Facile synthesis of MnOx nanoparticles sandwiched between nitrogen-doped carbon plates for lithium ion batteries with stable capacity and high-rate capability†
Xiangyang Zhoua,
Tao Baia,
Feng Chena,
JingJing Tanga,
Qunchao Liaoa,
Yingrui Zhaob and
Juan Yang*a
aSchool of Metallurgy and Environment, Central South University, Changsha 410083, China. E-mail: j-yang@csu.edu.cn
bSchool of Materials Science and Engineering, Central South University, Changsha 410083, China
First published on 27th January 2016
Abstract
In this work, a material consisting of MnOx nanoparticles sandwiched between nitrogen-doped carbon plates (C/MnOx/C) has been successfully synthesized via a step-by-step strategy. It is demonstrated that the MnOx nanoparticles are well sandwiched between the double nitrogen-doped platelike carbon sheets. As an anode material for lithium-ion batteries, the double nitrogen-doped platelike carbon sheets encapsulating MnOx can not only address the issues related to the aggregation and volumetric changes of manganese oxides during the Li+ insertion/extraction, but also effectively shorten the transport path of Li+ ions and enhance the conductivity. As a result, the prepared C/MnOx/C composite exhibits stable cycling performance and superior high rate capability. The reversible capacity of C/MnOx/C after 100 cycles is as high as 770.9 mA h g−1, which is comparable with the initial capacity at 0.2 A g−1, and even at a high rate at 1 A g−1, it can deliver a high reversible of 443.9 mA h g−1, demonstrating the rational architecture design of the encapsulation of MnOx with nitrogen-doped platelike carbon layers.
Introduction
With the growing market demand for electric vehicles and energy storage systems, lithium-ion batteries (LIBs) have attracted great attention and become a promising power source.1–3 The electrode materials are one of the most critical influences on electrochemical performances of LIBs.4 Therefore, it is important to exploit electrode materials with long cycle life, low cost, high reversible capacity and excellent rate capability.5,6 Transition metal oxides display higher theoretical reversible capacities than commercial graphite materials due to an unusual conversion reaction mechanism.7–11 Manganese oxides including MnO, Mn3O4, Mn2O3 and MnO2 benefit from high theoretical capacity, low cost, low toxicity, natural abundance and environmental friendliness, have been widely studied as potential anode materials for LIBs.12–16 However, the application of pure manganese oxides as practical electrodes is still hampered, which is due to their low rate capability arising from the limited electrical conductivity and rapid capacity fading arising from the huge volume change upon cycling.17–19To date, many strategies have been made to improve these problems. One of the effective methods is reducing the dimension of the particles to nanometer to shorten the diffusion length of lithium ions and electrons.20,21 Meanwhile, the synthesis of various nanostructures such as nanotubes, hollow spheres or other porous nanostructures in electrode materials is also an effective way, which can enhance the capacity retention by alleviating the volume changes due to their interior hollow spaces.14,22–24 In addition, combining manganese oxides with carbon materials is one of the most used methods. Generally, carbonaceous materials such as amorphous carbon, carbon nanotubes, carbon nanofibers and graphene sheets are often used as both a volume buffer to absorb the internal stress and a conductive network to increase ion and electron transport in the electrodes.25 These approaches, such as MnO/reduced graphene oxide composite,26 N-doped MnO/graphene nanosheets,27 MnO/multi-walled carbon nanotubes composite28 and 3D porous MnO/C microspheres,6 have been proved to be effective for enhancing the rate performance and cycling stability of manganese oxides-based hybrid electrode. However, most of the manganese oxides and carbonaceous materials were combined in loose state. It is inevitable that manganese oxides will fall off from carbonaceous materials during the repeated charge and discharge process. As a result, those manganese oxides will lose the stress absorption and high conductivity benefited from carbonaceous materials, which will affect the electrochemical performances. Therefore, a rational design of manganese oxides/carbon hybrid is of great significant to solve these problems.
In this work, MnOx nanoparticles sandwiched between nitrogen-doped carbon plates architecture (C/MnOx/C) was synthesized. In this structure, MnOx nanoparticles are evenly dispersed between two thin layers of carbon plates. This structure has novel advantages. On one hand, the double carbonized poly-dopamine sheets can efficiently prevent manganese oxides aggregation and buffer the volume change of MnOx during the Li-ion insertion/extraction process. On the other hand, the highly conductive N-doped carbon plates could shorten the transport path of Li+ ions and enhance the conductivity. In order to obtain the structure, we use basic magnesium carbonate as hard template and dopamine as carbon source to synthesize N-doped platelike carbon, which acts as supporting framework for C/MnOx/C sandwich plates composite. And then, the precursor of MnOx nanoparticles were grew on the platelike carbon through the reaction between hydrazine and Mn(Ac)2 in ethylene glycol. After coating on poly-dopamine, the as-obtained product was annealed in H2/Ar atmosphere. As expected, the C/MnOx/C sandwich plates composite exhibits excellent lithium storage performance. It could deliver a reversible capacity of 770.9 mA h g−1 after 100 cycles at a current density of 0.2 A g−1, which is much higher than platelike carbon/MnOx (543.6 mA h g−1) and MnOx (81.6 mA h g−1).
Experimental
Synthesis of the platelike carbon
In a typical synthesis, 1 g of basic magnesium carbonate (4MgCO3Mg(OH)2·5H2O) was dispersed in 400 mL of 10 mM Tris solution (pH = 8.5) containing 2 g of polyethylene polypropylene glycol (F127). Then, 1 g of dopamine hydrochloride was added in with continuous stirring in air for about 12 h. Similarly, the precipitate was collected by filtration, washed with deionized water and absolute ethanol then dried at 60 °C. Afterwards, the resulting product was heated at 800 °C for 2 h in N2 atmosphere with a heating rate of 5 °C min−1. After washing the resulting sample with 1 mol L−1 HCl solutions for 12 h, the carbon was collected by filtration, washed with deionized water and dried at 70 °C for 12 h.Synthesis of platelike carbon/MnOx composite
0.1 g of the as prepared platelike carbon (carbonized poly-dopamine) was dispersed in 150 mL ethylene glycol and sonicated for 0.5 h, and then 0.9 g Mn(CH3COO)2·4H2O was added in under stirring, followed by adding 1 mL hydrazine hydrate (80 wt%). The resultant solution was kept stirring for 6 h at 110 °C. After cooling to room temperature, the precipitate was collected by filtration, washed with deionized water. Finally, the precursor material was annealed in Ar/H2 mixing atmosphere (Ar![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2 = 95%
H2 = 95%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5%,volume) at 550 °C for 2 h at a heating rate of 2 °C min−1. The carbonized poly-dopamine/MnOx was designated as CM. For comparison, the pure MnOx was obtained in the absence of porous carbon, in which other conditions were kept the same.
5%,volume) at 550 °C for 2 h at a heating rate of 2 °C min−1. The carbonized poly-dopamine/MnOx was designated as CM. For comparison, the pure MnOx was obtained in the absence of porous carbon, in which other conditions were kept the same.
Synthesis of the C/MnOx/C sandwich plates composite
0.1 g of the as prepared platelike carbon was dispersed in 150 mL ethylene glycol and sonicated for 0.5 h, and then 1.2 g Mn(CH3COO)2·4H2O was added in under stirring, followed by adding 1 mL hydrazine hydrate (80 wt%). The resultant solution was kept stirring for 6 h at 110 °C. Afterwards, the precipitate was dispersed in 400 mL of 10 mM Tris solution containing 0.4 g of F127. After that, 0.2 g of dopamine hydrochloride was added with continuous stirring in air for about 12 h. A precursor material was obtained by filtration, washed with deionized water and dried at 60 °C. Finally, the precursor material was annealed in Ar/H2 mixing atmosphere (Ar![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) H2 = 95%
H2 = 95%![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 5%, volume) at 550 °C for 2 h at a heating rate of 2 °C min−1, and the carbonized poly-dopamine/MnOx/carbonized poly-dopamine sandwich plates composite was designated as CMCS.
5%, volume) at 550 °C for 2 h at a heating rate of 2 °C min−1, and the carbonized poly-dopamine/MnOx/carbonized poly-dopamine sandwich plates composite was designated as CMCS.
Material characterization
The products were characterized by X-ray diffraction (XRD, Rigaku-TTRIII), scanning electron microscope (SEM, JSM-6360LV), transmission electron microscopy (TEM, JEM-2100F), X-ray photoelectron spectra (XPS, K-Alpha 1063), thermogravimetric analysis (TGA, SDTQ600), Raman spectroscopy (LabRAM Hr800) and elemental analysis (EuroEA3000 analyzer).Electrochemical measurement
Electrochemical measurements were performed by using coin-type 2025 cells. The working electrodes were prepared as follows. First the as-synthesized active materials, carbon black and polyvinyl-difluoride (PVDF) were mixed in NMP with a weight ratio of 8![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1
1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1, and then the slurry was pasted onto Cu foil. The electrolyte consists of a solution of 1 M LiPF6 in ethylene carbonate (EC)/dimethyl carbonate (DMC) (volume ratio of 1
1, and then the slurry was pasted onto Cu foil. The electrolyte consists of a solution of 1 M LiPF6 in ethylene carbonate (EC)/dimethyl carbonate (DMC) (volume ratio of 1![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) :
:![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 1). Pure lithium foils were used as counter electrodes, and polypropylene membranes from Celgard were used as the separators. The mass loading of the active materials are about 1.0 mg cm−2. The cells were assembled in a glove-box filled with argon. The charge and discharge performances were carried out on a LAND battery measurement system within a range of 0.01–3 V at different current densities. Cyclic voltammograms experiments were performed in the potential window of 0.01–3.0 V at a scanning rate of 0.2 mV s−1. Electrochemical impedance spectroscopy measurements were performed with electrochemical workstation in the frequency window of 100
1). Pure lithium foils were used as counter electrodes, and polypropylene membranes from Celgard were used as the separators. The mass loading of the active materials are about 1.0 mg cm−2. The cells were assembled in a glove-box filled with argon. The charge and discharge performances were carried out on a LAND battery measurement system within a range of 0.01–3 V at different current densities. Cyclic voltammograms experiments were performed in the potential window of 0.01–3.0 V at a scanning rate of 0.2 mV s−1. Electrochemical impedance spectroscopy measurements were performed with electrochemical workstation in the frequency window of 100![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) 000–0.01 Hz.
000–0.01 Hz.
Results and discussion
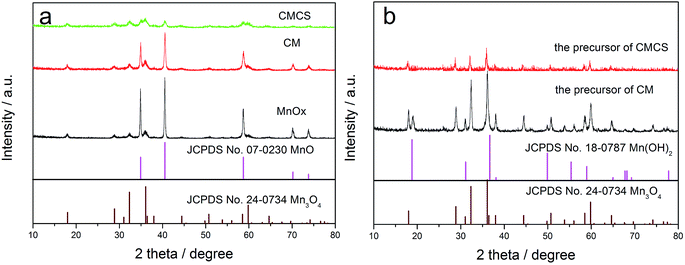
The XRD patterns of the as-prepared MnOx, CM and CMCS are shown in Fig. 1a. The dominant diffractions can be indexed to the cubic MnO (JCPDS no. 07-0230), and the other weak diffractions are ascribed to the hausmannite structure of Mn3O4 (JCPDS no. 24-0734) probably owing to the oxidation of MnO in air.29 It can be seen from Fig. 1a that diffraction peaks of CMCS are much lower than those of MnOx and CM, this is probably due to the surface of CMCS is coated by carbonized poly-dopamine. Fig. 1b shows the XRD patterns of the precursors of CM and CMCS. The dominant diffractions can be indexed to Mn3O4 (JCPDS no. 24-0734), and the other weak diffractions are ascribed to Mn(OH)2 (JCPDS no. 18-0787). The main phase in the precursor of CMCS is Mn3O4, which means that most of Mn(OH)2 in the precursor of CM are oxidized to be Mn3O4 in the process of coating poly-dopamine.The final decomposition products of heating CM and CMCS in air are Mn2O3.30 According to the TGA curves shown in Fig. S1,† the final weight of CM and CMCS is 71.7% and 63.0% respectively, which means that the Mn content in CM and CMCS is 49.9 wt% and 43.9 wt%. As shown in Table S1,† the O, C and N in CM evaluated by CHONS element analysis are 16.7 wt%, 30.39 wt% and 3.21 wt%, respectively. Thus, the content of Mn in CM is 49.7 wt% under ignoring other elements, which is consistent with the result of TGA.
Raman spectra of the as-prepared CM and CMCS are shown in Fig. 2a. Two broaden peaks at ca. 1590 cm−1 and 1360 cm−1 assigning to the G band (graphitic carbon) and D band (disordered carbon) of carbon materials31 can be observed in the spectra of the composites. Meanwhile, the characteristic peak at 640 cm−1 should be ascribed to the Mn–O vibration mode.27 The signal intensity for the metal–oxygen bond of the sample CM is higher than that of the sample CMCS, which may be due to the fact that the Mn content in CM is higher than that in CMCS and there is carbonized poly-dopamine coated on the surface of CMCS. XPS spectra (Fig. 2b) of the as-prepared of CM and CMCS show the presence of C, N, O and Mn elements. The high-resolution C 1s, N 1s, O 1s and Mn 2p spectra for CMCS are shown in Fig. 2c–f. The C 1s peak could be deconvoluted into three peaks centered at ca. 289.1, 285.5, 284.7 eV corresponding to the C–O, N–sp2C, sp2–sp2C type bonds, respectively. The N 1s spectrum could be deconvoluted into three peaks at 403.9, 400.4 and 398.3 eV (Fig. 2d), which are attributed to graphitic N, pyrrolic N and pyridinic N bonds, respectively. In the O 1s spectrum (Fig. 2e), three deconvoluted peaks at 533, 530.9 and 529.7 eV can be assigned to H–O, C–O and Mn–O, respectively. The H–O bond originates from water absorbed on the sample surface after exposed in air.27,29,32,33 The high-resolution Mn 2p spectrum (Fig. 2f) exhibits two signals at 641.5 eV for Mn 2p3/2 and 653.0 eV for Mn 2p1/2, respectively. However, the binding energies of Mn 2p3/2 for MnO, Mn3O4, Mn2O3 and MnO2 locate in the small range of 642.2–640.4 eV, which are difficult to distinguish due to the measurement resolution in experiment.34,35 The high-resolution C 1s, N 1s, O 1s and Mn 2p spectra for CM are shown in Fig. S2.†
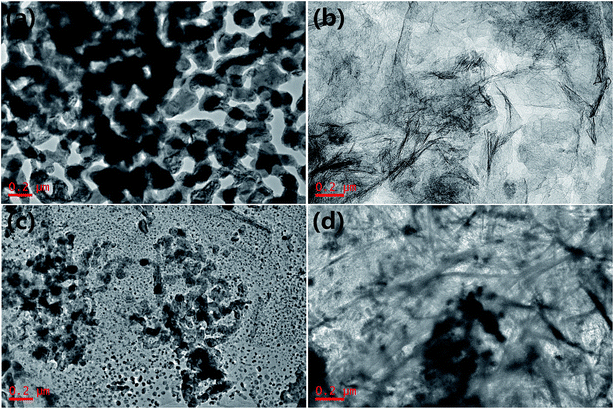
The morphologies and nanostructures of the synthesized products were investigated by SEM and TEM. As shown in Fig. 3a and 4a, pure MnOx is composed of small particles with an average size of about 200 nm and these nanoparticles aggregate slightly. In this work, basic magnesium carbonate acted as hard template for carbon, will largely determine the morphology of the as-prepared carbon. Fig. S3† displays the SEM image of basic magnesium carbonate, demonstrating the rough surfaces of interconnected thick sheets. Fig. 3b and 4b show the morphology of the as-prepared carbon. The platelike carbon is composed of large numbers of interconnected thin carbon sheets, indicating that poly-dopamine was synthesized and coated on the surfaces of basic magnesium carbonate. As revealed in Fig. 3c and 4c, the morphology of carbon matrix in CM is maintained. Meanwhile, the MnOx nanoparticles in CM have two different average sizes, ca. 120 nm and ca. 20 nm, respectively. This is probably due to that the platelike carbon with rough surface acts as supporting material for the MnOx nanoparticles and restricts these particles size, while the MnOx nanoparticles grew in the solution without restriction from carbon sheets have a larger size and these particles distribute in the interlayer of the thin carbon sheets. From Fig. 3d, one can see that the surface of CMCS is coated by carbonized poly-dopamine, and there are no MnOx nanoparticles on the surface of CMCS, demonstrating that MnOx nanoparticles are well sandwiched between the double nitrogen-doped platelike carbon sheets. Fig. 4d shows that the nanostructure under carbonized poly-dopamine is similar to CM, demonstrating that coating poly-dopamine would not change the nanostructure of CM. Therefore, the difference between CM and CMCS is that MnOx nanoparticles in CM are distributed on the surface of platelike carbon and the MnOx nanoparticles in CMCS are sandwiched between the double nitrogen-doped platelike carbon sheets. Fig. S4† is the high-resolution TEM (HRTEM) image of CMCS. The lattice distance of 0.496 nm and 0.258 nm correspond to the (1 0 1) planes of Mn3O4 and the (1 1 1) planes of MnO, respectively.30,36 Mn3O4 is outside of MnO probably owing to the oxidation of MnO in air.
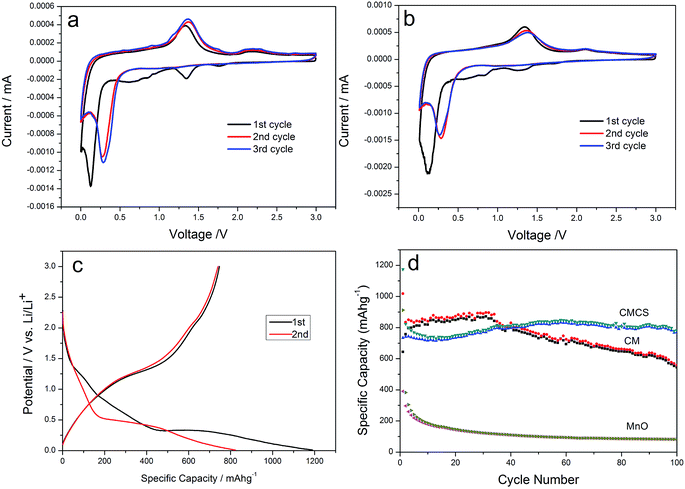
The electrochemical performances of the composites were investigated as anode materials in lithium ion batteries. Their electrochemical properties were first studied by the cyclic voltammogram curves. Fig. 5a and b show typical CV curves of CM and CMCS electrodes for the initial three cycles at a scan rate of 0.2 mV s−1 in the voltage range of 0.01–3.0 V vs. Li/Li+. It is clear to see that CM and CMCS have a reduction peak at ca. 1.3 V, which can be ascribed to the reduction of Mn3+ (Mn3O4) to Mn2+.37 From the second cycle, the reduction peak disappeared, indicating that few or no Mn3+ is generated in the oxidation process. In addition, two irreversible reduction peaks at around 0.85 V and 0.6 V correspond to the irreversible reduction of the electrolyte and the formation of a solid electrolyte interphase (SEI) layer, respectively.38 In addition, the sharp reduction peak at ca. 0.13 V refers to the transformation from Mn2+ to Mn0. The reduction peak shifts to ca. 0.28 V in the subsequent cycles, indicating a microstructure alteration arising from the formation of Mn and Li2O.39 The main oxidation peak at ca. 1.35 V could be ascribed to the oxidation reaction of Mn0 to Mn2+. A weak peak appears at ca. 2.1 V corresponding to the decomposition of the polymer/gel layer at high oxidation potential above 2.0 V.40 The CV profiles demonstrate that metallic Mn and Li2O are the end products of discharge to 0.01 V, while MnO is the end product of recharge to 3.0 V for CM and CMCS. Fig. 5c shows the first and second galvanostatic charge–discharge profiles of CMCS at a current density of 0.2 A g−1. The charge–discharge profiles match very well with the above CV. The initial discharge and charge specific capacities capacity are 1190.6 and 746.7 mA h g−1, respectively. The formation of solid electrolyte interphase (SEI) films is the mainly reason for leading the irreversible capacity.33
The cycling stability of CMCS electrode was investigated by comparing with that of CM and MnOx at a current density of 0.2 A g−1. The formation of SEI films is the mainly reason for the capacity loss after the initial cycle, while the diffusion length and volume change for lithium insertion/extraction is normally the influence for the further capacity loss.36 As shown in Fig. 5d, it can be clearly observed that CMCS shows the most excellent cycling performance. After 100 cycles, the capacity of CMCS is still as high as 770.9 mA h g−1. On the contrary, the capacity of CM and MnOx drops to 543.6 mA h g−1 and 81.6 mA h g−1 after 100 cycles. As shown in Table S2,† the electrochemical performance of the CMCS is superior among those of the reported manganese oxide materials. The theoretical capacity of MnO is 755 mA h g−1 and the capacity of carbon is mostly less than that of MnO. However, the reversible capacities of CMCS and CM are higher than the theoretical value (755 mA h g−1). This can be attributed to the synergistic effect between MnOx and nitrogen-doped carbon plates, that is polymeric gel-like film formed on the surface of active materials during the repeated charge and discharge processes.7
The SEM and TEM were applied to further examine the morphologies of CMCS and CM after 100 cycles. Fig. 6a and b show the morphology of CMCS electrode before discharge/charge and after 100 cycles. It can be seen that the structure of active materials in CMCS electrode surrounded by PVDF can still be maintained in the electrode. In contrast, the morphology of CM electrode after 100 cycles shown in Fig. 5Sb† exhibits a more loose structure and many holes appear on the surface of electrode, which is possible that MnO particles fell from the carbon plate during the process of volume expansion/contraction. It is worth noting that TEM results (Fig. 6d and S5d†) show that both the MnOx nanoparticles in CM and CMCS turned into a diameter of about 5 nm small particles after 100 cycles. The lattice distance of 0.216 nm correspond to the (2 0 0) planes of MnO. These small particles tended to be larger particles due to the huge surface energy, and then fell from the electrode. This is the primary reason for the capacity loss. As shown in Fig. 6c and S5c,† MnO particles in CMCS still uniformly distributed in double carbonized poly-dopamine sheets, while MnO particles in CM aggregated slightly due to the protection is incomplete came from a single platelike carbon. These large particles fell easily from the electrode, and then formed loose structure and many holes (Fig. S5b†). These results indicate that platelike carbon acts as supporting material, which could alleviate the gradual aggregation of metal grains and the loss of electronic contact of active particles resulting from large volume expansion/contraction during the repeated conversion reaction. However, the MnOx nanoparticles in CM distributed on the surfaces of platelike carbon will still suffer these issues. Therefore, the capacity of CM electrode drops rapidly after 35 cycles. The MnOx nanoparticles in CMCS are well encapsulated between the double carbonized poly-dopamine sheets. Even though the volume expansion of MnOx still occurs, the carbonized poly-dopamine will ensure the structural stability of MnOx during the Li-ion insertion/extraction process. That's the reason why CMCS could keep a stable reversible capacity after 100 cycles.
 | ||
| Fig. 6 SEM images of CMCS electrode (a) before discharge/charge, (b) after discharge/charge. (c) TEM image of CMCS after discharge/charge. (d) HRTEM image of CMCS after discharge/charge. | ||
The consecutive cycling behavior of CMCS at various charge and discharge current densities, measured after 10 cycles in ascending steps from 0.1 A g−1 to 1.0 A g−1, followed by a repetitive process, is presented. As shown in Fig. 7a, the capacity fades gradually with the increasing of the current density, but retains stable at each current density. Even at 1.0 A g−1, the reversible capacity is 443.9 mA h g−1 in the first procedure and 328.8 mA h g−1 in the second procedure. Importantly, when the two measuring procedures is end and the current density is returned to 0.1 A g−1, the capacity of CMCS is recovered to 713.4 mA h g−1 and remains 732.4 mA h g−1 after 130 cycles. The electrochemical results suggest that the double nitrogen-doped platelike carbon sheets can efficiently buffer the volume change of MnOx during the Li-ion insertion/extraction process and improve the electrical conductivity.
 | ||
| Fig. 7 (a) Rate performances (discharge) of CMCS at various rates. (b) The electrochemical impedance spectra of CMCS, CM and MnOx. | ||
EIS is an effective way to evaluate the electron conductivity and Li diffusion of materials. Prior to the EIS tests, the cells were activated for 3 cycles at a current density of 0.2 A g−1 to obtain stable electrodes. The resistance values were evaluated by the equivalent circuit (Fig. S6†). In the equivalent circuit, Ro, RS, Rct and ZW represent the ohmic resistance of the cell, the SEI resistance, the charge-transfer resistance and Warburg diffusion impedance, respectively. According to the pervious papers, Rct directly reflects the kinetics of electrode reactions and low Rct generally favors fast charge-transfer response.41 As shown in Fig. 7b, Rct of the pure MnOx, CM and CMCS electrodes are 63.59 Ω, 35.6 Ω and 24.78 Ω, which suggested that CMCS electrode possess a lowest charge transfer resistance. It means that the double carbonized poly-dopamine layer could greatly improve the activation and kinetics of conversion reaction upon Li-ion insertion/extraction. Thus, CMCS shows the best reversible capacity and wonderful rate capability.
Conclusion
In summary, we have demonstrated a facile strategy to fabricate C/MnOx/C sandwich plates composite using a solvothermal method followed by annealing treatment. The resultant composite exhibits high reversible capacity, excellent cycling stability and exceptional rate performance when used as anode materials in LIBs. It can deliver a reversible capacity of 770.9 mA h g−1 at 0.2 A g−1 after 100 cycles and a stable reversible capacity of 443.9 mA h g−1 at a high current density of 1.0 A g−1. Compared with CM and MnOx, CMCS has the best electrochemical performances because of the encapsulation of double nitrogen-doped platelike carbon sheets, which can not only address the issues related to manganese oxides aggregation and volumetric changes during the Li+ insertion/extraction, but also effectively shorten the transport path of Li+ ions and enhance the conductivity.Acknowledgements
This study were Supported by the National Nature Science Foundation of China (Grant no. 51204209 and 51274240) and Grants from the Project of Innovation-driven Plan in Central South University.Notes and references
- K. Su, C. Wang, H. Nie, Y. Guan, F. Liu and J. Chen, J. Mater. Chem. A, 2014, 2, 10000 CAS.
- V. Etacheri, R. Marom, R. Elazari, G. Salitra and D. Aurbach, Energy Environ. Sci., 2011, 4, 3243 CAS.
- W.-M. Chen, L. Qie, Y. Shen, Y.-M. Sun, L.-X. Yuan, X.-L. Hu, W.-X. Zhang and Y.-H. Huang, Nano Energy, 2013, 2, 412–418 CrossRef CAS.
- Y. Sun, X. Hu, W. Luo and Y. Huang, J. Mater. Chem., 2012, 22, 19190 RSC.
- Y. Xiao, X. Wang, W. Wang, D. Zhao and M. Cao, ACS Appl. Mater. Interfaces, 2014, 6, 2051–2058 CAS.
- H. Hu, H. Cheng, Z. Liu and Y. Yu, Electrochim. Acta, 2015, 152, 44–52 CrossRef CAS.
- Q. Wang, D.-A. Zhang, Q. Wang, J. Sun, L.-L. Xing and X.-Y. Xue, Electrochim. Acta, 2014, 146, 411–418 CrossRef CAS.
- L.-L. Xing, B. He, Y.-X. Nie, P. Deng, C.-X. Cui and X.-Y. Xue, Mater. Lett., 2013, 105, 169–172 CrossRef CAS.
- I. Nam, N. D. Kim, G.-P. Kim, J. Park and J. Yi, J. Power Sources, 2013, 244, 56–62 CrossRef CAS.
- Y. Xiao, C. Hu and M. Cao, J. Power Sources, 2014, 247, 49–56 CrossRef CAS.
- J. Qu, Y. X. Yin, Y. Q. Wang, Y. Yan, Y. G. Guo and W. G. Song, ACS Appl. Mater. Interfaces, 2013, 5, 3932–3936 CAS.
- J. Yue, X. Gu, L. Chen, N. Wang, X. Jiang, H. Xu, J. Yang and Y. Qian, J. Mater. Chem. A, 2014, 2, 17421–17426 CAS.
- X. Zhang, Z. Xing, L. Wang, Y. Zhu, Q. Li, J. Liang, Y. Yu, T. Huang, K. Tang, Y. Qian and X. Shen, J. Mater. Chem., 2012, 22, 17864 RSC.
- Z. Bai, N. Fan, Z. Ju, C. Guo, Y. Qian, B. Tang and S. Xiong, J. Mater. Chem. A, 2013, 1, 10985 CAS.
- Z. Bai, Y. Zhang, Y. Zhang, C. Guo, B. Tang and D. Sun, J. Mater. Chem. A, 2015, 3, 5266–5269 CAS.
- L. Xing, C. Cui, C. Ma and X. Xue, Mater. Lett., 2011, 65, 2104–2106 CrossRef CAS.
- J. Huang, J. Xie, K. Chen, L. Bu, S. Lee, Z. Cheng, X. Li and X. Chen, Chem. Commun., 2010, 46, 6684–6686 RSC.
- X. Sun, Y. Xu, P. Ding, M. Jia and G. Ceder, J. Power Sources, 2013, 244, 690–694 CrossRef CAS.
- G. L. Xu, Y. F. Xu, J. C. Fang, F. Fu, H. Sun, L. Huang, S. Yang and S. G. Sun, ACS Appl. Mater. Interfaces, 2013, 5, 6316–6323 CAS.
- F. J. Douglas, D. A. MacLaren, F. Tuna, W. M. Holmes, C. C. Berry and M. Murrie, Nanoscale, 2014, 6, 172–176 RSC.
- D. Qiu, L. Ma, M. Zheng, Z. Lin, B. Zhao, Z. Wen, Z. Hu, L. Pu and Y. Shi, Mater. Lett., 2012, 84, 9–12 CrossRef CAS.
- Z. Bai, X. Zhang, Y. Zhang, C. Guo and B. Tang, J. Mater. Chem. A, 2014, 2, 16755–16760 CAS.
- G. Jian, Y. Xu, L.-C. Lai, C. Wang and M. R. Zachariah, J. Mater. Chem. A, 2014, 2, 4627 CAS.
- W. Luo, X. Hu, Y. Sun and Y. Huang, ACS Appl. Mater. Interfaces, 2013, 5, 1997–2003 CAS.
- T. Wang, Z. Peng, Y. Wang, J. Tang and G. Zheng, Sci. Rep., 2013, 3, 2693 Search PubMed.
- G. Zhao, X. Huang, X. Wang, P. Connor, J. Li, S. Zhang and J. T. S. Irvine, J. Mater. Chem. A, 2015, 3, 297–303 CAS.
- K. Zhang, P. Han, L. Gu, L. Zhang, Z. Liu, Q. Kong, C. Zhang, S. Dong, Z. Zhang, J. Yao, H. Xu, G. Cui and L. Chen, ACS Appl. Mater. Interfaces, 2012, 4, 658–664 CAS.
- W. B. Luo, S. L. Chou, W. Jia-Zhao, Y. C. Zhai and H. K. Liu, Sci. Rep., 2015, 5, 8012 CrossRef CAS PubMed.
- G. Lu, S. Qiu, H. Lv, Y. Fu, J. Liu, X. Li and Y.-J. Bai, Electrochim. Acta, 2014, 146, 249–256 CrossRef CAS.
- C. Yang, Q. Gao, W. Tian, Y. Tan, T. Zhang, K. Yang and L. Zhu, J. Mater. Chem. A, 2014, 2, 19975–19982 CAS.
- J. Zang, H. Qian, Z. Wei, Y. Cao, M. Zheng and Q. Dong, Electrochim. Acta, 2014, 118, 112–117 CrossRef CAS.
- C. Zhang, L. Fu, N. Liu, M. Liu, Y. Wang and Z. Liu, Adv. Mater., 2011, 23, 1020–1024 CrossRef CAS PubMed.
- S. Qiu, X. Wang, G. Lu, J. Liu and C. He, Mater. Lett., 2014, 136, 289–291 CrossRef CAS.
- Z. Cui, X. Guo and H. Li, J. Power Sources, 2013, 244, 731–735 CrossRef CAS.
- X. Fang, X. Lu, X. Guo, Y. Mao, Y.-S. Hu, J. Wang, Z. Wang, F. Wu, H. Liu and L. Chen, Electrochem. Commun., 2010, 12, 1520–1523 CrossRef CAS.
- S. Z. Huang, J. Jin, Y. Cai, Y. Li, H. Y. Tan, H. E. Wang, G. Van Tendeloo and B. L. Su, Nanoscale, 2014, 6, 6819–6827 RSC.
- B. Sun, Z. Chen, H.-S. Kim, H. Ahn and G. Wang, J. Power Sources, 2011, 196, 3346–3349 CrossRef CAS.
- X. Li, D. Li, L. Qiao, X. Wang, X. Sun, P. Wang and D. He, J. Mater. Chem., 2012, 22, 9189 RSC.
- Y. Sun, X. Hu, W. Luo, F. Xia and Y. Huang, Adv. Funct. Mater., 2013, 23, 2436–2444 CrossRef CAS.
- S. Wang, Y. Xing, H. Xu and S. Zhang, ACS Appl. Mater. Interfaces, 2014, 6, 12713–12718 CAS.
- J. Yang, Q. Liao, X. Zhou, X. Liu and J. Tang, RSC Adv., 2013, 3, 16449 RSC.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra26411a |
| This journal is © The Royal Society of Chemistry 2016 |