Iron triad (Fe, co, Ni) trinary phosphide nanosheet arrays as high-performance bifunctional electrodes for full water splitting in basic and neutral conditions†
Zhe Zhanga,
Jinhui Haoab,
Wenshu Yangab and
Jilin Tang*a
aState Key Laboratory of Electroanalytical Chemistry, Changchun Institute of Applied Chemistry, Chinese Academy of Sciences, Changchun 130022, People's Republic of China. E-mail: jltang@ciac.ac.cn; Fax: +86 431-85262734; Tel: +86 431-85262734
bUniversity of Chinese Academy of Sciences, Beijing, 100049, People's Republic of China
First published on 18th January 2016
Abstract
The development of an efficient and affordable bifunctional electrode for full water splitting in basic and neutral conditions is still a challenging issue in obtaining clean and sustainable chemical fuels. Herein, a unique bifunctional electrode consisting of 3D trinary transition-metal phosphide nanosheets with abundant reactive sites anchored on Ni foam, referred to as FexCoyNizP, has been developed through a facile two-step process of electrodeposition and low-temperature phosphidation. When used as a bifunctional electrode for the full water splitting, an applied voltage of 1.57 V is required for a current density of 10 mA cm−2.
Hydrogen generation from electrocatalytic and photoelectrocatalytic water splitting is an appealing way to address the continuous depletion of fossil fuels and the associated exacerbating environmental concerns.1–3 In order to achieve electrochemical water splitting, an applied voltage higher than the thermodynamic potential (1.23 V at 25 °C and 1 atm) must be applied. So far, Pt-based materials are the best electrocatalysts for hydrogen evolution reaction (HER), and IrO2 and RuO2 are the benchmark oxygen evolution reaction (OER) electrocatalysts.4,5 Nevertheless, these precious metals are not suitable for large-scale applications because of their scarcity and high costs. Therefore, extensive research efforts have been dedicated to rationally design and synthesize HER or OER electrocatalysts based on low-cost and earth-abundant elements with highly catalytic activity close to, or even better than, that of state-of-the-art precious metals.6,7 Recent studies have shown that transition-metal phosphates,8–12 chalcogenides,13–22 oxides,23–30 hydro(oxy)oxides,5,31–35 carbides,36–40 borides,41 nitrides,42–44 phosphides,45–55 perovskite oxides,56–59 and metal-free materials60–63 have relatively good HER or/and OER activities. Among them, transition metal phosphides (TMPs) have been found to be promising substitutes for precious metal-based HER electrocatalysts.
Over the past years, first-row transition metal-based materials, particularly iron triad (Fe, Co, Ni) materials have been widely investigated in the fields of drug delivery, biosensors, water treatment, catalysts, energy conversion and storage, etc.64,65 For example, Schaak and colleagues demonstrated that Ni2P nanoparticles, which have previously been predicted to be an active HER electrocatalyst, exhibited among the highest HER activity of any non-noble metal electrocatalysts reported to date.54 On the other hand, integration of two or more transition metals into heterogeneous materials to reduce the overpotential and increase OER activity in comparison with single-component materials has attracted a great deal of research attention. As one of the most abundant transition metals in the earth's crust, Fe is of particular interest as a dopant. For example, Smith and co-workers have confirmed that the presence of iron in binary and ternary films can significantly improve the OER activity and stability of electrocatalysts.66 The high electrochemical activities of heterogeneous materials maybe due to the presence of multiple valences of the cations with more complex electronic structure and higher electrical conductivity.67 However, the performance of these transition metal-based electrocatalysts for water splitting is still inferior to precious metal-based catalysts, and the complicated synthetic procedures and sophisticated fabrication techniques prevent for viable practical applications. Thus, effective and simple strategies are highly desirable to develop transition metal-based electrocatalysts with improved activity and stability.
Up to now, most of the non-noble metal catalysts have the best HER catalytic activity in the acidic electrolyte. Contrarily, the best non-noble metal catalysts for OER are required to operate under alkaline conditions. However, to realize practical applications, both the HER and OER electrocatalysts must operate in an integrated electrolyser to realize sustainable water splitting. Therefore, in order to simplify synthetic procedures and lower the cost, preparation of bifunctional electrocatalysts based on non-noble metal for both HER and OER in the same electrolyte is highly desirable. However, the research of bifunctional electrocatalysts is in its infancy, and most of them work well only in basic media and suffer from their low catalytic performance for either HER or OER.68–83 Therefore, it is still a great challenge to design new bifunctional materials for overall water splitting with low overpotential and long-term stability in a wide pH range. Additionally, the development of three-dimensional (3D) architectures of full water splitting catalysts on a conducting substrate (such as Ni foam with rich macroporosity, high conductivity and low price) is of great interest due to exposing more electrochemically active sites to the electrolyte and allowing easy diffusion of evolved gas bubbles to timely release active sites.84 All of the aforementioned problems are important and provide us useful directions to further design novel bifunctional electrocatalysts. One possibility to meet these requirements at the same time is the use of hybrid structures.
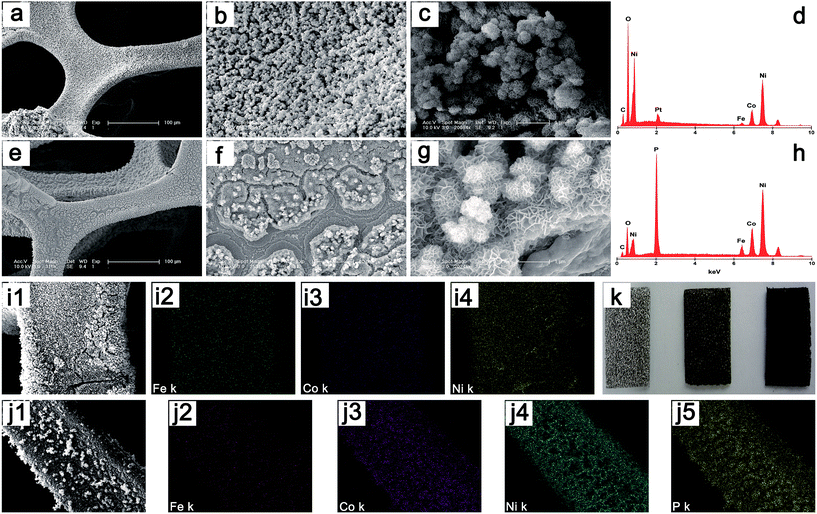
Inspired by the high HER activity of cobalt phosphides, good OER activity of nickel oxide/hydroxide, and the synergistic effect of iron doping, herein, for the first time, we synthesized iron triad-based phosphide (FexCoyNizP, x, y, and z stand for the molar concentration of Fe3+, Co2+ and Ni2+ in the electrolyte) nanosheet arrays supported on nickel foam simultaneously for HER and OER in basic and neutral media. The synthesis of FexCoyNizP nanosheet arrays on Ni foam could be divided into two steps: (1) FexCoyNiz nanosheet arrays are prepared by co-electrodeposition of Fe, Co, and Ni hydroxides onto the Ni foam support; (2) FexCoyNiz nanosheet arrays are converted into FexCoyNizP nanosheet arrays by a simple low-temperature phosphidation. Taking the Fe10Co40Ni40P as example, the morphologies of the Fe10Co40Ni40 nanostructures were inspected by scanning electron microscopy (SEM). As shown in Fig. 1a, during the convenient, energy-saving, and efficient electrodeposition process, Fe10Co40Ni40 with dense structure are successfully grown on Ni foam and a reliable contact between the Fe10Co40Ni40 and Ni foam are formed. Fig. 1b shows a high magnification top-view SEM image, it can be seen that the dense structure is comprised of numerous microspheres with the size of hundreds of nanometers. To further enlarge the SEM image (Fig. 1c), evidently, the Fe10Co40Ni40 microspheres are constructed by a large number of nanosheets that are interconnected with each other to self-assembled into flower-like structures. The chemical composition and structure of F Fe10Co40Ni40 nanosheet arrays were analyzed by energy-dispersive X-ray spectroscopy (EDX) and elemental mapping of field-emission SEM (FE-SEM). The corresponding EDX spectrum (Fig. 1d) verifies that these nanosheet arrays consist of Fe, Co, Ni, O and C. The peak representing Pt is from the sputtered Pt to increase the electrical conductivity for SEM measurements. Elemental mapping analysis taken from the nanosheet arrays (Fig. 1i) reveals that a uniform distribution of Fe, Co, and Ni throughout the whole region.
Fig. 1e shows a low-magnification SEM image of Fe10Co40Ni40P nanosheet arrays, in which the Ni foam is covered by a layer of Fe10Co40Ni40P film with some cracks induced by the high-temperature during phosphidation step. A close view of such film (Fig. 1f) reveal they are composed of a number of island-like structures with diameter up to several micrometers. A further magnification of the SEM image reveals that Fe10Co40Ni40P well inherits the nanosheet morphology of Fe10Co40Ni40 and self-assembled flowerlike structures are maintained (Fig. 1g), and the color of which changes to black (Fig. 1k). Compared with Fe10Co40Ni40, the EDX of Fe10Co40Ni40P (Fig. 1h) shows obvious peak corresponding to the elemental P, which indicates the successful phosphidation of Fe10Co40Ni40. Elemental mapping analysis (Fig. 1j) shows the uniform distribution of Fe, Co, Ni, and P throughout the whole region. The crystal structures of the Fe10Co40Ni40 and Fe10Co40Ni40P were examined by the X-ray diffraction patterns (XRD). Since it is hard to collect the powdered Fe10Co40Ni40 and Fe10Co40Ni40P from the Ni foam, the XRD patterns of bare Ni foam substrate, Fe10Co40Ni40 and Fe10Co40Ni40P nanosheets on substrate are measured. By subtracting the peaks from Ni foam substrate, the peak positions of Fe10Co40Ni40 (Fig. 2a) are in agreement with the well-known NiFe-LDH (JCPDF: 51-0463), which indicates that the Fe10Co40Ni40 has a LDH structure and is similar to NiFe-LDH.85 Transmission electron microscopy (TEM) and high-resolution TEM (HRTEM) images of the Fe10Co40Ni40 scraped from the Ni foam exhibit a layered morphology (Fig. 2b and c). The near transparency to the electron beams and existence of a large number of corrugations are indicative of the ultrathin nature. Fig. 2d is the XRD patterns of the Fe10Co40Ni40P, showing that the peaks are indexed to the complex phosphides of Ni2P (JCPDF: 03-0953) and CoP (JCPDF: 29-0497) doped with Fe element. The broad diffraction peaks suggests that the nanoscale dimensions of Fe10Co40Ni40P nanosheets. TEM and HRTEM show that these nanosheets are a polycrystalline structure consisted of a large number of nanoparticles with size about 5 nm (Fig. 2e and f), which is consistent with XRD results.
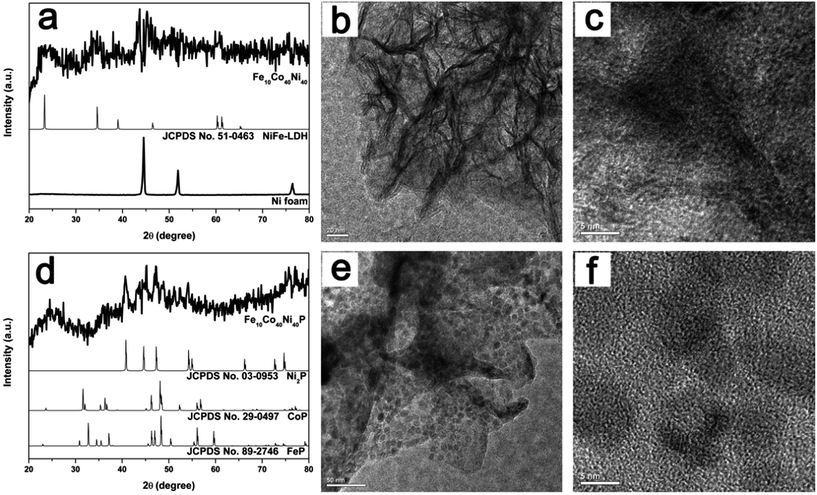 | ||
| Fig. 2 XRD patterns of Fe10Co40Ni40 (a) and Fe10Co40Ni40P (d) after subtraction of Ni foam signal. TEM and HRTEM images of Fe10Co40Ni40 (b and c) and Fe10Co40Ni40P (e and f). | ||
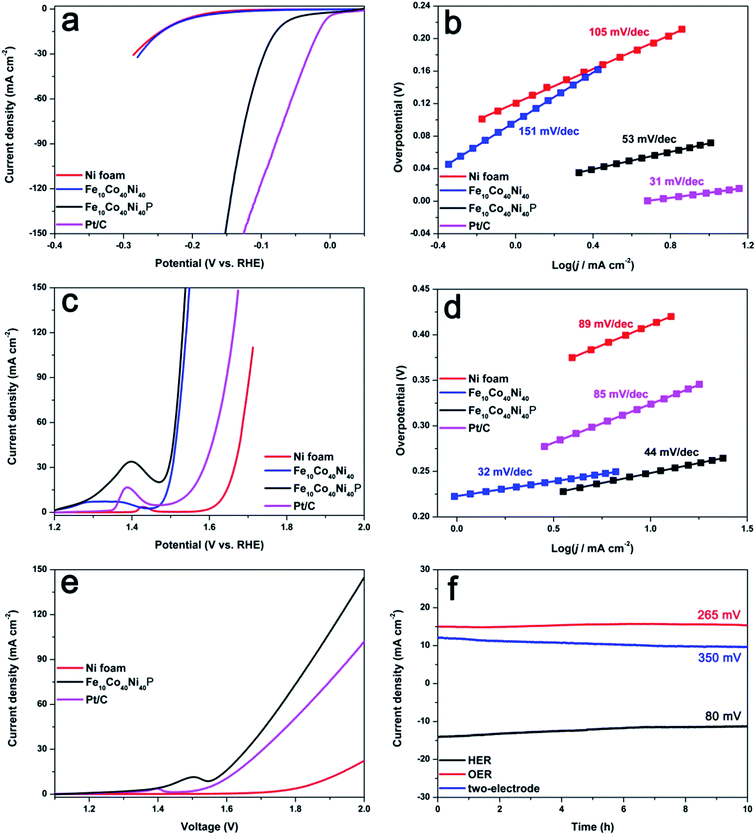
To verify whether Fe10Co40Ni40P could serve as high-performance bifunctional electrodes for full water splitting, the electrocatalytic activity in 1 M KOH solution was first evaluated by using a conventional three-electrode system, in which Fe10Co40Ni40P was used directly as the working electrode without a binding agent or conducting additive. Firstly, we optimize the catalytic property of the FexCoyNizP by synthesis of a series of samples with different amount of Fe and different molar ratio of Co and Ni. The ratios of the three metals were facilely tuned by varying the electrolyte employed, and the ratios in the final samples were determined by inductively coupled plasma optical emission spectrometer (ICP-OES, Table S1, see the ESI†). The electrochemical behavior of a series of FexCoyNizP is shown in Fig. S1 and S2.† We found that Fe10Co40Ni40P yielded the highest electrocatalytic activity. Fig. 3a shows the linear sweep voltammetry (LSV) curves of the samples with a scan rate of 2 mV s−1 at room temperature. As would be expected, the Pt/C catalyst exhibits extraordinary HER activity with an onset overpotential close to zero. For the Fe10Co40Ni40P electrode, it is shows much smaller onset overpotential of 42 mV vs. RHE, and beyond which the cathodic current rises rapidly under more negative potentials. The LSV curve recorded by the Fe10Co40Ni40P electrode shows a low overpotential of 68, 110, and 135 mV at current densities of 10, 50, and 100 mA cm−2, respectively. In sharp contrast, bare Ni foam and Fe10Co40Ni40 electrodes need overpotentials of 227 and 225 mV at a current density of 10 mA cm−2, respectively, which indicates their inferior HER catalytic activity. In order to investigate the kinetics of HER, the Tafel plots in the low current density region for all the catalyst samples are measured and shown in Fig. 3b. According to the Tafel equation (η = b![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) log
log![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) j + a, where j is the current density and b is the Tafel slope), it exhibits Tafel slopes of 31, 53, 151, and 105 mV dec−1 for Pt/C, Fe10Co40Ni40P, Fe10Co40Ni40, and Ni foam, respectively. Thus, Tafel curve of Fe10Co40Ni40P suggests that the HER process occurs through a Volmer–Heyrovsky mechanism, where the electrochemical desorption of Hads and H3O+ to form hydrogen is the rate-limiting step. As another key descriptor of an electrocatalyst, exchange current density (j0), is also carefully calculated by extrapolating the Tafel plots to overpotential of 0 V. The j0 value of Fe10Co40Ni40P was determined to be 4.6 × 10−1 mA cm−2, and is thus over 7 and 2 times that of Ni foam (7.0 × 10−2 mA cm−2) and Fe10Co40Ni40 (2.2 × 10−1 mA cm−2), respectively.
j + a, where j is the current density and b is the Tafel slope), it exhibits Tafel slopes of 31, 53, 151, and 105 mV dec−1 for Pt/C, Fe10Co40Ni40P, Fe10Co40Ni40, and Ni foam, respectively. Thus, Tafel curve of Fe10Co40Ni40P suggests that the HER process occurs through a Volmer–Heyrovsky mechanism, where the electrochemical desorption of Hads and H3O+ to form hydrogen is the rate-limiting step. As another key descriptor of an electrocatalyst, exchange current density (j0), is also carefully calculated by extrapolating the Tafel plots to overpotential of 0 V. The j0 value of Fe10Co40Ni40P was determined to be 4.6 × 10−1 mA cm−2, and is thus over 7 and 2 times that of Ni foam (7.0 × 10−2 mA cm−2) and Fe10Co40Ni40 (2.2 × 10−1 mA cm−2), respectively.
The multi-electron process of OER makes it the bottleneck in water splitting for a sustainable hydrogen evolution. Fig. 3c shows the typical LSV curves for OER at scan rate of 2 mV s−1, the current rises rapidly as the potential becomes more positive. To avoid the oxidation peak (Ni2+/Ni3+), we reversely swept the LSV curve (Fig. S3†), which very close to the forward LSV curve. It is observed that Fe10Co40Ni40P show a more negative OER onset potential (1.47 V vs. RHE) than the Pt/C (1.54 V) and Ni foam (1.64 V). For Fe10Co40Ni40P, the overpotentials required to generate a current density of 10, 50, and 100 mA cm−2 are 250, 277, and 295 mV, respectively, which is similar to Fe10Co40Ni40 and dramatically surpasses both Pt/C and Ni foam. As shown in Fig. 3d, the Tafel slope of Fe10Co40Ni40P is 44 mV dec−1, while those of Pt/C and Ni foam are 85 and 89 mV dec−1, respectively, suggesting that Fe10Co40Ni40P enables a faster deprotonation of OH−. These results demonstrate that Fe10Co40Ni40P is a highly active electrocatalyst for both HER and OER in basic media. To illustrate the dual function of the Fe10Co40Ni40P, the full water splitting reaction in a two-electrode setup was investigated using Fe10Co40Ni40P as both anode and cathode. Fe10Co40Ni40P achieves current density of 10 mA cm−2 at a voltage of 1.57 V (Fig. 3e and S4†), whereas Pt/C and Ni foam need voltages of 1.60 and 1.89 V to reach a current density of 10 mA cm−2, respectively (Fig. 3e). To the best of our knowledge, this is one of the most efficient bifunctional electrocatalyst for full water splitting in basic medium reported thus far (Table S2†). Stability is another important criterion of a catalyst. The current–time (I–t) curves operated for HER, OER, and two-electrode electrolyzer are shown in Fig. 3f. After a long period of 10 h, only a slight decrease in the current density is observed, which confirms the excellent stability of Fe10Co40Ni40P.
Besides operating well as a bifunctional electrocatalyst for full water splitting in basic medium, Fe10Co40Ni40P was also found to be an excellent bifunctional electrocatalyst in neutral media. Compared with basic solutions, the neutral solutions have the advantages of low corrosion and small environmental pollution, which is the key for large-scale applications. However, few bifunctional electrocatalysts operated in neutral aqueous media have been reported. Fig. 4a shows the LSV curves of Ni foam, Fe10Co40Ni40, Fe10Co40Ni40P, and Pt/C for HER under neutral media (pH 7.0). Fe10Co40Ni40P produces a current density of 10 mA cm−2 under an overpotential of 88 mV, which is much smaller than overpotentials measured with Ni foam (398 mV) and Fe10Co40Ni40 (300 mV). The Tafel slope of Fe10Co40Ni40P is 62 mV dec−1 (Fig. 4b), which also indicates that it has great performances in electrocatalytic HER under neutral conditions. As shown in Fig. 4c, Fe10Co40Ni40P also shows electrochemically active toward the OER under neutral media (pH 7.0). The overpotential of 466 mV is needed to afford a current density of 10 mA cm−2, which is much smaller than that of Fe10Co40Ni40 (554 mV), Pt/C (575 mV), Ni foam (652 mV), and is superior to most reported non-noble-metal OER catalysts in neutral media (Table S3†).
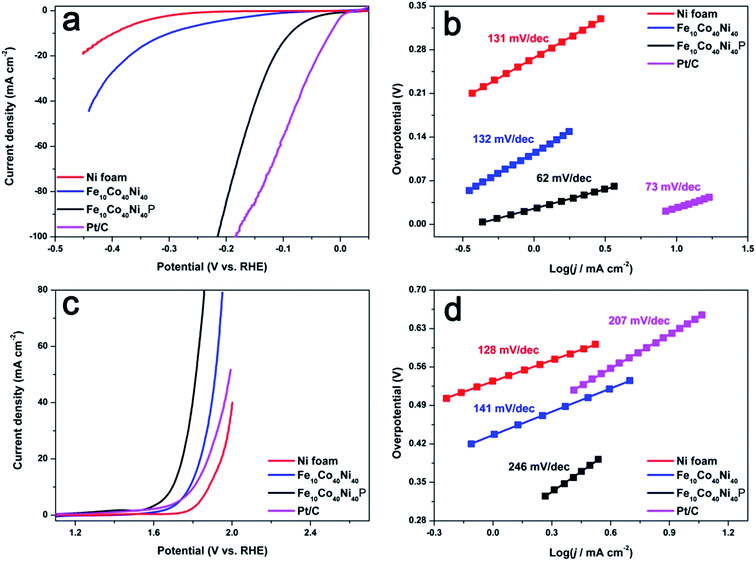 | ||
| Fig. 4 LSV curves for HER (a) and OER (c) of Ni foam, Fe10Co40Ni40, Fe10Co40Ni40P, and Pt/C with a scan rate of 2 mV s−1, and corresponding Tafel plots for HER (b) and OER (d) in 1 M PBS at pH 7.0. | ||
In order to investigate the effect of each component on the catalytic activity, monometallic phosphide samples, Fe90Co00Ni00P, Fe00Co90Ni00P, and Fe00Co00Ni90P, were prepared and their HER and OER activity were evaluated. As shown in Fig. S7a,† the Fe00Co90Ni00P catalyst exhibits the highest HER performance among the three samples and has similar catalytic activity to Fe10Co40Ni40P. Fig. 7Sb† presents the OER process: the LSV curves display a remarkable small overpotential to generate a current density of 50 mA cm−2 for Fe00Co00Ni90P with the smallest value of only 278 mV. This is significantly lower than that of Fe00Co90Ni00P (326 mV) and Fe90Co00Ni00P of (345 mV) as well as similar to Fe10Co40Ni40P. Experimental activity trends of Co > Fe > Ni for HER and Ni > Co > Fe for OER were found in alkaline media. Overall, among these monometallic phosphide samples, Fe00Co90Ni00P demonstrates the highest activity for HER and Fe00Co00Ni90P exhibits the highest activity for OER, suggesting that Co species enable optimized HER activity and Ni species serve as excellent OER components. To investigate the synergistic effect between different species, we also synthesized a series of binary phosphides with same molar ratios to Fe10Co40Ni40P to examine their activities for HER and OER. Fig. S8a† shows the HER operation for each catalyst. While all of the samples are active for HER, the unique synergistic effect between all binary phosphides increases their activity over the monometallic phosphides (Fig. S7a†). Among the binary phosphides, Fe18Co00Ni72P is the most inactive catalyst than either Fe18Co72Ni00P or Fe00Co45Ni45P, which suggests that Co species may be necessary for maintaining good HER activity. In addition, OER activities of binary phosphides were also evaluated and compared in Fig. S8b,† Fe18Co00Ni72P exhibits the highest activity, superior to Fe18Co72Ni00P and Fe00Co45Ni45P, and even better than Fe10Co40Ni40P. Previous reports have confirmed that the presence of small amounts of iron in binary and ternary catalyst can significantly improve the OER catalytic performance,66,86,87 and our results clearly support the fact that high-performance OER catalyst can be obtained via the Fe-doped complex catalyst.
To better understand the origin of the excellent bifunctional activity of Fe10Co40Ni40P, the surface compositions were further analyzed by X-ray photoelectron spectroscopy (XPS). The P 2p high-resolution spectrum of Fe10Co40Ni40P shown in Fig. 5a1 can be deconvoluted into three peaks at 128.6, 129.5, and 132.9 eV, which correspond to the binding energies (BEs) of P 2p3/2 and P 2p1/2 in phosphides and phosphorus oxide, respectively. Fig. 5a2 shows the high-resolution region of Fe 2P for Fe10Co40Ni40P and the peak at 706.4 eV is close to Fe 2p3/2 in FeP. Compared to monometallic phosphides, the BEs of P 2p3/2 and Fe 2p3/2 for Fe10Co40Ni40P downshift slightly to the lower direction. The downshift causes electron localization on P and Fe and increases their electron donating ability, which makes the electron transfer more favorable. For HER in basic media, three principle steps for converting H2O to H2 have been proposed:
| H2O + e− → Hads + OH− (Volmer) | (1) |
| H2O + Hads + e− → H2 + OH− (Heyrovsky) | (2) |
| Hads + Hads → H2 (Tafel) | (3) |
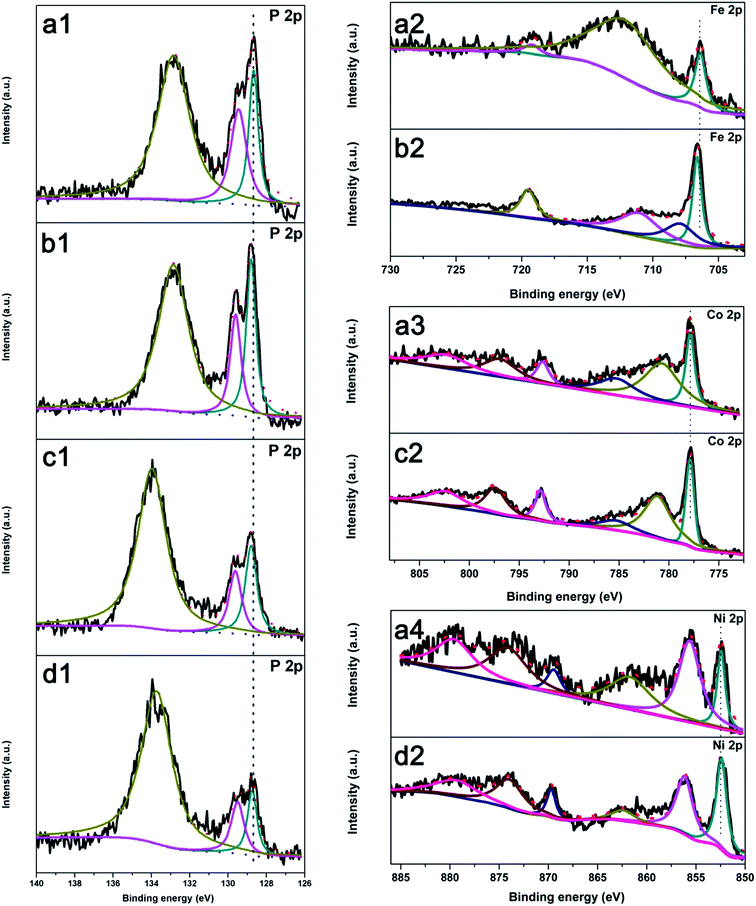 | ||
| Fig. 5 High-resolution XPS spectra of Fe10Co40Ni40P (a), Fe90Co00Ni00P (b), Fe00Co90Ni00P (c), and Fe00Co00Ni90P (d) in the different regions. | ||
Tafel curve of Fe10Co40Ni40P suggests the HER occurs through a Volmer–Heyrovsky mechanism, which involves the adsorption of H2O molecules on catalyst surface and dissociation of adsorbed H2O molecules into Hads and OH− species, followed by desorption of OH− and release H2 from catalyst surface. The electron localization on P and Fe increases their electron-donating ability and makes the electron transfer more favorable to H2O molecules, while the OH− species dissociated from H2O molecules can facilitate desorption from catalyst to refresh the surface due to electrostatic repulsion. We further analyzed the activity of catalyst toward OER. As shown in Fig. S6d,† EDX measurement performed on the Fe10Co40Ni40P after OER test in 1 M KOH showed a significant drop in P intensity and rise in O intensity. Previous researches had indicated that the original phosphides can be partially oxidized to hydro(oxy)oxides and phosphate during OER and a core–shell heterostructure was obtained.70,76,88 In our case, the catalytic mechanism of Fe10Co40Ni40P is similar to previously reported transition-metal phosphides during the OER process in basic solution. Initially formed hydro(oxy)oxides and phosphate serve as the active skin for OER and high conductivity of transition-metal phosphides core can make sure electron transport from conductive support to the active phases.
In conclusion, a highly efficient, freestanding bifunctional electrode for full water splitting is prepared via a two-step process of electrodeposition and low-temperature phosphidation. In basic solution, the electrode can deliver a catalytic current of 10 mA cm−2 at overpotentials of just 68 and 250 mV for HER and ORE, respectively. An applied voltage of 1.57 V is required to obtain a 10 mA cm−2 full water splitting current via an electrolyzer constructed by Fe10Co40Ni40P for both anode and cathode. This electrode also displays excellent activity for catalyzing the HER and OER in neutral media and overpotentials of 88 and 466 mV are needed to afford a 10 mA cm−2 current for HER and OER, respectively. Excellent catalytic performance of FeCoNiP has been supposed as: (i) 3D structure of the electrode with open space can provide more reactive sites and is beneficial for diffusion of electrolyte and bubbles escape from the electrode surface. (ii) Binder-free electrode structured via directly growth 2D nanosheets on Ni foam offers lower contact resistance and facilitates interfacial electron transport between catalyst and current collector. (iii) The synergistic and strong couple effects from the iron triad (Fe, Co, Ni) trinary phosphides also contribute to the enhanced activity.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (no. 21405152, 21275140) and the Science and Technology Development Plan of Jilin Province (20130101126JC).Notes and references
- J. Kibsgaard, Z. Chen, B. N. Reinecke and T. F. Jaramillo, Nat. Mater., 2012, 11, 963–969 CrossRef CAS PubMed.
- H. M. Chen, C. K. Chen, R.-S. Liu, L. Zhang, J. Zhang and D. P. Wilkinson, Chem. Soc. Rev., 2012, 41, 5654–5671 RSC.
- X. Zou and Y. Zhang, Chem. Soc. Rev., 2015, 44, 5148–5180 RSC.
- Y. Zheng, Y. Jiao, M. Jaroniec and S. Z. Qiao, Angew. Chem., Int. Ed., 2015, 54, 52–65 CrossRef CAS PubMed.
- J. Wang, H.-x. Zhong, Y.-l. Qin and X.-b. Zhang, Angew. Chem., Int. Ed., 2013, 52, 5248–5253 CrossRef CAS PubMed.
- C. G. Morales-Guio, L.-A. Stern and X. Hu, Chem. Soc. Rev., 2014, 43, 6555–6569 RSC.
- M. S. Faber and S. Jin, Energy Environ. Sci., 2014, 7, 3519–3542 CAS.
- M. W. Kanan and D. G. Nocera, Science, 2008, 321, 1072–1075 CrossRef CAS PubMed.
- D. A. Lutterman, Y. Surendranath and D. G. Nocera, J. Am. Chem. Soc., 2009, 131, 3838–3839 CrossRef CAS PubMed.
- Y. Surendranath, M. Dinca and D. G. Nocera, J. Am. Chem. Soc., 2009, 131, 2615–2620 CrossRef CAS PubMed.
- M. Risch, V. Khare, I. Zaharieva, L. Gerencser, P. Chernev and H. Dau, J. Am. Chem. Soc., 2009, 131, 6936–6937 CrossRef CAS PubMed.
- K. Jin, J. Park, J. Lee, K. D. Yang, G. K. Pradhan, U. Sim, D. Jeong, H. L. Jang, S. Park, D. Kim, N.-E. Sung, S. H. Kim, S. Han and K. T. Nam, J. Am. Chem. Soc., 2014, 136, 7435–7443 CrossRef CAS PubMed.
- T. F. Jaramillo, K. P. Jorgensen, J. Bonde, J. H. Nielsen, S. Horch and I. Chorkendorff, Science, 2007, 317, 100–102 CrossRef CAS PubMed.
- H. I. Karunadasa, E. Montalvo, Y. Sun, M. Majda, J. R. Long and C. J. Chang, Science, 2012, 335, 698–702 CrossRef CAS PubMed.
- Y. Li, H. Wang, L. Xie, Y. Liang, G. Hong and H. Dai, J. Am. Chem. Soc., 2011, 133, 7296–7299 CrossRef CAS PubMed.
- M.-R. Gao, J.-X. Liang, Y.-R. Zheng, Y.-F. Xu, J. Jiang, Q. Gao, J. Li and S.-H. Yu, Nat. Commun., 2015, 6, 5982 CrossRef PubMed.
- L. Liang, H. Cheng, F. Lei, J. Han, S. Gao, C. Wang, Y. Sun, S. Qamar, S. Wei and Y. Xie, Angew. Chem., Int. Ed., 2015, 54, 12004–12008 CrossRef CAS PubMed.
- Y. Sun, C. Liu, D. C. Grauer, J. Yano, J. R. Long, P. Yang and C. J. Chang, J. Am. Chem. Soc., 2013, 135, 17699–17702 CrossRef CAS PubMed.
- D.-Y. Wang, M. Gong, H.-L. Chou, C.-J. Pan, H.-A. Chen, Y. Wu, M.-C. Lin, M. Guan, J. Yang, C.-W. Chen, Y.-L. Wang, B.-J. Hwang, C.-C. Chen and H. Dai, J. Am. Chem. Soc., 2015, 137, 1587–1592 CrossRef CAS PubMed.
- J. Yuan, J. Wu, W. J. Hardy, P. Loya, M. Lou, Y. Yang, S. Najmaei, M. Jiang, F. Qin, K. Keyshar, H. Ji, W. Gao, J. Bao, J. Kono, D. Natelson, P. M. Ajayan and J. Lou, Adv. Mater., 2015, 27, 5605–5609 CrossRef CAS PubMed.
- K. Xu, F. Wang, Z. Wang, X. Zhan, Q. Wang, Z. Cheng, M. Safdar and J. He, ACS Nano, 2014, 8, 8468–8476 CrossRef CAS PubMed.
- J. Yang, D. Voiry, S. J. Ahn, D. Kang, A. Y. Kim, M. Chhowalla and H. S. Shin, Angew. Chem., Int. Ed., 2013, 52, 13751–13754 CrossRef CAS PubMed.
- S. Chen and S.-Z. Qiao, ACS Nano, 2013, 7, 10190–10196 CrossRef CAS PubMed.
- J. Masa, W. Xia, I. Sinev, A. Zhao, Z. Sun, S. Gruetzke, P. Weide, M. Muhler and W. Schuhmann, Angew. Chem., Int. Ed., 2014, 53, 8508–8512 CrossRef CAS PubMed.
- J. Rosen, G. S. Hutchings and F. Jiao, J. Am. Chem. Soc., 2013, 135, 4516–4521 CrossRef CAS PubMed.
- A. Indra, P. W. Menezes, I. Zaharieva, E. Baktash, J. Pfrommer, M. Schwarze, H. Dau and M. Driess, Angew. Chem., Int. Ed., 2013, 52, 13206–13210 CrossRef CAS PubMed.
- M.-R. Gao, Y.-F. Xu, J. Jiang, Y.-R. Zheng and S.-H. Yu, J. Am. Chem. Soc., 2013, 135, 6378 CrossRef CAS.
- Y. Yang, H. Fei, G. Ruan, C. Xiang and J. M. Tour, ACS Nano, 2014, 8, 9518–9523 CrossRef CAS PubMed.
- L. Kuai, J. Geng, C. Chen, E. Kan, Y. Liu, Q. Wang and B. Geng, Angew. Chem., Int. Ed., 2014, 53, 7547–7551 CrossRef CAS PubMed.
- M. Gao, W. Sheng, Z. Zhuang, Q. Fang, S. Gu, J. Jiang and Y. Yan, J. Am. Chem. Soc., 2014, 136, 7077–7084 CrossRef CAS PubMed.
- J. Huang, J. Chen, T. Yao, J. He, S. Jiang, Z. Sun, Q. Liu, W. Cheng, F. Hu, Y. Jiang, Z. Pan and S. Wei, Angew. Chem., Int. Ed., 2015, 54, 8722–8727 CrossRef CAS PubMed.
- X. Zou, A. Goswami and T. Asefa, J. Am. Chem. Soc., 2013, 135, 17242–17245 CrossRef CAS PubMed.
- C. Tang, H.-S. Wang, H.-F. Wang, Q. Zhang, G.-L. Tian, J.-Q. Nie and F. Wei, Adv. Mater., 2015, 27, 4516–4522 CrossRef CAS PubMed.
- N. Danilovic, R. Subbaraman, D. Strmcnik, K.-C. Chang, A. P. Paulikas, V. R. Stamenkovic and N. M. Markovic, Angew. Chem., Int. Ed., 2012, 51, 12495–12498 CrossRef CAS PubMed.
- X. Lu and C. Zhao, Nat. Commun., 2015, 6, 6616 CrossRef CAS PubMed.
- C. Wan, Y. N. Regmi and B. M. Leonard, Angew. Chem., Int. Ed., 2014, 53, 6407–6410 CrossRef CAS PubMed.
- J. Zhu, K. Sakaushi, G. Clavel, M. Shalom, M. Antonietti and T.-P. Fellinger, J. Am. Chem. Soc., 2015, 137, 5480–5485 CrossRef CAS PubMed.
- P. Xiao, X. Ge, H. Wang, Z. Liu, A. Fisher and X. Wang, Adv. Funct. Mater., 2015, 25, 1520–1526 CrossRef CAS.
- S. T. Hunt, T. Nimmanwudipong and Y. Roman-Leshkov, Angew. Chem., Int. Ed., 2014, 53, 5131–5136 CAS.
- Y. Liu, G.-D. Li, L. Yuan, L. Ge, H. Ding, D. Wang and X. Zou, Nanoscale, 2015, 7, 3130–3136 RSC.
- H. Vrubel and X. Hu, Angew. Chem., Int. Ed., 2012, 51, 12703–12706 CrossRef CAS PubMed.
- W.-F. Chen, K. Sasaki, C. Ma, A. I. Frenkel, N. Marinkovic, J. T. Muckerman, Y. Zhu and R. R. Adzic, Angew. Chem., Int. Ed., 2012, 51, 6131–6135 CrossRef CAS PubMed.
- J. Xie, S. Li, X. Zhang, J. Zhang, R. Wang, H. Zhang, B. Pan and Y. Xie, Chem. Sci., 2014, 5, 4615–4620 RSC.
- B. Cao, G. M. Veith, J. C. Neuefeind, R. R. Adzic and P. G. Khalifah, J. Am. Chem. Soc., 2013, 135, 19186–19192 CrossRef CAS PubMed.
- E. J. Popczun, C. G. Read, C. W. Roske, N. S. Lewis and R. E. Schaak, Angew. Chem., Int. Ed., 2014, 53, 5427–5430 CrossRef CAS PubMed.
- Q. Liu, J. Tian, W. Cui, P. Jiang, N. Cheng, A. M. Asiri and X. Sun, Angew. Chem., Int. Ed., 2014, 53, 6710–6714 CrossRef CAS PubMed.
- Z. Zhang, J. Hao, W. Yang and J. Tang, ChemCatChem, 2015, 7, 1920–1925 CrossRef CAS.
- J. Tian, Q. Liu, N. Cheng, A. M. Asiri and X. Sun, Angew. Chem., Int. Ed., 2014, 53, 9577–9581 CrossRef CAS PubMed.
- P. Jiang, Q. Liu, Y. Liang, J. Tian, A. M. Asiri and X. Sun, Angew. Chem., Int. Ed., 2014, 53, 12855–12859 CrossRef CAS PubMed.
- Z. Zhang, B. Lu, J. Hao, W. Yang and J. Tang, Chem. Commun., 2014, 50, 11554–11557 RSC.
- Z. Zhang, J. Hao, W. Yang, B. Lu and J. Tang, Nanoscale, 2015, 7, 4400–4405 RSC.
- Z. Xing, Q. Liu, A. M. Asiri and X. Sun, Adv. Mater., 2014, 26, 5702–5707 CrossRef CAS PubMed.
- X. Wang, Y. V. Kolen'ko, X.-Q. Bao, K. Kovnir and L. Liu, Angew. Chem., Int. Ed., 2015, 54, 8188–8192 CrossRef CAS PubMed.
- E. J. Popczun, J. R. McKone, C. G. Read, A. J. Biacchi, A. M. Wiltrout, N. S. Lewis and R. E. Schaak, J. Am. Chem. Soc., 2013, 135, 9267–9270 CrossRef CAS PubMed.
- Z. Pu, Q. Liu, A. M. Asiri and X. Sun, ACS Appl. Mater. Interfaces, 2014, 6, 21874–21879 CAS.
- J.-I. Jung, H. Y. Jeong, J.-S. Lee, M. G. Kim and J. Cho, Angew. Chem., Int. Ed., 2014, 53, 4582–4586 CrossRef CAS PubMed.
- J. Kim, X. Yin, K.-C. Tsao, S. Fang and H. Yang, J. Am. Chem. Soc., 2014, 136, 14646–14649 CrossRef CAS PubMed.
- F. Cheng, J. Shen, B. Peng, Y. Pan, Z. Tao and J. Chen, Nat. Chem., 2011, 3, 79–84 CrossRef CAS PubMed.
- J. Suntivich, K. J. May, H. A. Gasteiger, J. B. Goodenough and Y. Shao-Horn, Science, 2011, 334, 1383–1385 CrossRef CAS PubMed.
- Y. Zhao, R. Nakamura, K. Kamiya, S. Nakanishi and K. Hashimoto, Nat. Commun., 2013, 4, 2390 Search PubMed.
- Y. Zheng, Y. Jiao, Y. Zhu, L. H. Li, Y. Han, Y. Chen, A. Du, M. Jaroniec and S. Z. Qiao, Nat. Commun., 2014, 5, 3783 Search PubMed.
- Y. Zhao, F. Zhao, X. Wang, C. Xu, Z. Zhang, G. Shi and L. Qu, Angew. Chem., Int. Ed., 2014, 53, 13934–13939 CrossRef CAS PubMed.
- X. Lu, W.-L. Yim, B. H. R. Suryanto and C. Zhao, J. Am. Chem. Soc., 2015, 137, 2901–2907 CrossRef CAS PubMed.
- W. Zhou and L. Guo, Chem. Soc. Rev., 2015, 44, 6697–6707 RSC.
- H. Wang, H. Yuan, S. S. Hong, Y. Li and Y. Cui, Chem. Soc. Rev., 2015, 44, 2664–2680 RSC.
- R. D. L. Smith, M. S. Prevot, R. D. Fagan, S. Trudel and C. P. Berlinguette, J. Am. Chem. Soc., 2013, 135, 11580–11586 CrossRef CAS PubMed.
- C. Yuan, H. B. Wu, Y. Xie and X. W. Lou, Angew. Chem., Int. Ed., 2014, 53, 1488–1504 CrossRef CAS PubMed.
- S. Cobo, J. Heidkamp, P.-A. Jacques, J. Fize, V. Fourmond, L. Guetaz, B. Jousselme, V. Ivanova, H. Dau, S. Palacin, M. Fontecave and V. Artero, Nat. Mater., 2012, 11, 802–807 CrossRef CAS PubMed.
- W. Ma, R. Ma, C. Wang, J. Liang, X. Liu, K. Zhou and T. Sasaki, ACS Nano, 2015, 9, 1977–1984 CrossRef CAS PubMed.
- M. Ledendecker, S. Krick Calderon, C. Papp, H.-P. Steinruck, M. Antonietti and M. Shalom, Angew. Chem., Int. Ed., 2015, 54, 12361–12365 CrossRef CAS PubMed.
- Y. Hou, Z. Wen, S. Cui, S. Ci, S. Mao and J. Chen, Adv. Funct. Mater., 2015, 25, 872–882 CrossRef CAS.
- X. Liu, W. Liu, M. Ko, M. Park, M. G. Kim, P. Oh, S. Chae, S. Park, A. Casimir, G. Wu and J. Cho, Adv. Funct. Mater., 2015, 25, 5799–5808 CrossRef CAS.
- Y. Yang, H. Fei, G. Ruan and J. M. Tour, Adv. Mater., 2015, 27, 3175–3180 CrossRef CAS PubMed.
- J. Ren, M. Antonietti and T.-P. Fellinger, Adv. Energy Mater., 2015, 5, DOI:10.1002/aenm.201401660.
- X. Yu, T. Hua, X. Liu, Z. Yan, P. Xu and P. Du, ACS Appl. Mater. Interfaces, 2014, 6, 15395–15402 CAS.
- N. Jiang, B. You, M. Sheng and Y. Sun, Angew. Chem., Int. Ed., 2015, 54, 6251–6254 CrossRef CAS PubMed.
- C. Tang, N. Cheng, Z. Pu, W. Xing and X. Sun, Angew. Chem., Int. Ed., 2015, 54, 9351–9355 CrossRef CAS PubMed.
- M. Ledendecker, S. Krick Calderón, C. Papp, H.-P. Steinrück, M. Antonietti and M. Shalom, Angew. Chem., Int. Ed., 2015, 54, 12361–12365 CrossRef CAS PubMed.
- S. Du, Z. Ren, J. Zhang, J. Wu, W. Xi, J. Zhu and H. Fu, Chem. Commun., 2015, 51, 8066–8069 RSC.
- L.-L. Feng, G. Yu, Y. Wu, G.-D. Li, H. Li, Y. Sun, T. Asefa, W. Chen and X. Zou, J. Am. Chem. Soc., 2015, 137, 14023–14026 CrossRef CAS PubMed.
- H. Jin, J. Wang, D. Su, Z. Wei, Z. Pang and Y. Wang, J. Am. Chem. Soc., 2015, 137, 2688–2694 CrossRef CAS PubMed.
- D. Liu, Q. Lu, Y. Luo, X. Sun and A. M. Asiri, Nanoscale, 2015, 7, 15122–15126 RSC.
- H. Wang, H.-W. Lee, Y. Deng, Z. Lu, P.-C. Hsu, Y. Liu, D. Lin and Y. Cui, Nat. Commun., 2015, 6, 7261 CrossRef CAS PubMed.
- Z. Lu, W. Zhu, X. Yu, H. Zhang, Y. Li, X. Sun, X. Wang, H. Wang, J. Wang, J. Luo, X. Lei and L. Jiang, Adv. Mater., 2014, 26, 2683–2687 CrossRef CAS PubMed.
- L. Qian, Z. Lu, T. Xu, X. Wu, Y. Tian, Y. Li, Z. Huo, X. Sun and X. Duan, Adv. Energy Mater., 2015, 5 Search PubMed.
- L. Trotochaud, S. L. Young, J. K. Ranney and S. W. Boettcher, J. Am. Chem. Soc., 2014, 136, 6744–6753 CrossRef CAS PubMed.
- D. Friebel, M. W. Louie, M. Bajdich, K. E. Sanwald, Y. Cai, A. M. Wise, M.-J. Cheng, D. Sokaras, T.-C. Weng, R. Alonso-Mori, R. C. Davis, J. R. Bargar, J. K. Norskov, A. Nilsson and A. T. Bell, J. Am. Chem. Soc., 2015, 137, 1305–1313 CrossRef CAS PubMed.
- L.-A. Stern, L. Feng, F. Song and X. Hu, Energy Environ. Sci., 2015, 8, 2347–2351 CAS.
Footnote |
| † Electronic supplementary information (ESI) available. See DOI: 10.1039/c5ra26748j |
| This journal is © The Royal Society of Chemistry 2016 |