Probing the corrosion inhibiting role of a thermophilic Bacillus licheniformis biofilm on steel in a saline axenic culture
Ubong Eduoka,
Mazen Khaled*a,
Amjad Khalilb,
Rami Suleimanc and
Bassam El Alia
aDepartment of Chemistry, King Fahd University of Petroleum & Minerals (KFUPM), Dhahran 31261, Saudi Arabia. E-mail: mkhaled@kfupm.edu.sa; Fax: +966-3-860-4277; Tel: +966-3-860-2454
bDepartment of Life Sciences, King Fahd University of Petroleum & Minerals (KFUPM), Dhahran 31261, Saudi Arabia
cCenter of Research Excellence in Corrosion, King Fahd University of Petroleum & Minerals (KFUPM), Dhahran 31261, Saudi Arabia
First published on 2nd February 2016
Abstract
The growth of some bacterial biofilms has been widely reported to have defined consequences on industrial metals, and their related metabolic activities affect the overall electrochemical process of these metals in any given medium. This work seeks to unravel the role of a thermophilic Bacillus licheniformis biofilm (an isolate from the Jazan spring of Saudi Arabia) on corrosion reduction for stainless steel (316 L grade) in a saline culture medium. Grown on the steel substrate, this bacterial biofilm and the nature of its extracellular polymeric substances have been probed chemically and electrochemically for their influences on the metal dissolution within an incubation period. Corrosion inhibition in the presence of varying concentrations (in CFU ml−1) of this bacterium in the biotic-inoculate systems is explained in terms of corrosion resistance and capacitance of the biofilm. The corrosion rate of steel is found to reduce significantly in the saline culture medium within the range of concentrations of bacterium under study compared with the sterile control. This is attributed to the adhesion of a relatively compact and dense “beneficial” biofilm as well as the secretion of corrosion inhibiting substances from the bacterial biofilm as revealed during surface analysis.
1 Introduction
Alloyed and non-alloyed steels are used as materials of choice for many engineering applications (including construction/fabrication, storage and transportation of industrial products, etc.) due to their resistance to corrosion.1,2 The ability of some of these grades of steel to resist corrosion is linked with their unique chemical composition formulated during their metallurgical processing. Though these metals passivate and form stable oxide layers as their constituent alloying elements (Ni, Mn, Cr, Mo, etc.) react with molecular oxygen and with water, they still corrode at a relatively slow rate and are also susceptible to microbially induced corrosion (MIC).3–5 In alloyed pipeline steel materials, this could be explained electrochemically via the two half reactions: anodic, where metallic Fe (could also involve any other metal) is oxidized to its ions and cathodic (where dissolved molecular oxygen catalyzes a cathodic half reaction; water might be involved as well as any other products of microbial activity). The health related risks associated with the release of metal ions into portable drinking water for domestic applications, and the destruction of the industrial oil pipelines by MIC have made this form of corrosion very devastating in the long run. Corrosion episodes involving MIC are directly related to the bacterial colonization as well as the formation of acidic type biofilms by these sessile communities on the surfaces of these ferrous alloys.6,7 Pioneering works related to bacterial biofilm are those reported by Costerton et al.8 in the mid 1970's where the initial theory of biofilm development and the mechanism of how it “sticks” were proposed. As the communities of these harmful bacteria adhere to metallic surfaces, they speedily grow (depending on the availability of nutrients) into larger colonies bound by heterogeneous matrices of extracellular polymeric substances secreted by individual bacterial cells. As far as they can still “quorum sense” within these complex structured microbial communities, other bioactivities are possible, including cellular growths, gene transcription and the secretion of products of metabolism capable of destroying the metallic surfaces. A bacterial biofilm is mostly made up of bacterial cells embedded within the extracellular polymeric substances (EPS) that allows for the attachment of the whole growing community to the substrate at the metal–liquid interface.9Some strains of bacteria can also inhibit metal corrosion.10 In fact, corrosion inhibition by spore-forming Gram-positive bacteria have been widely reported and reviewed in the literature for many metal substrates in both surface and ground water.10–15 These microbes alter the rate of metal dissolution as long as they remain viable in the media by reducing the concentration of oxygen at the metal surface via aerobic respiration. For this class of bacteria, the nature of their biofilms have been reported to have huge influences on the overall electrochemical process as a consequence of their metabolic activities.12,13 Some biofilm-forming strains of Bacillus brevis and Bacillus subtilis have been reported to secrete antimicrobial substances (e.g. gramicidin S antibiotics) from their biofilms that prevent the settlement of other bacteria capable of inducing corrosion.16,17 Arps et al.18 have reported the corrosion reduction of some steel alloys in an artificial service water using Bacillus strains capable of producing polymyxins and gramicidin S. Amongst these Gram-positive and spore-forming Bacillus species with the potency of reducing metal corrosion is Bacillus licheniformis.19
1.1 B. licheniformis
The taxonomic classification of B. licheniformis is presented in Table 1. B. licheniformis is generally a facultative anaerobic, mesophilic, Gram-positive, curved or straight rod-like (Bacillus) motile bacterium (with peritrichous flagella/hair-like outgrowths, hence the name “licheniformis”) with a full cellular size between 1.5–3.5 and 0.6–0.9 μm (Fig. 1). It is spore- and biofilm-forming B. licheniformis is endowed with enzymes that allows its spores to survive harsh conditions, including elevated temperatures, solvents (e.g. NaCl) and pH. This bacterium is widely found in the soil; since many of its strains can survive at elevated temperatures; vegetative growth is still possible between 30 and 50 °C. Higher thermophilic strains can strive in hot springs; and the strain employed in this study was isolated from the hot springs in the Jazan area of Saudi Arabia. B. licheniformis is penicillinase inducible and also known for secreting polypeptide antibiotic bacitracin (active against most Gram-positive pathogenic bacteria) (http://www.tgw1916.net/Bacillus/licheniformis.html). Some strains also possess proteases, lyases/lipases and polysaccharide degrading enzymes.| Classification | |
|---|---|
| Phylum | Firmicutes |
| Class | Bacilli |
| Order | Bacillales |
| Family | Bacillaceae |
| Genus | Bacillus |
 | ||
| Fig. 1 SEM micrograph showing cellular clusters/distribution (a) and dimension (b) of B. licheniformis vegetative cells on stainless steel substrate. | ||
Most corrosion inhibiting strains of B. licheniformis are biofilm-forming bacteria capable of secreting antibiotic compounds that can act as corrosion inhibitors.10 Ornek et al.19 have reported the inhibition of pitting corrosion of aluminum in the presence of B. licheniformis (specifically, the ATCC 9945A strain) in Luria Bertani culture medium. Significant reduction in aluminum pitting rate was observed after 7 days of substrate immersion in the culture medium; this was attributed to bacterial secretion of corrosion inhibiting polymer (γ-polyglutamate) by the B. licheniformis biofilm at medium pH. The effect of bacterial biofilm of nitrate reducing B. licheniformis strain has also been investigated on carbon steel by Xu et al.20 This bacterium acted as a corrosion nitrate reducer against carbon steel corrosion under anaerobic condition but showed severe pitting with 14.5 μm maximum depth while degrading the metal substrate in a one-week exposure period due to its unique biofilm. Marques et al.21 have reported the surface corrosion and bulk carbon steel pitting for isolates of B. licheniformis (and other forms of bacteria) carbon steel in a Brazilian oil platform water. Monitored by weight loss in reactors, authors studied the effect of nitrate on bacterial biofilms on the corrosion rates of steel substrate monitored by weight loss in reactors. Significant biofilm growth were observed on the steel coupons with lower weight loss observed from corrosion inhibiting bacteria in the nitrate reactor. Our group has also recently reported the effectiveness of encapsulated endospores of this bacterium within protective sol–gel coating against steel (S36 grade) corrosion and marine fouling in highly saline medium.22 The presence of these bacterial endospores in the coating ascribed an antibiotic property to the coating surface while also increasing its hydrophobicity against the passage of ionic currents of corrosive ions and molecules via the bulk of the coating to the metal. Though this bacterium-in-coating system was found to exhibit both anticorrosion and antifouling properties, the exact mechanism of action as related to bacterial biofilm formation was not further investigated.
In the field, the extent of microbial influence on metals when exposed to their service environments depends on the type of bacterium (or more) they come in contact with, their prevalence and the nature of such environment. Since the formation of bacterial biofilms on exposure alters the chemistry of these surfaces, there is a need to study the underlining impact of the bioactivities of these microbes at the substrate/solution interfaces in a view to proffering lasting solutions to MIC. This work seeks to unravel the role of biofilm produced from thermophilic strain of B. licheniformis isolated from the Jazan spring of Saudi Arabia. The biofilm of this bacterium is grown on stainless steel (316 L grade) and the nature of EPS as well as the possible bacterial metabolites produced from its physiological activity is monitored chemically and electrochemically in an axenic culture. This culture and corrosion medium is also described as being “saline” since it consists of 1.5 wt% NaCl (in combination with other salts) deployed to accelerate the corrosion of stainless steel with and without single species (axenic) of bacterium, B. licheniformis, which acts as an inhibitor for steel incubated at 37 °C for three weeks. Stainless steel (316 L grade) is chosen as the test substrate due to its susceptibility to pitting and galvanic corrosion in solutions of high ionic (e.g. chlorides) concentrations. By dissolving in corrosive media without huge corrosion products (unlike mild steel), stainless steel allows for the study of the morphology and chemistry of adhering bacterial biofilm with limited interfacial influences.
2 Experimental
2.1 Culture medium/composition
The sourcing of this thermophilic bacterial strain has been previously reported,23,24 and in the present study, the culturing procedure in a fresh minimal salt medium (pH 5.0) has been elaborated elsewhere;25 without modification, except for the replacement of diluted water with a sterile type. Components of the medium includes: 7.5 g NaCl, 10 g glucose, 0.2 g MgSO4·7H2O, 0.01 g FeSO4·7H2O, 1 g (NH4)2SO4, 0.5 g K2HPO4. The B. licheniformis concentration deployed in this study measured 2.79 × 107 CFU ml−1, and 1 ml of the bacterial suspension was inoculated in 500 ml of the sterile minimal salt culture medium (labelled as System A; pH 4.6) and cultured at 37 °C for three weeks with a sterile and highly polished stainless steel substrate (316 L grade). The other biotic set up (System 2A; with 6.80 × 107 CFU ml−1 at pH 3.8) had double the amount (in volume) of the bacterial suspension in System A, and was cultured in similar condition as presented in the flow chart in Fig. 2. The sterile minimal salt solution (in the absence of the bacterium) was employed as the control with a pH of 5.0. Bacterial incubation was done in a closed incubator devoid of external influences. Electrochemical biocorrosion and surface analytical evaluations of the steel coupons followed at the end of the third week.2.2 Electrochemical biocorrosion studies
The SS 316 L steel substrate used in this study has the chemical composition presented in Table 2. It was polished with a procedure previously reported22 for both electrochemical and surface analytical tests after bacterial culture. Both ac and dc experiments were conducted using a three-electrode system with a graphite rod counter electrode (Gamry, US), the polished stainless steel coupon (4 cm × 4 cm) employed as the working electrode, and with a standard calomel reference electrode (SCE). Electrodes were assembled in a 500 ml capacity conducting cell used as the bioreactor for bacterial culturing containing the bacterial suspension/minimal salt culture medium. In this bioreactor, electrochemical analyses were conducted in order to determine the effect of bacterial adhesion/maturation on steel corrosion at the end of the three week culturing period. Electrochemical impedance spectroscopy (EIS) was measured using a Gamry Instrument potentiostat/galvanostat/ZRA (GAMRY 3000, Gamry Instruments, US) corrosion measuring system with ac 10 mV amplitude perturbation at Eoc applied between 10 mHz and 100 kHz; impedance data were collected at 10 points per decade. This test was followed by Tafel polarization (on the same steel coupon), and it involved the measuring of current responses while polarizing the surface of the stainless steel electrode between −250 and +250 mV relative to Ecorr (vs. SCE) at a 1 mV s−1 scan rate. EChem Analyst software package 6.0 (Gamry, US) was used for all data collection and fitting as well as other simulations. Before the electrochemical analyses, values of pH of the bacterial culture solutions were also taken periodically from the onset of incubation until the third week, relative to the control.| Elements | C | Cr | Ni | Mo | Mn | Si | P | S |
|---|---|---|---|---|---|---|---|---|
| Percentage | <0.03 | 16–18.5 | 10–14 | 2–3 | <2 | <1 | <0.045 | <0.03 |
2.3 Surface analytical evaluations
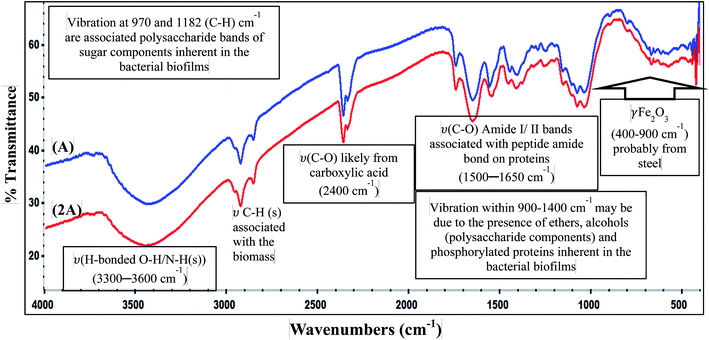
All surface analytical measurements were conducted after the 3 week exposure of the steel substrate to the bacterial-inoculate minimal salt medium in order to study the morphology and chemistry of the adhering bacterial biofilm on steel. The steel substrates were removed from the inoculated culture medium to analyze the chemical composition of the bacterial EPS/biofilm on their surfaces using Fourier transform infra-red (FTIR) spectroscopy (Nicolet 6700 Fourier Transform (FT) spectrometer, Thermo Electron Corporation, UK) in transmittance mode recorded between 400–4000 cm−1, at the resolution and scan rate of 8 cm−1 and 64, respectively. Scanning electron microscopy (U9320A 8500 Field Emission Scanning Electron Microscope, Agilent Technology, UK) coupled with energy dispersive spectroscopy (EDS) for elemental analysis (Oxford 7424 solid-state detector) was employed for the morphological/compositional study of the bacterial biofilm matrix as well as the evaluation of possible corrosion products on the steel surface before and after bacterial culturing.3 Results and discussion
3.1 Electrochemical analyses
The mechanisms of bacterial cell adhesion and biofilm formation on the solid steel surface were monitored electrochemically (both ac and dc techniques) after culturing in the minimal salt medium for three weeks.![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 200 and 18
200 and 18![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 500 ohm per square centimeter (Ω cm2) recorded for the control, System A and System 2A, respectively, at 0.01 Hz. Higher magnitudes of Zmod represents improved protection and the lower values of Zmod obtained for the control denotes corrosion activation in the presence of the chloride ions in minimal salt medium without the bacterial cells. The phase angle plots (Fig. 3c) show distinct phase deviation in the presence of the bacteria as the biofilm is formed on steel with minima observed between 0.01 and 1 Hz for the biotic systems. From the impedance spectra, although a passive film which represents a single layer forms on 316 L SS in the minimal salt medium (control), there is substantial evidence of a second and more compact film/layer after bacterial attachment and biofilm formation for the bacterial-inoculated systems at the culture condition under study.27 With this in mind, equivalent circuit models displayed in Fig. 4 were deployed to fit the experimental (a) control and (b) biofilm impedance data; values of the electrochemical parameters derived are presented in Table 3. These circuit models were designed with CPE components in other to account for possible inhomogeneity inherent in the double layers, the non-uniform thicknesses of the formed biofilm as well as other electrode surface irregularities (not limited to roughness and porosity). The impedance expression of a CPE (ZCPE) is given in eqn (1).28
500 ohm per square centimeter (Ω cm2) recorded for the control, System A and System 2A, respectively, at 0.01 Hz. Higher magnitudes of Zmod represents improved protection and the lower values of Zmod obtained for the control denotes corrosion activation in the presence of the chloride ions in minimal salt medium without the bacterial cells. The phase angle plots (Fig. 3c) show distinct phase deviation in the presence of the bacteria as the biofilm is formed on steel with minima observed between 0.01 and 1 Hz for the biotic systems. From the impedance spectra, although a passive film which represents a single layer forms on 316 L SS in the minimal salt medium (control), there is substantial evidence of a second and more compact film/layer after bacterial attachment and biofilm formation for the bacterial-inoculated systems at the culture condition under study.27 With this in mind, equivalent circuit models displayed in Fig. 4 were deployed to fit the experimental (a) control and (b) biofilm impedance data; values of the electrochemical parameters derived are presented in Table 3. These circuit models were designed with CPE components in other to account for possible inhomogeneity inherent in the double layers, the non-uniform thicknesses of the formed biofilm as well as other electrode surface irregularities (not limited to roughness and porosity). The impedance expression of a CPE (ZCPE) is given in eqn (1).28| ZCPE = (jω)−α/Yo; | (1) |
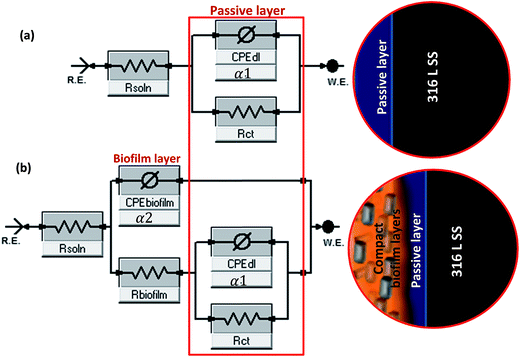 | ||
| Fig. 4 Equivalent circuit models used in fitting the experimental (a) control and (b) biofilm impedance curves (Systems A and 2A); magnitudes of α1 and α2 represent the homogeneity factors of the systems as expressed in eqn (1); both circuit models are adopted from Gamry Echem Analyst software's model editor. | ||
| Electrochemical parameters | Control | System A | System 2A |
|---|---|---|---|
| ac (EIS) | |||
| Rsoln (Ω cm2) | 1.062 | 4.045 | 4.567 |
| Rbiofilm (kΩ cm2) | — | 5.166 | 10.116 |
| CPEbiofilm (Yo, mF cm−2 s−(1−αc)) | — | 0.832 | 0.254 |
| α2 | — | 0.890 | 0.900 |
| Rct (kΩ cm2) | 0.241 | 2.001 | 2.982 |
| CPEdl (Yo, mF cm−2 s−(1−αc)) | 0.945 | 0.594 | 0.299 |
| α1 | 0.867 | 0.866 | 0.873 |
| Circuit type | R(Q(R)) | R(Q(R(QR))) | R(Q(R(QR))) |
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) |
|||
| dc (Tafel) | |||
| Icorr (μA cm−2) | 8.07 | 4.00 | 0.878 |
| Ecorr (mV) | −143 | −124 | −59 |
| CR (mpy) × 10−3 | 409.600 | 203.300 | 44.580 |
Magnitudes of solution resistance (Rsoln), resistance of the biofilm layer (Rbiofilm), charge transfer resistance (Rct), and the capacitances of the double (CPEdl) and biofilm (CPEbiofilm) layers are represented in the equivalent circuit models used in fitting the experimental impedance data for the B. licheniformis inoculate in the culture medium compared to the sterile control (Table 3). For the abiotic (control) system, only a single adsorption layer is represented with the following components: Rsoln, CPEdl and Rct. Rsoln is measured between the working and reference electrodes while charge transfer resistance accounts for current leakages. Higher values of Rsoln obtained for the bacterial-inoculated systems compared to the sterile control could be linked with the bacterial metabolic processes occurring in the culture medium;25 1.062, 4.045 and 4.567 Ω cm2 are the Rsoln values for the control, System A and System 2A, respectively. Higher values of Rct and Rbiofilm for the biotic systems compared to the control denotes corrosion resistance of 316 L stainless steel in the saline medium.25,29 With values of Rct for the control, System A and System 2A recorded as 0.241, 2.001 and 2.982 kΩ cm2, very compact bacterial biofilm–metal oxide layer is conceived to have formed at the metal surface for System 2A compared to System A; this is expected as the concentration of bacterial cells in System 2A doubles that System A in the starter culture.25 The formation of thicker and more compact biofilm layers on steel for System 2A must be the reason for higher Rbiofilm values compared to System A. Rbiofilm represents the formation of EPS as well as the resistance of the formed bacterial biofilm layer to the passage of ionic current of corrosive chloride ions (in combination with other aggressive ions/molecules inherent in the minimal salt medium at 37 °C) after 3 week immersion. More bacteria cells must have adhered unto the surface of steel thereby impeding electron-transfer; this can also be explained in terms of increased metabolic activities as more cells (thicker biofilms) are formed.30 Constant phase element (CPE) replaces pure capacitor in the equivalent circuit so as to account for metal surface irregularities/inhomogeneity as well as those of the electrode/electrolyte interface. In this study, the trend of CPE for the bacterial-inoculated systems compared to the sterile control, could be linked with surface inhomogeneity as the bacterial biofilms are formed on the metal surface. Values of CPEbiofilm for the System A and System 2A are 0.832 and 0.254 mF cm−2 s−(1−αc) while 0.594 and 0.299 mF cm−2 s−(1−αc) represents values of CPEdl; lower CPE values for System 2A denote reduced water absorptivity due to the presence of compact and denser biofilm on steel compared to System A.25 Normally for most bacteria, biofilm formation begins with cellular attachment in clusters and as they colonize the surface. If surface attachment is irreversible, a more complex community of bacteria cells in the EPS biomatrix could mature into a biofilm at a particular stage in their development depending on the availability of nutrients. EIS presents a suitable real time and online technique for monitoring biofilm formation since the application of minute voltage perturbation accompanying this technique has little or no effect on the attached bacterial cells. Polarization technique can also be used to study the same process at Ecorr.
3.2 Variation of pH with culture duration
The variation of culture media pH for the uninhibited and inhibited systems with immersion period was evaluated between the onsets of bacterial incubation until the third week. The bacterial biofilm formation/maturation led to reduction in pH of the media as the more cells adhere on the steel substrate; and Fig. 5 shows reducing culture media pH as metabolic products are formed from inherent physiological bioactivity in the presence of the bacterial-inoculated systems (compared to the control) for 316 L at with exposure periods. These byproducts (alongside inorganic nutrients in the culture) could have also supported the protective biofilm formation on surface of the metal.25 As displayed in Fig. 5, the pH of the control was relatively unchanged within the culture period (between 4.7 and 5) while those of bacterial inoculate systems drastically dropped throughout the culture period; pH units of 2.75 and 2.68 were recorded for Systems A and 2A, respectively, after the third week. The pH at the onset of incubation were 5.0, 4.67 and 3.86 for the control, System A and 2A, respectively. The reduction of pH of the biotic systems also denotes the formation of biofilm layers; and could also be linked with the retardation of interfacial electron-transfer kinetics and increased the electron-transfer resistance as the attachment of cells and development of a biofilm on the metal surface increases.31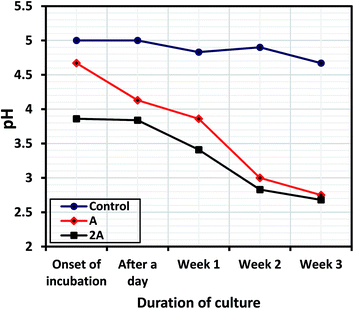 | ||
| Fig. 5 Variation in culture media pH containing B. licheniformis inoculates (compared to the control) for SS 316 L corrosion at different exposure periods. | ||
3.3 Surface analyses
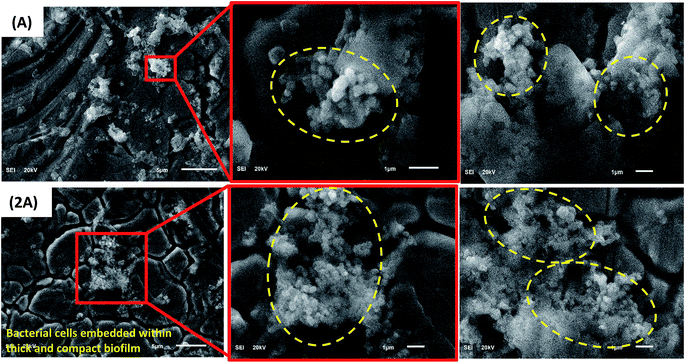 | ||
| Fig. 6 SEM images showing adhering B. licheniformis biofilms (circled) on surfaces of SS 316 L coupons after 3 weeks immersion in the culture medium. | ||
 | ||
| Fig. 7 Schematic illustration of the proposed mechanism of corrosion inhibition induced by B. licheniformis biofilm for steel; (a) onset of bacterial incubation in the culture medium with SS 316 L steel substrate; (b) gradual adhesion of relatively few biofilm-forming bacterial cellular clusters on steel after three week culture period for System A; and (c) the appearance of dense and compact axenic bacterial biofilms on steel for System 2A; (the mode/pathway of biofilm/cellular adhesion on steel is not restricted to the direction of the arrow). Formation of compact bacterial biofilm layers on steel increases the corrosion resistance of the surface against the conductive ionic currents capable of inducing pitting and other possible corrosion episodes. Naturally during culturing, B. licheniformis has been reported to secrete γ-polyglutamate, an antimicrobial and corrosion inhibiting polymer, from its biofilm capable of reducing metal surface corrosion around the pH reported in this study.19 | ||
4 Conclusions
The following conclusions are drawn from the experimental results:(1) Higher values of Rsoln obtained for the B. licheniformis inoculate in the culture medium compared to the sterile control could be linked with inherent bacterial metabolic activities in the axenic culture medium. Also compared to the control, the trend in Rct values for these biotic systems (2.001 and 2.982 kΩ cm2 for System A and System 2A) could be attributed to the formation of B. licheniformis biofilm/metal oxide bilayer on steel. This also accounts for more than 90% corrosion inhibition efficiency for System 2A relative to the control.
(2) Higher values of Rbiofilm for System 2A compared to System A could be attributed to the formation of denser and more compact biofilm layers on steel for System 2A. Rbiofilm also represents the formation of EPS as well as the resistance of the formed bacterial biofilm layer to the passage of ionic current of corrosive chloride ions (in combination with other aggressive ions/molecules in the minimal salt medium at 37 °C) after 3 week immersion.
(3) Reduced magnitudes of corrosion current density and corrosion rates for the bacterial-inoculated systems also suggest that the formation of the bacterial biofilm could be the primary reason for steel corrosion inhibition in the minimal salt culture medium. The variation in the magnitude of Icorr is in this order: Icorr (System 2A) < Icorr (System A) < Icorr (control).
(4) SEM images of the metal substrates in the bacterial-inoculate culture media reveal the adhesion of this relatively compact and dense biofilm (groups of bacterial cells enclosed in an EPS matrix) on its surface. This further affirms the reason for corrosion inhibition of 316 L steel proposed from the electrochemical results; FTIR results suggest that the EPS biomatrix could be dominantly proteins and polysaccharides.
(5) The biofilm of B. licheniformis isolated from the Jazan spring of Saudi Arabia inhibits corrosion of 316 L stainless steel to a great extent after culturing at 37 °C for 3 weeks.
Acknowledgements
Authors are grateful to King Fahd University of Petroleum & Minerals (KFUPM) for providing the needed facilities for this work.References
- A. Moteshakker and I. Danaee, J. Mater. Sci. Technol. DOI:10.1016/j.jmst.2015.11.021.
- U. M. Eduok and M. M. Khaled, Construct. Build. Mater., 2014, 68, 285–290 CrossRef.
- J. J. Kim and Y. M. Young, Int. J. Electrochem. Sci., 2013, 8, 11847–11859 Search PubMed.
- V. Maurice and P. Marcus, Adsorption layers and passive oxide films on metals, in Tribocorrosion of Passive Metals and Coatings, ed. D. Landolt and S. Mischler, A volume in Woodhead Publishing Series in Metals and Surface Engineering, 2011, ch. 2, pp. 29–64, ISBN: 978-1-84569-966-6 Search PubMed.
- H. A. Videla, Int. Biodeterior. Biodegrad., 1994, 34(3–4), 245–257 CrossRef CAS.
- C. W. Keevil, Water Sci. Technol., 2004, 49(2), 91–98 CAS.
- B. J. Webster, S. E. Werner, D. B. Wells and P. J. Bremer, Corrosion, 2000, 56(9), 942–950 CrossRef CAS.
- J. W. Costerton, G. G. Geesey and K. J. Cheng, Sci. Am., 1978, 238, 86–95 CrossRef CAS PubMed.
- H. Zhang, Y. Tian, J. Wan and P. Zhao, Appl. Surf. Sci., 2015, 357, 236–247 CrossRef CAS.
- K. A. Zarasvand and V. R. Rai, Int. Biodeterior. Biodegrad., 2014, 87, 66–74 CrossRef.
- H. A. Videla and L. K. Herrera, Int. Biodeterior. Biodegrad., 2009, 63(7), 896–900 CrossRef CAS.
- N. Aimeur, K. Houali, L. Hamadou, N. Benbrahim and A. Kadri, Corros. Eng., Sci. Technol., 2015, 50(8), 579–588 CrossRef.
- F. Mansfeld, Electrochim. Acta, 2007, 52(27), 7670–7680 CrossRef CAS.
- D. Ornek, A. Jayaraman, B. C. Syrett, C. H. Hsu, F. B. Mansfeld and T. K. Wood, Appl. Microbiol. Biotechnol., 2002, 58, 651–657 CrossRef CAS PubMed.
- M. Sancy, A. Abarzua, M. I. Azócar, J. M. Blamey, F. Boehmwald, G. Gómez, N. Vejar and M. Páez, J. Electroanal. Chem., 2015, 737, 212–217 CrossRef CAS.
- R. Zuo and T. K. Wood, Appl. Microbiol. Biotechnol., 2004, 65, 747–753 CrossRef CAS PubMed.
- A. Jayaraman, F. B. Mansfeld and T. K. Wood, J. Ind. Microbiol. Biotechnol., 1999, 22, 167–175 CrossRef CAS.
- P. J. Arps, J. C. Earthman, L. C. Xu, B. C. Syrett, R. Green, T. Wood and F. B. Mansfeld, CORROSION/03, NACE International, San Diego, CA, Paper no. 03714, 2003 Search PubMed.
- D. Ornek, A. Jayaraman, B. C. Syrett, C. H. Hsu, F. B. Mansfeld and T. K. Wood, Appl. Microbiol. Biotechnol., 2002, 58, 651–657 CrossRef CAS PubMed.
- D. Xu, Y. Li, F. Song and T. Gu, Corros. Sci., 2013, 77, 385–390 CrossRef CAS.
- J. M. Marques, F. P. deAlmeida, U. Lins, L. Seldin and E. Korenblum, World J. Microbiol. Biotechnol., 2012, 28, 2355–2363 CrossRef CAS PubMed.
- U. Eduok, R. Suleiman, J. Gittens, M. Khaled, T. J. Smith, R. Akid, B. El Ali and A. Khalil, RSC Adv., 2015, 5, 93818–93830 RSC.
- A. Khalil, Afr. J. Biotechnol., 2011, 10, 8834–8839 CrossRef CAS.
- A. Khalil, G. Anfoka and S. Bdour, World J. Microbiol. Biotechnol., 2003, 19, 239–241 CrossRef CAS.
- H. Z. Wadood, A. Rajasekar, Y. P. Ting and A. N. Sabari, Arabian J. Sci. Eng., 2015, 40, 1825–1836 CrossRef CAS.
- H. Zhang, Y. Tian, J. Wan and P. Zhao, Appl. Surf. Sci., 2015, 357, 236–247 CrossRef CAS.
- J. E. Gonzalez, F. J. H. Santana and J. C. Mirza-Rosca, Corros. Sci., 1998, 40(12), 2141–2154 CrossRef CAS.
- S. Kirtay, Prog. Org. Coat., 2014, 77, 1861–1866 CrossRef CAS.
- E. Miranda, M. Bethencourt, F. Botana, M. Cano, J. Sánchez-Amaya, A. Corzo, J. G. DeLomas, M. L. Fardeau and B. Ollivier, Corros. Sci., 2006, 48, 2417–2431 CrossRef CAS.
- A. Dheilly, I. Linossier, A. Darchen, D. Hadjiev, C. Corbel and V. Alonso, Appl. Microbiol. Biotechnol., 2008, 79, 157–164 CrossRef CAS PubMed.
- M. Moradi, Z. Song, X. Nie, M. Yan and F. Q. Hu, Int. J. Adhes. Adhes., 2016, 65, 70–78 CrossRef CAS.
- I. T. Vargas, M. A. Alsina, J. P. Pavissich, G. A. Jeria, P. A. Pastén, M. Walczak and G. E. Pizarro, Bioelectrochemistry, 2014, 97, 15–22 CrossRef CAS PubMed.
- A. Rajasekar and Y. P. Ting, Ind. Eng. Chem. Res., 2010, 49, 6054–6061 CrossRef CAS.
- L. Marcotte, G. Kegelaer, C. Sandt, J. Barbeau and M. Lafleur, Anal. Biochem., 2007, 361, 7–14 CrossRef CAS PubMed.
- M. O. Suraju, S. L. Barnes, S. Sanamvenkata, M. Esmaeili, S. Shishodia and J. A. Rosenzweig, Sci. Total Environ., 2015, 538, 949–958 CrossRef CAS PubMed.
- F. Mansfeld, H. Hsu, D. Ornek, T. K. Wood and B. C. Syrett, J. Electrochem. Soc., 2002, 149(4), B130–B138 CrossRef CAS.
| This journal is © The Royal Society of Chemistry 2016 |