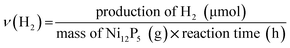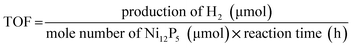Efficient visible-light-induced hydrogen evolution from water splitting using a nanocrystalline nickel phosphide catalyst†
Weiming Wu,
Xianyang Yue,
Xiao-Yuan Wu and
Can-Zhong Lu*
Key Laboratory of Design and Assembly of Functional Nanostructures, Fujian Institute of Research on the Structure of Matter, Chinese Academy of Sciences, Fuzhou 350002, P. R. China. E-mail: czlu@fjirsm.ac.cn; Fax: +86 59183714946; Tel: +86 591837705794
First published on 25th February 2016
Abstract
Nanocrystalline nickel phosphide (Ni12P5) was successfully synthesized by a simple hydrothermal method via using NiCl2 and red phosphorus as raw materials. The crystal structure, morphology and surface chemical compositions of the as-prepared sample were characterized by X-ray diffraction, scanning electron microscope and X-ray photoelectron spectroscopy techniques, respectively. Its catalytic activity for the hydrogen evolution from water was investigated under visible light irradiation (λ ≥ 420 nm) with fluorescein sodium as the photosensitizer and triethanolamine as the sacrificial electron donor, respectively. The results indicated that the Ni12P5 sample showed high catalytic activity (10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 760 μmol h−1 g−1, TOF = 9.3 h−1) and good stability (15 h) in the present system. The electrochemical results revealed that Ni12P5 had a high cathodic current and small charge transfer resistance, which further suggested Ni12P5 indeed could efficiently catalyze the evolution of hydrogen.
760 μmol h−1 g−1, TOF = 9.3 h−1) and good stability (15 h) in the present system. The electrochemical results revealed that Ni12P5 had a high cathodic current and small charge transfer resistance, which further suggested Ni12P5 indeed could efficiently catalyze the evolution of hydrogen.
1. Introduction
Hydrogen is considered as the ideal clean green fuel. Its large-scale application can solve the energy and environment issues at a global level. Three component systems for light-induced hydrogen evolution have received considerable attention in the field of chemistry,1–3 because hydrogen can be efficiently produced from water by utilizing visible light in these systems. A typical three component system consists of a catalyst, photosensitizer, and sacrificial electron donor. Generally, nobel metal Pt is often used as the catalyst in the previous studies. For real wide-spread application, catalysts made from elementally abundant and less expensive materials are urgently required. Earth-abundant nickel based homogeneous systems have received special attention in recent years, such as nickel-phosphine complex,4 nickel-thiolate complexes5,6 and nickel substituted polyoxometalates.7,8 These nickel based homogeneous systems can efficiently split water into H2 by utilizing visible light. However, considering to the reusability of catalysts, discovery of heterogeneous catalyst based on earth-abundant nickel element would be highly useful.Previous works suggest nickel phosphides exhibit high catalytic activities in electrochemical hydrogen generation.9–12 In 2014, Fu et al. have found that Ni2P can efficiently catalyze the evolution of hydrogen from water splitting under visible light irradiation.13 Recently, Sun and his co-workers further confirm that Ni2P is an active heterogeneous catalyst for the visible-light-induced H2 evolution from water splitting.14 These works provide a new way to construct visible-light-induced hydrogen production systems, which should not only be efficiently, but also be came from abundant sources. However, as far as we known, nickel phosphides are generally obtained by using toxic chemicals (such as white phosphorus14 or organophosphorus compounds9,10,13,15) or via rigorous reaction conditions (such as oxygen-free environment9–13,15 or high-temperature calcination11,12).
Herein, nanocrystalline Ni12P5 was simply prepared by a hydrothermal method via using NiCl2 and red phosphorus as raw materials. Its catalytic activity and stability for the hydrogen evolution from water were evaluated under visible light irradiation (λ ≥ 420 nm) with fluorescein sodium as the photosensitizer and triethanolamine as the sacrificial electron donor, respectively. Furthermore, the electrochemical technique was introduced to investigate the charge transfer process of Ni12P5. Our results may allow us to provide a simple and feasible approach for the preparation of metal phosphides, and highlight their promising application potential in visible-light-induced hydrogen evolution from water.
2. Results and discussion
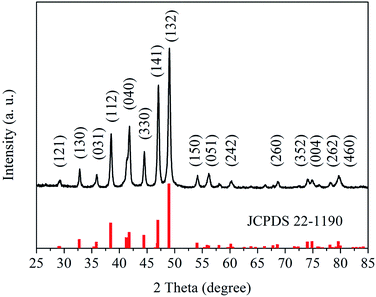
Fig. 1 shows the X-ray diffraction (XRD) pattern of the as-prepared sample. All diffraction peaks of the pattern are well indexed to cubic nickel phosphide (Ni12P5, JCPDS card no. 22-1190). The main peaks at 2θ value about 29.15, 32.80, 35.91, 38.36, 41.67, 44.45, 47.06, 49.15, 54.03, 56.13, 60.30, 68.67, 73.90, 75.12, 78.07 and 79.82° can be assign to the diffraction peaks of the (121), (130), (031), (112), (040), (330), (141), (132), (150), (051), (242), (260), (352), (004), (262) and (460) planes, respectively. Furthermore, the morphology the as-prepared sample has been characterized by scanning electron microscope (SEM) (see Fig. S1 in ESI†). It is found that the sample prepared by the hydrothermal method is packed by Ni12P5 nanoparticles (20–50 nm). These results suggest that pure Ni12P5 nanocrystalline can be obtained by the hydrothermal method in this work.Surface chemical compositions of the as-prepared sample have also been studied by X-ray photoelectron spectroscopy (XPS). The XPS survey spectrum is shown in Fig. S2.† All peaks on the survey spectra can be ascribed to Ni, P, C and O elements, and no peaks with others elements are observed. The C and O elements come from the hydrocarbon contaminants which commonly exist for XPS. Therefore, the as-prepared sample is a pure Ni12P5 sample, which is in agreement with the analysis result of XRD. Fig. 2 shows the high-resolution XPS spectra of Ni 2p3/2 and P 2p for the as-prepared sample. Three binding energy peak at 853.2, 856.0 and 861.2 eV are observed in the high-resolution XPS spectrum of Ni 2p3/2 (see Fig. 2a). The peak at 853.2 eV can be attributed to the Ni species in Ni12P5, and the binding energy value is close to that of metallic Ni (852.8 eV).16 The result indicates that the Ni species in Ni12P5 have a weakly positive charge (Niδ+, 0 < δ < 2).10 As shown in Fig. 2b, there are two doublets in the high-resolution XPS spectrum of P 2p. The doublet at 129.8 eV can be assigned to the P in the nickel phosphide. It suggests that the related P species possess a weakly negative charge (Pδ−, 0 < δ < 1), because the binding energy of this doublet is very closed to that of elemental P.10,17 Furthermore, the additional peaks at 856.0 and 861.2 eV in the high-resolution XPS spectrum of Ni 2p3/2 and the doublet at 132.9 eV in the high-resolution XPS spectrum of P 2p may come from the nickel phosphate formed on the surface of Ni12P5 due to the exposure of the sample to air.10,18 The analysis results of XPS reveal that there are weakly charged species in the as-prepared sample, including positively charged Ni (Niδ+, 0 < δ < 2) and negatively charged P (Pδ−, 0 < δ < 1).
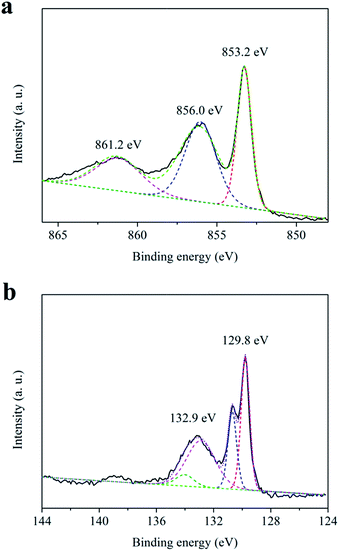 | ||
| Fig. 2 High-resolution XPS spectra of (a) Ni 2p3/2 and (b) P 2p for the as-prepared sample (solid line: experimental data; dash line: curve fitting). | ||
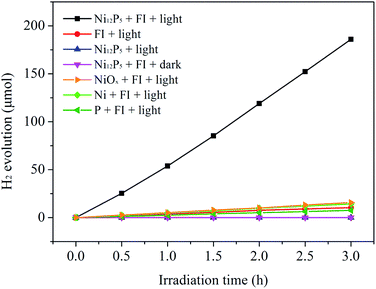
Catalytic activity of the Ni12P5 sample for the H2 evolution from water splitting has been evaluated under visible light irradiation (λ ≥ 420 nm) with fluorescein sodium (FI) as the photosensitizer and triethanolamine (TEOA) as the sacrificial electron donor, respectively. As shown in Fig. 3, the as-prepared sample exhibits highly efficient activity for the visible-light-driven H2 evolution from water splitting. The rate of the H2 evolution is about 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 760 μmol h−1 g−1, corresponding to a turnover frequency (TOF) of 9.3 h−1. Control experiments show that the hydrogen yield can be ignored in the absence of the light, Ni12P5 or FI. This suggests that the reaction system is a typical three component system for visible-light-induced hydrogen production. In order to evaluate the catalytic efficiency of the catalyst, the catalytic activities of metallic Ni, elemental P and NiO (obtained by calcining Ni(NO3)2·6H2O at 400 °C for 2 h, XRD pattern see Fig. S3†) for the H2 evolution from water splitting have also been evaluated under same conditions. It is found that the catalytic activity of the as-prepared sample is much higher than those of the metallic Ni (964 μmol h−1 g−1), elemental P (514 μmol h−1 g−1) and NiO (1044 μmol h−1 g−1). Although the commercial Pt/C catalyst (Aladdin Co.) shows a higher activity (∼19
760 μmol h−1 g−1, corresponding to a turnover frequency (TOF) of 9.3 h−1. Control experiments show that the hydrogen yield can be ignored in the absence of the light, Ni12P5 or FI. This suggests that the reaction system is a typical three component system for visible-light-induced hydrogen production. In order to evaluate the catalytic efficiency of the catalyst, the catalytic activities of metallic Ni, elemental P and NiO (obtained by calcining Ni(NO3)2·6H2O at 400 °C for 2 h, XRD pattern see Fig. S3†) for the H2 evolution from water splitting have also been evaluated under same conditions. It is found that the catalytic activity of the as-prepared sample is much higher than those of the metallic Ni (964 μmol h−1 g−1), elemental P (514 μmol h−1 g−1) and NiO (1044 μmol h−1 g−1). Although the commercial Pt/C catalyst (Aladdin Co.) shows a higher activity (∼19![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 340 μmol h−1 g−1, see Fig. S4†) than the Ni12P5 sample, the noble metal Pt is the active species in this catalyst for the visible-light-induced H2 evolution. For real wide-spread application, catalysts made from elementally abundant and less expensive materials are urgently required. Ni12P5 is composed of earth-abundant Ni and P elements. Therefore, it can be an attractive material as a candidate of the cheap catalysts for the efficient visible-light-induced H2 evolution from water.
340 μmol h−1 g−1, see Fig. S4†) than the Ni12P5 sample, the noble metal Pt is the active species in this catalyst for the visible-light-induced H2 evolution. For real wide-spread application, catalysts made from elementally abundant and less expensive materials are urgently required. Ni12P5 is composed of earth-abundant Ni and P elements. Therefore, it can be an attractive material as a candidate of the cheap catalysts for the efficient visible-light-induced H2 evolution from water.
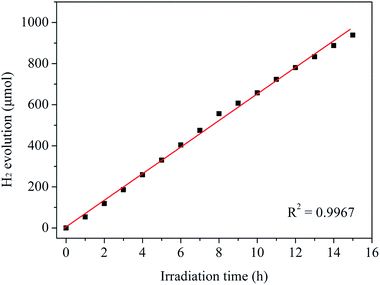
It is widely regarded that the stability of a catalyst is a very important factor for its practical applications. Therefore, the stability of Ni12P5 for the visible-light-induced H2 evolution from water splitting has been carried out. As shown in Fig. 4, the production rate of hydrogen does not obviously decrease after 15 h of visible light irradiation. Furthermore, the crystal structure and surface chemical compositions of the as-prepared sample after the reaction have been studied by XRD and XPS techniques, respectively. XRD patterns (see Fig. S5†) indicate the crystal structure of Ni12P5 catalyst is intact before and after the reaction. The XRD patterns of the catalyst can be well indexed to cubic nickel phosphide (Ni12P5, JCPDS card no. 22-1190). And the analysis result of XPS reveals that the binding energies of Ni 2p3/2 and P 2p on the surface of the sample have no obvious changes in their position after the catalytic test (see Fig. S6†). These results suggest that the catalyst exhibits good stability in the present system.
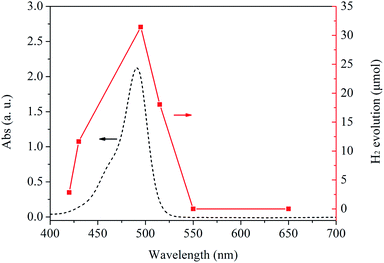
As shown in Fig. 5, the dependence of the wavelength of the incident light on the H2 evolution from water splitting has also been investigated in this work. It is found that the hydrogen yield match well with the photon absorption characteristics of FI (see the dash line in Fig. 5). The result confirms the reaction is indeed induced by the light excitation of FI as a photosensitizer, and H2 comes from the visible-light-induced water splitting on the Ni12P5 catalyst.
Electrochemical measurements have been conducted in a typical three-electrode cell to get further insight into the hydrogen evolution reaction process of Ni12P5 nanocrystalline. Fig. 6a shows the polarization curve of the as-prepared sample loaded on a fluorine-doped tin oxide (FTO) transparent conductive film glass, which is measured in the N2-saturated 0.1 M Na2SO4 with a scan rate of 100 mV s−1. The metallic Ni, elemental P and NiOx electrodes prepared with similar contents have also been examined for comparison. It is obvious that the Ni12P5 sample shows the highest cathodic current in the range of −1.0 to 0 V vs. saturated calomel electrode (SCE) as compared with the metallic Ni, elemental P and NiOx, indicating Ni12P5 can efficiently catalyze the evolution of H2. Furthermore, the charge transfer rate in the dark has been studied by electrochemical impedance spectroscopy (EIS; Fig. 6b) and the expected semicircular Nyquist plots for different sample, with a significantly decreased diameter for Ni12P5, have been obtained. It is generally accepted that a small diameter gives rise to fast charge transfer kinetics.12,19 This result implies that the Ni12P5 sample indeed is an efficient catalyst for the visible-light-induced H2 evolution from water in this work.
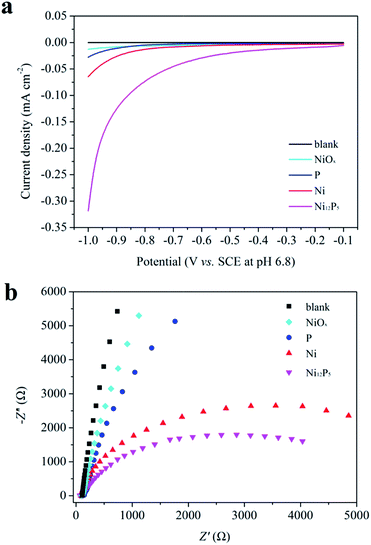 | ||
| Fig. 6 (a) Polarization curves of different samples load on FTO transparent conductive film glasses and (b) Nyquist plots of EIS data measured at −1.0 V vs. SCE. | ||
3. Conclusions
Pure Ni12P5 nanocrystalline was successfully synthesized by a simple hydrothermal method via using NiCl2 and red phosphorus as raw materials. The crystal structure, morphology and surface chemical compositions of the as-prepared sample were verified by XRD, SEM and XPS techniques, respectively. It could be used as an elementally abundant and less expensive catalyst for the efficient visible-light-induced H2 evolution from water with FI as the photosensitizer and TEOA as the sacrificial electron donor, respectively. The rate of the H2 evolution was about 10![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 760 μmol h−1 g−1, corresponding to a TOF of 9.3 h−1. And the production rate of hydrogen did not obviously decrease after 15 h of visible light irradiation. The analysis results of the XRD and XPS showed that the crystal structure and surface chemical compositions of the as-prepared sample had no obvious change. These results revealed that it exhibited good stability in the present system. Furthermore, the electrochemical results indicated that Ni12P5 had high cathodic current and small charge transfer resistance, suggesting Ni12P5 indeed could efficiently catalyze the evolution of hydrogen.
760 μmol h−1 g−1, corresponding to a TOF of 9.3 h−1. And the production rate of hydrogen did not obviously decrease after 15 h of visible light irradiation. The analysis results of the XRD and XPS showed that the crystal structure and surface chemical compositions of the as-prepared sample had no obvious change. These results revealed that it exhibited good stability in the present system. Furthermore, the electrochemical results indicated that Ni12P5 had high cathodic current and small charge transfer resistance, suggesting Ni12P5 indeed could efficiently catalyze the evolution of hydrogen.
4. Experimental section
4.1. Preparation
Ni12P5 nanocrystalline was prepared by a modified hydrothermal method.20,21 Typical, 0.237 g of NiCl2·6H2O (1.0 mmol, Sinopharm chemical reagent Co.) and 0.930 g of red phosphorus (30.0 mmol, Beijing hongxing chemical industry factory) were added into 50 mL of distilled water in a 100 mL Teflon liner. Then, the pH value of the suspension was adjusted to 13 by dropping 2 M NaOH aqueous solution (∼3.0 mL). After stirring for 30 min (600 rpm), the Teflon liner was sealed in a stainless steel autoclave and maintained at 200 °C for 12 h. Black Ni12P5 powders were collected, washed with distilled water and absolute ethyl alcohol several times, and dried in vacuum at 60 °C.4.2. Characterization
XRD patterns were collected on a Miniflex 600 X-ray diffractometer (Rigaku Co.) with Cu Kα radiation. The data were recorded in a 2θ range of 25–85°. SEM image was obtained on a JEOL JSM6700 field emission scanning electron microscope at an accelerating voltage of 10 kV. XPS measurement was carried out by using a ESCALAB 250Xi X-ray photoelectron spectrometer (Thermo fisher scientific Co.) equipped with Al Kα X-ray source. The binding energy calibration of the spectra was referred to C1s peak located at 284.6 eV for the analysis. Electrochemical measurements were performed in a typical three electrode cell, using Pt wire and SCE electrode as counter electrode and reference electrode, respectively. The working electrode was prepared on a FTO transparent conductive film glass (work area: 0.36 cm2). The electrolyte was a 0.1 mol L−1 Na2SO4 (pH = 6.8) aqueous solution and was purged with nitrogen gas for 30 min prior to the measurements. The electrochemical experiments were taken on a CHI660d workstation.4.3. H2 evolution from water splitting
H2 evolution from water splitting was performed in a closed gas-circulating system (Labsolar-IIIAG, Perfectlight Co.) equipped with an external-irradiation Pyrex reaction cell. 5 mg of Ni12P5 powders and 20 mg of FI (Aladdin Co.) were mixed in distilled water (80 mL) containing TEOA (10 vol%, Sinopharm chemical reagent Co.). Before the reaction, the system was evacuated by a vacuum pump (∼−101.0 kPa) for 10 min. Then, the reaction was carried out by irradiating the suspension using a 300 W Xe lamp with a cut-off filter (λ ≥ 420 nm). The production of hydrogen was analyzed by a gas chromatograph (GC2014C, Shimazu Co., TCD, molecular sieve 5A column, argon carrier).The rate of the H2 evolution (ν(H2)) and turnover frequency (TOF) were calculated by using the following equations:
Acknowledgements
This work was supported by the 973 Program (2010CB933501 and 2012CB821705), the Chinese Academy of Sciences (KJCX2-YW-319 and KJCX2-EW-H01), the National Natural Science Foundation of China (21373221, 21221001, 91022008, 91122027 and 51172232), the Natural Science Foundation of Fujian Province, China (2011HZ0001-1, 2012J06006 and 2006L2005), and the China Postdoctoral Science Foundation (2015M570561).Notes and references
- J. Kiwi and M. Grätzel, Nature, 1979, 281, 657 CrossRef CAS.
- H. Ozawa, M. Haga and K. Sakai, J. Am. Chem. Soc., 2006, 128, 4926 CrossRef CAS PubMed.
- P. Du, J. Schneider, P. Jarosz and R. Eisenberg, J. Am. Chem. Soc., 2006, 128, 7726 CrossRef CAS PubMed.
- M. P. McLaughlin, T. M. McCormick, R. Eisenberg and P. L. Holland, Chem. Commun., 2011, 47, 7989 RSC.
- Z. Han, W. R. McNamara, M. S. Eum, P. L. Holland and R. Eisenberg, Angew. Chem., Int. Ed., 2012, 51, 1667 CrossRef CAS PubMed.
- Z. Han, L. Shen, W. W. Brennessel, P. L. Holland and R. Eisenberg, J. Am. Chem. Soc., 2013, 135, 14659 CrossRef CAS PubMed.
- H. Lv, J. Song, Y. V. Geletii, J. W. Vickers, J. M. Sumliner, D. G. Musaev, P. Kogerler, P. F. Zhuk, J. Bacsa, G. Zhu and C. L. Hill, J. Am. Chem. Soc., 2014, 136, 9268 CrossRef CAS PubMed.
- W. Wu, X. Y. Wu, L. Zhang, J. Xiong, L. Wu and C. Z. Lu, Int. J. Hydrogen Energy, 2015, 41, 139 CrossRef.
- E. J. Popczun, J. R. McKone, C. G. Read, A. J. Biacchi, A. M. Wiltrout, N. S. Lewis and R. E. Schaak, J. Am. Chem. Soc., 2013, 135, 9267 CrossRef CAS PubMed.
- Z. Huang, Z. Chen, Z. Chen, C. Lv, H. Meng and C. Zhang, ACS Nano, 2014, 8, 8121 CrossRef CAS PubMed.
- J. Kibsgaard, C. Tsai, K. Chan, J. D. Benck, J. K. Norskov, F. Abild-Pedersen and T. F. Jaramillo, Energy Environ. Sci., 2015, 8, 3022 CAS.
- X. Wang, Y. V. Kolen'ko, X. Q. Bao, K. Kovnir and L. Liu, Angew. Chem., Int. Ed., 2015, 54, 8188 CrossRef CAS PubMed.
- S. Cao, Y. Chen, C. J. Wang, P. He and W. F. Fu, Chem. Commun., 2014, 50, 10427 RSC.
- Z. Sun, H. Zheng, J. Li and P. Du, Energy Environ. Sci., 2015, 8, 2668 CAS.
- E. Muthuswamy and S. L. Brock, J. Am. Chem. Soc., 2010, 132, 15849 CrossRef CAS PubMed.
- A. B. Mandale, S. Badrinarayanan, S. K. Date and A. P. B. Sinha, J. Electron Spectrosc. Relat. Phenom., 1984, 33, 61 CrossRef CAS.
- V. I. Nefedov, Y. V. Salyn, E. P. Domashevskaya, Y. A. Ugai and V. A. Terekhov, J. Electron Spectrosc. Relat. Phenom., 1975, 6, 231 CrossRef CAS.
- S. J. Sawhill, K. A. Layman, D. R. Van Wyk, M. H. Engelhard, C. Wang and M. E. Bussell, J. Catal., 2005, 231, 300 CrossRef CAS.
- Y. Hou, A. B. Laursen, J. Zhang, G. Zhang, Y. Zhu, X. Wang, S. Dahl and I. Chorkendorff, Angew. Chem., Int. Ed., 2013, 52, 3621 CrossRef CAS PubMed.
- Y. Ni, L. Jin and J. Hong, Nanoscale, 2011, 3, 196 RSC.
- Y. Y. Dou, G. R. Li, J. Song and X. P. Gao, Phys. Chem. Chem. Phys., 2012, 14, 1339 RSC.
Footnote |
| † Electronic supplementary information (ESI) available: SEM image of the as-prepared sample; XPS survey spectrum for the obtained Ni12P5 sample; XRD pattern for NiO obtained by calcining Ni(NO3)2·6H2O at 400 °C for 2 h; H2 evolution from water splitting in the presence of the commercial Pt/C catalyst under visible light irradiation (λ ≥ 420 nm); XRD patterns for the as-prepared sample before and after the catalytic reaction; high-resolution XPS spectra of Ni 2p3/2 and P 2p for the as-prepared sample before and after the catalytic test. See DOI: 10.1039/c5ra25286e |
| This journal is © The Royal Society of Chemistry 2016 |