Whey protein isolate/gum arabic intramolecular soluble complexes improving the physical and oxidative stabilities of conjugated linoleic acid emulsions†
Xiaolin Yaoab,
Shengping Xianga,
Ke Niea,
Zhiming Gaoab,
Weiqi Zhanga,
Yapeng Fang*ab,
Katsuyoshi Nishinariab,
Glyn O. Phillipsa and
Fatang Jianga
aGlyn O. Phillips Hydrocolloid Research Centre, School of Food and Pharmaceutical Engineering, Faculty of Light Industry, Hubei University of Technology, Wuhan 430068, China. E-mail: fangypphrc@163.com; Fax: +86 27 88015996; Tel: +86 27 88015996
bHubei Collaborative Innovation Centre for Industrial Fermentation, Hubei University of Technology, Wuhan 430068, China
First published on 20th January 2016
Abstract
Protein/polysaccharide electrostatic complexes have been widely used in food products to confer structure and stability. Intramolecular soluble complexes (ISCs) have superior emulsifying properties in stabilizing oil-in-water (o/w) emulsions. This paper investigates the potential application of ISCs to stabilize polyunsaturated fatty acids that were difficult to disperse and liable to oxidation. The idea was demonstrated using whey protein isolate/gum arabic (WPI/GA) ISCs and conjugated linoleic acid (CLA). Zeta potential measurements indicated a stoichiometry of r = 1.0 for the electrostatic complexation of WPI/GA. Excess of GA (r < 1.0) ensured the formation of stable ISCs in a specific pH range, e.g. pH 4.0–5.4 at r = 0.5. The nano-sized ISCs significantly improved the physical and oxidative stabilities of CLA emulsions in comparison with individual WPI or GA. Optimal stabilization was found at a WPI/GA concentration of 2.0 wt% for emulsions with CLA = 15 wt%. NaCl tended to dissociate ISCs when NaCl > 20 mM and therefore seriously reduced the stability of ISCs-stabilized CLA emulsions. The superiority of ISCs in stabilizing polyunsaturated fatty acids is due to the cooperative adsorption of protein and polysaccharide at the emulsion interface, providing strong steric and electrostatic effects against droplet aggregation and coalescence and thus excellent physical stability. The improved oxidative stability should arise from the free radical scavenging ability of the protein at the emulsion interface, reducing lipid oxidation.
Introduction
Polyunsaturated fatty acids (PUFAs), e.g. conjugated linoleic acid (CLA; C18:2), have been demonstrated to possess numerous health benefits, including sustaining infant development, supporting cardiovascular health, cancer prevention, weight control and immunomodulation.1 Many PUFAs are essential because they cannot be synthesized by humans and thus must be derived from the diet.1 PUFAs are often incorporated into food products in the form of oil-in-water (o/w) emulsions due to their low water dispersibility and high sensitivity to oxidation. Oxidation of PUFAs in food matrices was accelerated by the presence of oxygen, heat, light, enzymes, metals, metalloproteins etc., and could cause the development of off-flavors, change in color, loss of other nutrients, and the formation of potentially toxic compounds.2 Physically and chemically stabilizing PUFAs still proves to be a challenge in the food industry with regards to the development of PUFAs fortified functional foods.Protein/polysaccharide combinations have been widely used in food products to confer structure and stability. Non-covalent electrostatic complexes formed by charged protein and polysaccharide attract much interest due to their important biological implications and ease to use in product formulation.3 For example, electrostatic complexation between gelatin and pectin was used to create hydrogel particles with similar dimensions and functional attributes as starch granules for formulation of low-calorie and low-starch foods.4 Bosnea et al. applied protein/polysaccharide electrostatic complexation as a novel microencapsulation technique to improve the viability of probiotics under different stresses.5 Selective complexation of proteins with polysaccharide was employed as an effective tool to enrich and purify proteins.6 Due to the combined advantages of hydrophilic polysaccharide and hydrophobic protein, protein/polysaccharide electrostatic complexes emerged as novel and unique emulsifier and foaming agent, exhibiting excellent interfacial properties.7,8
Protein/polysaccharide electrostatic complexation has been well characterized by using complementary techniques including turbidimetry, light scattering, zeta potentiometers, spectroscopy and light microscopy, etc.9,10 The process involved the formation of soluble complexes and subsequently insoluble complexes (liquid coacervation or solid precipitate).11,12 In a previous study, we studied the structural transition of protein/polysaccharide electrostatic complexation in bovine serum albumin/sugar beet pectin system, and proposed a detailed phase diagram.10 A particular phase region, i.e., intramolecular soluble complexes (ISCs), was found to behave superbly in stabilizing o/w emulsions via cooperative adsorption.7
The present work aimed to evaluate the potential of protein/polysaccharide ISCs in stabilizing PUFAs-based emulsions. The approach is demonstrated by using whey protein isolate (WPI)/gum arabic (GA) and CLA as a model system. GA is an anionic heterogeneous polysaccharide with branched structure. It contains a small amount of proteinaceous material that is covalently attached to the polysaccharide.13,14 WPI is mainly composed of β-lactoglobulin (β-lg) and α-lactalbumin.15 It can inhibit the oxidative deterioration of limonene in o/w emulsions due to its ability to scavenge free radical and chelate prooxidative metals.16 Both GA and WPI exhibit surface activities and are used in the food industry as emulsifiers.17,18 In the work, the electrostatic complexation between WPI/GA was characterized and the experimental conditions to produce ISCs identified. The ISCs were then used to stabilize CLA emulsions, and their physical and chemical stabilities were evaluated by acceleration test and oxygen consumption measurements. The results were compared with those obtained with individual protein or polysaccharide to reveal the superiority of ISCs in stabilizing PUFAs emulsions. The results obtained in the present study are expected to shed lights on the protection of other PUFAs.
Materials and methods
Materials
GA in spray-dried form with a purity of >94.6% was supplied by San Ei Gen F.F.I. Inc., Japan. WPI with a purity of >95.0% was obtained from Davisco, USA. The biopolymers were used without further purification. Food grade CLA (C18:2) with the principal isomer form of cis-9, trans-11 was purchased from Beijing Health Science and Technology Co. Ltd., China. The purity of CLA was 80%. The remaining components contained 12.5% of oleic acid, and palmitic acid, stearic acid, and linoleic acid accounted for the rest, which were measured by a gas chromatograph (Varian 3900, USA). Glucono-δ-lactone (GDL) with a purity of 99.0% was purchased from Sigma, USA. All other chemicals used in the study were of analytical grade. Millipore water was used for the preparation of solutions.Characterization of WPI/GA electrostatic complexes
 | (1) |
Light scattering measurement was conducted at 25 °C using Zetasizer Nano-ZS apparatus (Malvern Instruments, UK). The average scattered light intensity at 173° (I173) and intensity autocorrelation function during in situ acidification were recorded every 30 s for 150 min. Z-averaged diffusion coefficient (DZ), obtained from the analysis of autocorrelation function,19 was used to calculate Z-averaged hydrodynamic diameter (Dh) of particles through the Stokes–Einstein equation:11
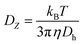 | (2) |
Turbidity measurement was performed on a UV/visible spectrophotometer (TU-1900, PERSEE, China) at a wavelength of 500 nm. The change in turbidity (τ) during in situ acidification was recorded every 30 s for 150 min at 25 °C. τ was defined as:
| τ = (−1/L)ln(I0/It) | (3) |
Characterization of CLA emulsions stabilized with WPI/GA complexes
![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000 rpm min−1 for 3 min. The primary emulsions were further homogenized by a high-pressure homogenizer (Microfluidics M-110L, USA) at 75 MPa for one pass. The homogenization was carried out in an ice bath to minimize the extent of lipid oxidation.
000 rpm min−1 for 3 min. The primary emulsions were further homogenized by a high-pressure homogenizer (Microfluidics M-110L, USA) at 75 MPa for one pass. The homogenization was carried out in an ice bath to minimize the extent of lipid oxidation.Based on D[3,2], the specific surface area (Sν) of emulsions was calculated according to the following equations:
| Sν = 6φ/D[3,2] (m2 per mL emulsion) | (4) |
| φ = (ρaq − ρem)/(ρaq − ρoil) | (5) |
Results and discussion
Electrostatic complexation of WPI/GA
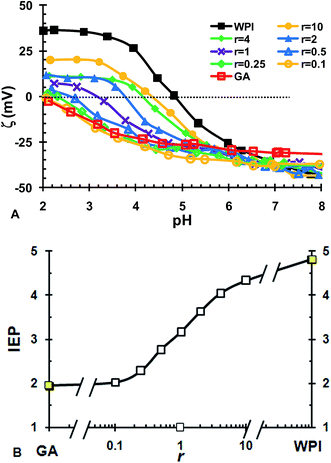
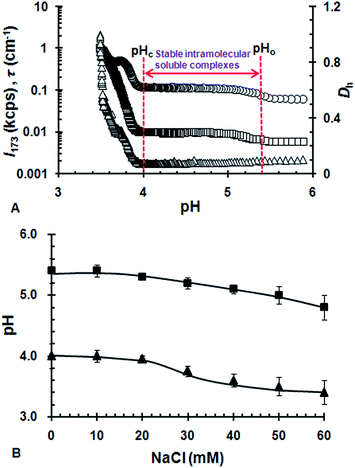
Fig. 2B shows the influence of ionic strength on the formation of ISCs. When NaCl ≤ 20 mM, pHo and pHc was nearly independent of ionic strength. It suggests that the electrostatic complexation between WPI and GA is insensitive to the addition of NaCl at lower ionic strength. With increasing concentration of NaCl, pHo and pHc started to decrease considerably when NaCl > 20 mM. With further increasing NaCl above 60 mM, the transitions associated with pHo and pHc nearly disappeared (data not shown). This indicated that the ISCs started to be unstable when NaCl > 20 mM and was nearly dissociated completely when NaCl > 60 mM. Similar effects of ionic strength was observed for the electrostatic complexation of bovine serum albumin/sugar beet pectin and gelatin/κ-carrageenan systems.10,23 It was generally believed that ionic strength reduced protein/polysaccharide complexation by exerting an electrostatic screening effect.11 The microions presented in the solution screened the charges of the polymers and thus reduced the range of their associative interactions.
Physical stability of CLA emulsions stabilized with ISCs
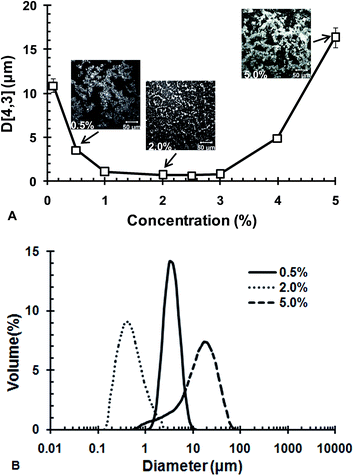
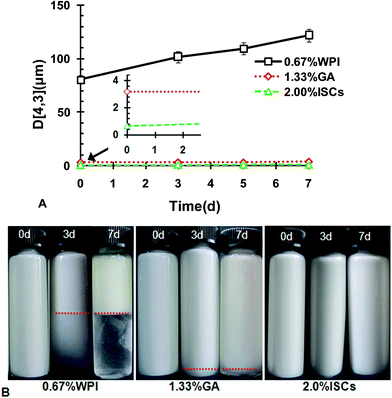
Fig. 3B shows the particle size distribution of CLA emulsions at typical concentrations of WPI/GA ISCs. The emulsions at ISCs = 0.5 and 2.0 wt% both had a relatively monomodal distribution, while the emulsion at ISCs = 5.0% showed a bimodal distribution. Among the three ISCs concentrations, ISCs = 2.0 wt% yielded the smallest droplet size. The micrographs taken at the typical ISCs concentration, shown as insets in Fig. 3A, supported the results of particle size measurements. The microstructures of the emulsions at ISCs = 0.5 and 5.0 wt% exhibited a stronger tendency of aggregation. Distinctly large emulsion droplets could be observed at ISCs = 0.5 wt%. In contrast, the emulsion at ISCs = 2.0 wt% was well dispersed with relatively fine particles.
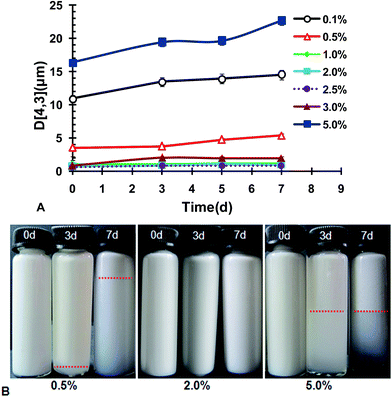
In brief summary, the potential conditions enabling production of fine CLA emulsions were 1.0 wt% < ISCs < 3.0 wt%, with an optimal concentration at 2.0 wt%. Further tests on the stability of the emulsions prepared under these conditions were carried out. Fig. 4 shows the volume weighted diameter D[4,3] as a function of storage time during acceleration test at 40 °C. In the concentration range of 1.0 wt% < ISCs < 3.0 wt%, the emulsions had rather good stability, with D[4,3] almost constant for 7 days during acceleration at 40 °C. When ISCs fell out of this particular concentration range, i.e., ISCs < 1.0 wt% or ISCs > 3.0 wt%, the emulsions showed considerable growth in D[4,3]. The instability could be due to the aggregation tendency that was augmented during acceleration test. The bulk appearance of the emulsions at the typical ISCs concentrations during acceleration test at 40 °C is shown in Fig. 4B. At 0.5 wt% ISCs, a creaming layer was observed on the 3rd day of storage, and its boundary moved up on the 7th day. This indicated an increased extent of creaming or phase separation. The emulsion with 5.0 wt% ISCs also exhibited significant creaming on the 3rd and 7th days of storage. In comparison, the emulsion with 2.0 wt% ISCs remained homogenous throughout the storage, without discernible creaming/phase separation. The results above showed that the WPI/GA ISCs could yield the best physical stability of CLA emulsions in an optimal concentration range of 1.0 wt% < ISCs < 3.0 wt%.
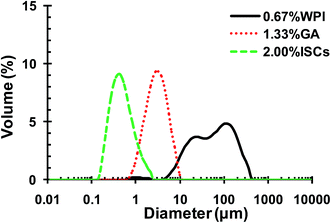 | ||
| Fig. 5 Particle size distributions of CLA emulsions stabilized by 0.67 wt% WPI, and 1.33 wt% GA and 2.0 wt% WPI/GA ISCs (r = 0.5) at pH 4.4. | ||
The stabilities of the emulsions with WPI, GA and ISCs against acceleration test are presented in Fig. 6A. D[4,3] of the ISCs-stabilized emulsion showed negligible change during 7 day storage at 40 °C, indicating a fairly stable emulsion. The D[4,3] of the GA-stabilized emulsion was larger than that of ISCs-stabilized emulsion, and increased slightly during the storage. However, the D[4,3] of the WPI-stabilized emulsion grew dramatically during the storage, indicating a poor stability. The macroscopic observations in Fig. 6B show clear phase separations in the emulsions stabilized with WPI and GA, while there is no sign of any phase separation in the emulsion stabilized with ISCs. The acceleration tests suggested an increased physical stability of CLA emulsions with ISCs > GA > WPI. The superiority of ISCs in stabilizing CLA emulsion was due to the cooperative adsorption of WPI and GA at the oil–water interface, providing strong steric and electrostatic effects against droplet aggregation and coalescence and thus improved physical stability.7
Oxidative stability of CLA emulsions stabilized with ISCs
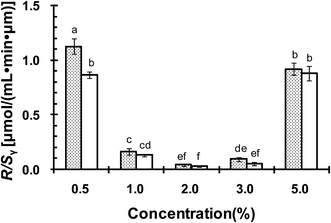
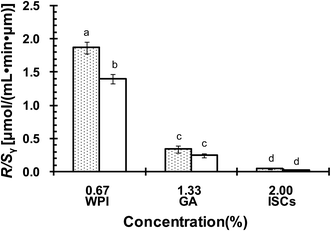
The oxidative stability of CLA emulsions stabilized with ISCs, WPI or GA alone was evaluated. Oxygen consumption rate (R) was calculated from the slopes of oxygen concentration–time curves. R/Sν represented the oxygen consumption rate per unit area of the emulsion droplet surface, and thus normalized the effect arising from the difference in emulsion droplet size distributions.28 R/Sν was linked to CLA oxidation that consumed oxygen to form lipid peroxyl radicals, according to the oxidation mechanism reported previously.22 Fig. 8 compares R/Sν for CLA emulsions stabilized with ISCs at different concentrations at 40 °C with or without exposure to light. The R/Sν for emulsion exposed to light was higher than that without exposure to light. It demonstrated that light might promoted lipid oxidation, but the effect was not significant (P > 0.05). The lowest R/Sν was found for emulsions with 2.0 wt% ISCs at both conditions. It was inferred that the oxidation of CLA emulsions was minimal at 2.0 wt% ISCs. The optimal concentration for oxidative stability was the same with that for physical stability (Fig. 3A). Moreover, compared with individual WPI (0.67 wt%) and GA (1.33 wt%), the emulsions stabilized with 2.0 wt% ISCs showed a significant lower R/Sν (P < 0.05) (Fig. 9), indicating the superiority of ISCs in preventing polyunsaturated fatty acid-based emulsions from being oxidized.In a previous study,22 we made a supposition that a physically stable emulsion was a prerequisite for the chemical stability of CLA, which also applied to the ISCs. The cooperative adsorption of WPI/GA ISCs onto the oil–water interface formed a thick and compact interfacial layer around CLA emulsion droplets, leading to an improved emulsifying functionality and stability. The interface could provide a strong steric and electrostatic stabilization effects against the aggregation and coalescence of emulsion droplets. On the other hand, CLA oxidation was highly dependent on the interaction between lipid hydroperoxides at emulsions droplet interface and transition metals present in the aqueous phase.29,30 The thick and compact interface layer could act as a physical barrier to the metals, isolating them from lipid hydroperoxides and thus preventing the formation of free radicals to attack CLA.
It should be pointed out that although the emulsion with 0.5 wt% ISCs was much finer and stable than that with 5 wt% ISCs (Fig. 3 and 4), the two emulsions showed more or less the same value of R/Sν. This could be explained by the presence of excessive ISCs and hence protein in the emulsion with 5.0 wt% ISCs. It is well known that proteins have ability to chelate metal ions and to scavenge free radicals, reducing lipid oxidation.31–35 CLA was reported to be efficiently protected from oxidative attack by complexation with amino acids (lysine or arginine), mainly attributed to the antioxidant effect of the amino acids through scavenging the oxygen radicals.36 The higher amount of protein in the emulsion with 5.0 wt% ISCs might counteract the increased lipid oxidation resulting from its poor physical stability. Similar effects had been observed in β-lg stabilized emulsions.37,38 The oxygen uptake in the β-lg stabilized emulsion with excessive β-lg was much lower, due to the antioxidant effect of the non-adsorbed β-lg. The antioxidant mechanisms of protein were thought to be dependent on protein tertiary structure. In order for a protein to chelate aqueous metals, the amino acid residues responsible for metal binding must be sufficiently exposed.39,40 However, protein oxidation could lead to the formation of carbonyls, intra- and intermolecular cross-linking through the formation of disulphide bonds and dityrosine, a decrease in protein solubility, and the fragmentation of peptide backbone.41 The negative impact of protein oxidation appeared to have limited effect on the physicochemical stabilities of the CLA emulsions.
Conclusion
This paper evaluated the potential of the WPI/GA ISCs in stabilizing PUFAs-based emulsions. The results showed that the nano-sized ISCs (∼50 nm) could significantly improve the physicochemical stabilities of CLA emulsions in comparison with individual protein or polysaccharide. The superiority of ISCs originated from the cooperative adsorption of protein and polysaccharide on to the emulsion interfaces, providing steric and electrostatic stabilization as well as free radicals-scavenging ability. The results can guide the design of protective delivery system for polyunsaturated fatty acids based on oil-in-water emulsion technique.Acknowledgements
We acknowledge financial support from the National Natural Science Foundation of China (31470096, 31101260, 31322043, 31501430), Projects from Hubei Provincial Department of Science and Technology (2014CFB602).References
- B. Chen, D. J. McClements and E. A. Decker, Annu. Rev. Food Sci. Technol., 2013, 4, 35–56 CrossRef CAS PubMed.
- F. Shahidi and Y. Zhong, Chem. Soc. Rev., 2010, 39, 4067–4079 RSC.
- C. Schmitt and T. Sl, Adv. Colloid Interface Sci., 2011, 167, 63–70 CrossRef CAS PubMed.
- B. C. Wu, B. Degner and D. J. McClements, J. Phys.: Condens. Matter, 2014, 26, 464104 CrossRef PubMed.
- L. A. Bosnea, T. Moschakis and C. G. Biliaderis, Food Bioprocess Technol., 2014, 7, 2767–2781 CrossRef CAS.
- Y. Xu, M. Mazzawi, K. Chen, L. Sun and P. L. Dubin, Biomacromolecules, 2011, 12, 1512–1522 CrossRef CAS PubMed.
- X. Li, Y. Fang, S. Al-Assaf, G. O. Phillips and F. Jiang, J. Colloid Interface Sci., 2012, 388, 103–111 CrossRef CAS PubMed.
- X. Li, Y. Fang, G. O. Phillips and S. Al-Assaf, J. Agric. Food Chem., 2013, 61, 1388–1396 CrossRef CAS PubMed.
- G. Mekhloufi, C. Sanchez, D. Renard, S. Guillemin and J. Hardy, Langmuir, 2004, 21, 386–394 CrossRef PubMed.
- X. Li, Y. Fang, S. Al-Assaf, G. O. Phillips, X. Yao, Y. Zhang, M. Zhao, K. Zhang and F. Jiang, Langmuir, 2012, 28, 10164–10176 CrossRef CAS PubMed.
- F. Weinbreck, R. de Vries, P. Schrooyen and C. G. de Kruif, Biomacromolecules, 2003, 4, 293–303 CrossRef CAS PubMed.
- F. Weinbreck, H. S. Rollema, R. H. Tromp and C. G. D. Kruif, Langmuir, 2004, 20, 6389–6395 CrossRef CAS PubMed.
- N. Garti and M. E. Leser, Polym. Adv. Technol., 2001, 12, 123–135 CrossRef CAS.
- T. Mahendran, P. A. Williams, G. O. Phillips, S. Al-Assaf and T. C. Baldwin, J. Agric. Food Chem., 2008, 56, 9269–9276 CrossRef CAS PubMed.
- A. K. Stone and M. T. Nickerson, Food Hydrocolloids, 2012, 27, 271–277 CrossRef CAS.
- D. Djordjevic, L. Cercaci, J. Alamed, D. J. Mcclements and E. A. Decker, J. Food Sci., 2008, 73, 167–172 CrossRef PubMed.
- A. Benichou, A. Aserin and N. Garti, J. Dispersion Sci. Technol., 2002, 23, 93–123 CrossRef CAS.
- A. Paraskevopoulou, D. Boskou and V. Kiosseoglou, Food Chem., 2005, 90, 627–634 CrossRef CAS.
- F. Weinbreck, V. R. De, P. Schrooyen and C. G. de Kruif, Biomacromolecules, 2003, 4, 293–303 CrossRef CAS PubMed.
- R. A. Buffo, G. A. Reineccius and G. W. Oehlert, Food Hydrocolloids, 2001, 15, 53–66 CrossRef CAS.
- L. Wang, Y. Cao, K. Zhang, Y. Fang, K. Nishinari and G. O. Phillips, Colloids Surf., A, 2015, 482, 604–610 CrossRef.
- X. Yao, Q. Xu, D. Tian, N. Wang, Y. Fang, Z. Deng, G. O. Phillips and J. Lu, J. Agric. Food Chem., 2013, 61, 4639–4645 CrossRef CAS PubMed.
- Y. Fang, L. Li, C. Inoue, L. Lundin and I. Appelqvist, Langmuir, 2006, 22, 9532–9537 CrossRef CAS PubMed.
- S. Xiang, X. Yao, W. Zhang, K. Zhang, Y. Fang, K. Nishinari, G. O. Phillips and F. Jiang, Food Hydrocolloids, 2015, 48, 110–116 CrossRef CAS.
- E. Dickinson, Food Hydrocolloids, 2009, 23, 1473–1482 CrossRef CAS.
- T. Moschakis, B. S. Murray and E. Dickinson, Langmuir, 2006, 22, 4710–4719 CrossRef CAS PubMed.
- S. Marze, Food Funct., 2015, 6, 3218–3227 CAS.
- A. Goki, K. Naoko, H. Masashi and M. Kazuo, J. Oleo Sci., 2009, 58, 329–338 CrossRef.
- J. R. Mancuso, D. J. Mcclements and E. A. Decker, J. Agric. Food Chem., 1999, 47, 4112–4116 CrossRef CAS PubMed.
- L. Mei, D. J. Mcclements and E. A. Decker, J. Agric. Food Chem., 1999, 47, 2267–2273 CrossRef CAS PubMed.
- L. L. Wang and Y. L. Xiong, J. Agric. Food Chem., 2005, 53, 9186–9192 CrossRef CAS PubMed.
- F. Habibollah, M. D. Julian and E. A. Decker, J. Agric. Food Chem., 2004, 52, 4558–4564 CrossRef PubMed.
- V. Angélique, V. Michèle, B. Isabelle, M. Nathalie and G. Claude, J. Agric. Food Chem., 2005, 53, 1514–1520 CrossRef PubMed.
- M. Sugiarto, A. Ye, M. W. Taylor and H. Singh, Dairy Sci. Technol., 2010, 90, 87–98 CrossRef CAS.
- M. R. Clausen, L. H. Skibsted and S. Jan, J. Agric. Food Chem., 2009, 57, 2912–2919 CrossRef CAS PubMed.
- S. Koohikamali and S. M. M. Kamal, Eur. J. Lipid Sci. Technol., 2014, 117, 637–645 CrossRef.
- C. Berton, M. H. Ropers, M. Viau and C. Genot, J. Agric. Food Chem., 2011, 59, 5052–5061 CrossRef CAS PubMed.
- R. Elias, D. McClements and E. Decker, Food Chem., 2007, 104, 1402–1409 CrossRef CAS.
- J. J. Baumy and G. Brule, Dairy Sci. Technol., 1988, 68, 409–417 CrossRef CAS.
- M. Diaz and E. A. Decker, J. Agric. Food Chem., 2004, 52, 8208–8213 CrossRef CAS PubMed.
- H. Chen, J. Diao, Y. Li, Q. Chen and B. Kong, Meat Sci., 2016, 111, 60–66 CrossRef CAS PubMed.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra26040j |
| This journal is © The Royal Society of Chemistry 2016 |