledgements
The financial support from Ministry of Higher Education Malaysia (MOHE), Mybrain15 is gratefully acknowledged. The authors would also like to acknowledge Universiti Putra Malaysia for the facilities provided throughout this research.
References
- L. Pickart and A. Margolina, J. Aging Res. Clin. Pract., 2012, 1, 13–15 Search PubMed.
- P. K. Mukherjee, N. Maity, N. K. Nema and B. K. Sarkar, Phytomedicine, 2011, 19, 64–73 CrossRef CAS PubMed.
- S. Duraivel, S. A. Shaheda, S. R. Basha, S. E. Pasha and S. Jilani, IOSR J. Pharm. Biol. Sci., 2014, 9, 58–73 Search PubMed.
- P. Leelapornpisid, S. Chansakaow, S. Na-boonlong and P. Jantrawut, Int. J. Pharm. Pharm. Sci., 2014, 6, 313–314 Search PubMed.
- C. L. Ngan, M. Basri, F. F. Lye, H. R. Fard Masoumi, M. Tripathy, R. Abedi Karjiban and E. Abdul-Malek, Ind. Crops Prod., 2014, 59, 309–317 CrossRef CAS.
- L. Pickart and A. Margolina, SOFW J., 2010, 136, 1–9 Search PubMed.
- J. H. Hamman, G. M. Enslin and A. F. Kotz, Drug Delivery, 2005, 19, 165–177 CAS.
- M. J. Tsai, Y. S. Fu, Y. H. Lin, B. H. Yaw and P. C. Wu, PLoS One, 2014, 9, 1–7 CAS.
- C. Solans, P. Izquierdo, J. Nolla, N. Azemar and M. J. Garcia-celma, Curr. Opin. Colloid Interface Sci., 2005, 10, 102–110 CrossRef CAS.
- S. Setya, S. Talegoankar and B. K. Razdan, World J. Pharm. Pharm. Sci., 2014, 3, 2214–2228 Search PubMed.
- T. Tadros, P. Izquierdo, J. Esquena and C. Solans, Adv. Colloid Interface Sci., 2004, 108–109, 303–318 CrossRef CAS PubMed.
- E. S. Mahdi, A. Mohd Noor, M. H. Sakeena, G. Z. Abdullah, M. F. Abdulkarim and M. Abdul Sattar, Int. J. Nanomed., 2011, 6, 2499–2512 CrossRef CAS PubMed.
- D. C. Sabrina Matos, N. Carolina Montanheiro, F. Caroline Louise, L. Renata Calegari, R. Gabriela, B. Ismael Casagrande, O. Paulo José and B. Pedro Luiz Manique, Ind. Crops Prod., 2013, 49, 278–285 CrossRef.
- H. R. Fard Masoumi, M. Basri, A. Kassim, D. K. Abdullah, Y. Abdollahi, S. S. Abdul Ghani and M. Rezaee, Sci. World J., 2013, 1–9 CrossRef PubMed.
- B. S. Kaith, R. Sharma, S. Kalia and M. S. Bhatti, RSC Adv., 2014, 4, 40339–40344 RSC.
- M. Porras, C. Solans, C. Gonzalez and J. M. Gutiérrez, Colloids Surf., A, 2008, 324, 181–188 CrossRef CAS.
- S. Rebolleda, M. T. Sanz, J. M. Benito, S. Beltrán, I. Escudero and M. L. G. San-Jose, Food Chem., 2015, 167, 16–23 CrossRef CAS PubMed.
- P. Sathishkumar, A. Mythili, T. Hadibarata, R. Jayakumar, M. S. Kantimathi, T. Palvannan, M. Ponraj, M. R. Salim and A. R. Mohd Yusoff, RSC Adv., 2014, 4, 11689–11697 RSC.
- T. Roldán-Carrillo, X. Martínez-García, I. Zapata-Peñasco, G. Castorena-Cortés, J. Reyes-Avila, M. Mayol-Castillo and P. Olguín-Lora, Colloids Surf., B, 2011, 86, 384–389 CrossRef PubMed.
- M. M. Ba-Abbad, A. A. H. Kadhum, A. B. Mohamad, M. S. Takriff and K. Sopian, J. Ind. Eng. Chem., 2013, 19, 99–105 CrossRef CAS.
- F. P. Borhan, S. S. Abdul Ghani and R. Shamsuddin, Sci. World J., 2014, 2014, 173979, DOI:10.1155/2014/173979.
- M. S. Kamble, V. G. Borwandkar, S. S. Bodade, P. P. Aute, P. D. Chaudhari and A. V. Bhosale, J. Biomed. Pharm. Res., 2013, 2, 100–108 Search PubMed.
- N. Kumar and S. Goindi, Eur. J. Pharm. Sci., 2015, 67, 97–112 CrossRef CAS PubMed.
- N. Kamairudin, S. S. Abdul Ghani, H. R. Fard Masoumi and P. Hashim, Molecules, 2014, 19, 16672–16683 CrossRef PubMed.
- H. R. Fard Masoumi, A. Kassim, M. Basri and D. K. Abdullah, Molecules, 2011, 16, 4672–4680 CrossRef PubMed.
- S. H. Ng, M. Basri, M. B. Abd Rahman, R. N. Z. Raja Abdul Rahman, A. B. Salleh and Z. Ismail, J. Dispersion Sci. Technol., 2010, 32, 1428–1433 Search PubMed.
- M. Rezaee, M. Basri, R. N. Z. Raja Abdul Rahman, A. B. Salleh, N. Chaibakhsh and R. Abedi Karjiban, Int. J. Nanomed., 2014, 9, 534–548 Search PubMed.
- D. de O. Dias, M. Colombo, R. G. Kelmann, S. Kaiser, L. G. Lucca, H. F. Teixeira, R. P. Limberger, V. F. Veiga Jr and L. S. Koester, Ind. Crops Prod., 2014, 59, 154–162 CrossRef CAS.
- S. H. Musa, M. Basri, H. R. Fard Masoumi, R. Abedi Karjiban, E. Abd Malek, H. Basri and A. F. Shamsuddin, Colloids Surf., B, 2013, 112, 113–119 CrossRef CAS PubMed.
- C. Qian and D. J. McClements, Food Hydrocolloids, 2011, 25, 1000–1008 CrossRef CAS.
- V. Krstonošić, L. Dokić, I. Nikolić and M. Milanović, Food Hydrocolloids, 2015, 45, 9–17 CrossRef.
- V. Krstonošić, L. Dokić, P. Dokić and T. Dapčević, Food Hydrocolloids, 2009, 23, 2212–2218 CrossRef.
- S. Da Costa, M. Basri, N. Shamsudin and H. Basri, J. Chem., 2014, 2014, 748680, DOI:10.1155/2014/748680.
- S. Ma, F. Chen, X. Ye, Y. Dong, Y. Xue, H. Xu, W. Zhang, S. Song, L. Ai, N. Zhang and W. Pan, Int. J. Nanomed., 2013, 8, 4045–4052 Search PubMed.
- R. C. De Azevedo Ribeiro, S. M. A. G. Barreto, E. A. Ostrosky, P. A. da Rocha-Filho, L. M. Veríssimo and M. Ferrari, Molecules, 2015, 20, 2492–2509 CrossRef PubMed.
- N. Mat Hadzir, M. Basri, M. B. Abdul Rahman, A. B. Salleh, R. N. Z. Raja Abdul Rahman and H. Basri, AAPS PharmSciTech, 2013, 14, 456–463 CrossRef CAS PubMed.
- B. S. X. Teo, M. Basri, M. R. S. Zakaria, A. B. Salleh, R. N. Z. R. A. Rahman and M. B. A. Rahman, J. Nanobiotechnol., 2010, 8, 1–11 CrossRef PubMed.
- S. P. Ng, O. M. Lai, F. Abas, H. K. Lim and C. P. Tan, Food Res. Int., 2014, 64, 919–930 CrossRef CAS.
- C. L. Ngan, M. Basri, F. F. Lye, H. R. Fard Masoumi, M. Tripathy, R. Abedi Karjiban and E. Abdul-Malek, Int. J. Nanomed., 2014, 4, 4375–4386 CrossRef PubMed.
- S. F. Ng, J. Rouse, D. Sanderson and G. Eccleston, Pharmaceutics, 2010, 2, 209–223 CrossRef CAS.
- J. Leanpolchareanchai, K. Padois, F. Falson, R. Bavovada and P. Pithayanukul, Molecules, 2014, 19, 17107–17129 CrossRef PubMed.
|
| This journal is © The Royal Society of Chemistry 2016 |
Click here to see how this site uses Cookies. View our privacy policy here. ![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) Pluronic PF68 (10–15%, w/w), xanthan gum (0.5–1.0%, w/w) and water (64.008–79.291), towards particle size as a response were studied. D-optimal mixture design analysis demonstrated that the variation in particle size as a response could be expressed as a quadratic function of the main components of the emulsion. Analysis of variance (ANOVA) showed that the quadratic polynomial model sufficiently fits the experimental data. The best combination of the composition factors that gave the optimum particle size was found to be VCO (10%, w/w), T80
Pluronic PF68 (10–15%, w/w), xanthan gum (0.5–1.0%, w/w) and water (64.008–79.291), towards particle size as a response were studied. D-optimal mixture design analysis demonstrated that the variation in particle size as a response could be expressed as a quadratic function of the main components of the emulsion. Analysis of variance (ANOVA) showed that the quadratic polynomial model sufficiently fits the experimental data. The best combination of the composition factors that gave the optimum particle size was found to be VCO (10%, w/w), T80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PF68 (15%, w/w), xanthan gum (0.867%, w/w) and water (74.133%, w/w). Under these optimum compositions, the actual particle size showed good agreement with predicted particle size with a residual standard error (RSE) less than 2%. The formulation containing copper peptide was successfully prepared with a particle size value of 120.7 nm, which was consistent with the result obtained by TEM. The final formulation also showed good conductivity and pH values as well as stability against phase separation. A rheology study demonstrates that the VCO nanoemulsion containing copper peptide exhibited shear thinning and pseudoplastic properties and showed more elastic rather than viscous characteristics due to the presence of a large linear viscoelastic region. Permeability test results showed that copper peptide in aqueous solution with and without penetration enhancer cannot be transported through the cellulose acetate membrane after 8 h application. The nanoemulsion increased the permeability of copper peptide with 21.89 ± 0.53% active matter release after 8 h application.
PF68 (15%, w/w), xanthan gum (0.867%, w/w) and water (74.133%, w/w). Under these optimum compositions, the actual particle size showed good agreement with predicted particle size with a residual standard error (RSE) less than 2%. The formulation containing copper peptide was successfully prepared with a particle size value of 120.7 nm, which was consistent with the result obtained by TEM. The final formulation also showed good conductivity and pH values as well as stability against phase separation. A rheology study demonstrates that the VCO nanoemulsion containing copper peptide exhibited shear thinning and pseudoplastic properties and showed more elastic rather than viscous characteristics due to the presence of a large linear viscoelastic region. Permeability test results showed that copper peptide in aqueous solution with and without penetration enhancer cannot be transported through the cellulose acetate membrane after 8 h application. The nanoemulsion increased the permeability of copper peptide with 21.89 ± 0.53% active matter release after 8 h application.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PF68 (40
PF68 (40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1) and VCO while for the aqueous phase, xanthan gum was added into deionized water. The oil and aqueous phase were initially heated separately until all ingredients dissolved. Using homogenizer, the aqueous phase was added drop wise into the oil phase until completed. The final mixture was homogenized for 3 hours using overhead stirrer. The emulsion obtained was further subjected to high shear homogenizer for 10 minutes at 15
1) and VCO while for the aqueous phase, xanthan gum was added into deionized water. The oil and aqueous phase were initially heated separately until all ingredients dissolved. Using homogenizer, the aqueous phase was added drop wise into the oil phase until completed. The final mixture was homogenized for 3 hours using overhead stirrer. The emulsion obtained was further subjected to high shear homogenizer for 10 minutes at 15![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm. Phenonip (0.7% w/w) was added as anti-microbial agent.
000 rpm. Phenonip (0.7% w/w) was added as anti-microbial agent.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm. Phenonip (0.7%, w/w) was added as anti-microbial agent.
000 rpm. Phenonip (0.7%, w/w) was added as anti-microbial agent.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PF68 (B), xanthan gum (C) and water (D) on response taken as particle size. For D-optimal design, there are restrictions on the component, Xj with general form as follows:
PF68 (B), xanthan gum (C) and water (D) on response taken as particle size. For D-optimal design, there are restrictions on the component, Xj with general form as follows:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PF68, B
PF68, B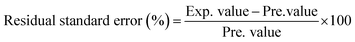
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PF68 and xanthan gum) were determined based on the data obtained while the level of water was determined by the software. The VCO based emulsions showed the size in the range of 100 nm–350 nm with composition of VCO, T80
PF68 and xanthan gum) were determined based on the data obtained while the level of water was determined by the software. The VCO based emulsions showed the size in the range of 100 nm–350 nm with composition of VCO, T80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PF68 and xanthan gum were 10–20% w/w, 10–15% w/w and 0.5–1.0% w/w respectively. Table 1 shows the values of variable used in the formulation. The upper- and lower-bound on the component proportion are needed for the construction and analysis of mixture design.
PF68 and xanthan gum were 10–20% w/w, 10–15% w/w and 0.5–1.0% w/w respectively. Table 1 shows the values of variable used in the formulation. The upper- and lower-bound on the component proportion are needed for the construction and analysis of mixture design.
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 882.69C + 314.20D − 5761.57AB + 19
882.69C + 314.20D − 5761.57AB + 19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 896.16AC − 113.91AD + 46
896.16AC − 113.91AD + 46![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 667.83BC − 3563.50BD + 11
667.83BC − 3563.50BD + 11![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 497.74CD
497.74CD

![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 962.41
962.41![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 552.04
552.04![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 552.04
552.04![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 131.02
131.02![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 131.02
131.02![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 883.69
883.69![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 896.16
896.16![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 667.83
667.83![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 497.74
497.74![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PF68), showed an opposite effect whereby the particle size decreased as the amount of T80
PF68), showed an opposite effect whereby the particle size decreased as the amount of T80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PF68 increased. This observation has been previously reported5,29,30 whereby the particle size decreased due to more emulsifier present to cover any new droplet surfaces formed during homogenization and leads to the reduction of the interfacial tension between oil and water. Increasing amount of emulsifier decreased the interfacial tension followed by reduction of Laplace pressure and stress required for droplet deformation. Similar trend was reported by Rebolleda et al., (2015).17 The plots demonstrated that a smaller particle size can be observed at high concentration of T80
PF68 increased. This observation has been previously reported5,29,30 whereby the particle size decreased due to more emulsifier present to cover any new droplet surfaces formed during homogenization and leads to the reduction of the interfacial tension between oil and water. Increasing amount of emulsifier decreased the interfacial tension followed by reduction of Laplace pressure and stress required for droplet deformation. Similar trend was reported by Rebolleda et al., (2015).17 The plots demonstrated that a smaller particle size can be observed at high concentration of T80![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PF68 and low concentration of VCO.
PF68 and low concentration of VCO.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PF68) %
PF68) %![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PF68, xanthan gum and water of 10% w/w, 15% w/w, 0.867% w/w and 74.133% w/w respectively as tabulate in Table 6. The predicted particle size given by the software for the optimum formulation was 120.882 nm. Nanoemulsions with particle size more than 100 nm (less than 200 nm) are considered good for cosmetic applications as those obtained by Ngan et al. (2014).5
PF68, xanthan gum and water of 10% w/w, 15% w/w, 0.867% w/w and 74.133% w/w respectively as tabulate in Table 6. The predicted particle size given by the software for the optimum formulation was 120.882 nm. Nanoemulsions with particle size more than 100 nm (less than 200 nm) are considered good for cosmetic applications as those obtained by Ngan et al. (2014).5
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PF68) %
PF68) %![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) PF68, xanthan gum and water. The effects of mixture components on particle size were investigated using D-optimal design. The analysis of variance showed the fitness of the model with a high F-value (428.18), a low p-value (<0.0001) and a non-significant lack of fit. The model also showed high coefficient of determination of R2 = 0.9982. This research provides not only the guideline on improving specific desirable characteristic using D-optimal design but also a guideline on the effects of ingredients towards the physical properties of the cosmeceutical products. Most importantly, the particle size value of the optimum formulation showed no significant difference when compared to the particle size of formulation containing copper peptide. The physicochemical properties of VCOCP showed suitability for topical application with excellent physical stability against phase separation. In addition, the permeation of copper peptide was enhanced through utilization of nanoemulsion as carrier vehicle.
PF68, xanthan gum and water. The effects of mixture components on particle size were investigated using D-optimal design. The analysis of variance showed the fitness of the model with a high F-value (428.18), a low p-value (<0.0001) and a non-significant lack of fit. The model also showed high coefficient of determination of R2 = 0.9982. This research provides not only the guideline on improving specific desirable characteristic using D-optimal design but also a guideline on the effects of ingredients towards the physical properties of the cosmeceutical products. Most importantly, the particle size value of the optimum formulation showed no significant difference when compared to the particle size of formulation containing copper peptide. The physicochemical properties of VCOCP showed suitability for topical application with excellent physical stability against phase separation. In addition, the permeation of copper peptide was enhanced through utilization of nanoemulsion as carrier vehicle.








